Abstract
Gamma-secretase inhibitor, N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester (DAPT) suppresses the activation of Notch 1 signaling, which is recognized as the cell fate signaling and may participate in inflammatory processes together with NF-κB pathway that contributes to the brain damage after stroke. DAPT has important pharmacological roles in many diseases. However, little is known about the effect of DAPT on NF-κB during cerebral ischemia. This study investigated the time course expression of Notch 1 and the effects of DAPT on Notch 1 and NF-κB after MCAO. The results showed that Notch 1 signaling was up-regulated at the early stage after MCAO, DAPT down-regulated the expression of Notch 1 and NF-κB and protected brain from damage caused by MCAO. These results may indicate that the downregulation of Notch 1–NF-κB pathway after ischemia by administration of DAPT is a potential mechanism for its protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Notch 1 signaling pathway is of fundamental importance in a wide variety of pathophysiological processes in adults including apoptosis and inflammatory processes, which are parts of the mechanism of brain injury resulting from cerebral ischemia. One of γ-secretase inhibitor, N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester (DAPT), efficiently inhibiting the activation of the Notch 1 signaling [1–5], has been used to treat neurodegenerative diseases [3, 6, 7] and modulated the differentiation of neural progenitor and the apoptotic cascades in neurons in cerebral ischemia which contribute to the neuroprotection of DAPT [8, 9]. Little is known weather DAPT protects brain from cerebral ischemia by interfering in inflammatory processes. NF-κB, a family of transcription factors [10, 11], participated in ischemic injury by promoting inflammatory processes and inducing the apoptosis of neurons [12]. But study on the relationship between Notch1 and NF-κB is sparse [13, 14]. The present project is to examine whether DAPT administration can protect cerebral ischemia and whether Notch1 and NF-κB are involved in DAPT protection in cerebral ischemia in rat.
Materials and methods
Animals
Male Sprague–Dawley rats (260–290 g) were purchased from Hebei Medical University. The protocol was approved by the institutional animal care and use committee and the local experimental ethics committee. All rats were allowed free access to food and water under controlled conditions (12/12 h light/dark cycle with humidity of 60 ± 5%, 22 ± 3°C).
Rat model of permanent focal cerebral ischemia
A modified model of MCAO was used to make permanent focal ischemia as previously described [15, 16]. Animals were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg). Body temperature was monitored and maintained at 36.5–37.5°C. Briefly, after midline skin incision, the right common carotid artery (CCA) and external carotid artery (ECA) were exposed and isolated by blunt dissection. ECAs were dissected and the distal branches ligated. The right middle cerebral artery (MCA) was permanent occluded by intraluminal placement of filament, as described previously. The common right carotid artery was exposed and isolated. MCA was occluded by inserting a filament into the internal carotid artery, which was advanced further until it closes the origin of the MCA. Sham-operated control rats received the same procedure except for intraluminal insertion of the filament.
Groups and drug administration
Experiment 1: dynamic expression of Notch 1 in cerebral ischemia
42 rats were randomly assigned to seven time course groups (n = 6 in each group), including a normal-control group (Normal) and 3, 6, 12, 24, 48 and 72 h after MCAO. Immunohistochemistry and Western blot were used to analyze the dynamic expression of Notch 1.
Experiment 2: DAPT’s protective effect in the acute phase of cerebral ischemia
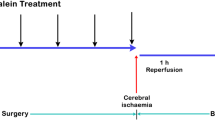
DAPT powder (Sigma-Aldrich, St. Louis, MO, USA), was dissolved in 0.01 M phosphate-buffered saline (PBS) including 5% DMSO to prepare concentrations of 8.3 mg/ml. DAPT solution was stereotactically injected into the lateral cerebral ventricle (LV) immediately after MCAO. The stereotactic injections into the LVs were performed at coordinates −0.8 mm anteroposterior, ±1.5 mm mediolateral and −4.5 mm dorsoventral from the bregma. 30 rats were randomly assigned to three operating groups (10 rats in each group): sham-operated group that received equal volume of PBS without MCAO operation (Sham); MCAO group that received equal volume PBS after MCAO (MCAO); and DAPT group that received DAPT as 0.03 mg/kg after MCAO. 24 h after operation the first neurological function was assessed and then 48 h after operation the second neurological function was assessed. Meanwhile, brain water content and infarction volume were measured and compared among different groups.
Experiment 3: the effect of DAPT on Notch 1 and NF-κB
In this part rats were still randomly divided into three groups as Sham group, MCAO group and DAPT group (n = 9 for each group). Rats were reanesthetized and killed 48 h after being successfully operated. Immunohistochemistry, Western blot and Immunofluorescence were used to detect the expression of Notch 1 and NF-κB.
Analysis of neurological deficit scores
A neurological test was carried out by an examiner blinded to the experimental groups. The deficits were scored on a modified scoring system based on that developed by Longa et al. (1989) as follows: 0, no deficits; 1, difficulty in fully extending the contralateral forelimb; 2, unable to extend the contralateral forelimb; 3, mild circling to the contralateral side; 4, severe circling; and 5, falling to the contralateral side. Rats with 0, 1 and 5 were excluded from the study.
Measurement of brain water content
Following neurological behavior tests, rats were killed at 3, 6, 12, 24, 48 and 72 h after operation. Brain tissues were rapidly obtained and first weighed wet on an electronic balance and then dried for 24 h at (100 ± 5)°C to measure dry weight. Results were calculated as follows: brain water content = (wet weight − dry weight)/wet weight × 100% [17].
Measurement of infarction volume
Rats were re-anesthetized after neurological behavior test at each time point and the brains were removed quickly. Coronal brain sections (2 mm thick) were stained with 2% TTC at 37°C for 20 min. The stained cerebral sections were photographed and ipsilateral and contralateral hemispheric volumes and infarct volumes were quantified with the use of Image Pro-Plus 5.1 analysis system. To compensate for the effect of brain edema, the infarction volumes were calculated by the following equations. Percentage hemisphere lesion volume (%HLV) = {[total infarct volume − (the volume of intact ipsilateral hemisphere − the volume of intact contralateral hemisphere)]/contralateral hemisphere volume} × 100% [18].
Immunohistochemistry
Brains were removed quickly, immersed with 4% paraformaldehyde in 0.01 M PBS for 3–7 days at ordinary temperature. Following embedding in paraffin, the tissues were serially sectioned in 5-μm-thick slices for application to the standard histological procedure as follows: the slices were blocked in 3% H2O2, 3% normal goat serum and incubated with Notch 1 rabbit polyclonal antibody (1:400, Abcam Biotechnology), NF-κB P65 rabbit polyclonal antibody (1:150, Santa Cruz Biotechnology) in 0.01 M phosphate-buffered saline over night. The secondary antibodies, secondary biotinylated conjugates and diaminobenzidine were from the Vect ABC kit (Zhongshan Biology Technology Company, China). Five visual fields of ischemic region of the infarct were selected and the immunoreactive cells were counted under a 400× light microscope.
Western blot
Rats were killed by decapitation under anesthesia. Total protein was extracted from infarction zone and peri-infarction cortex using a Total Protein Extraction Kit (Applygen Technologies Inc., Beijing) following the manufacturer’s protocols. Then the protein concentration was determined using a BCA Protein Assay reagent kit (Novagen, Madison, WI, USA). For polyacrylamide gel electrophoresis (PAGE), samples were boiled at 100°C for 5 min after the addition of the sample buffer. 50 µg of proteins was separated by SDS/PAGE, transferred 2 h on to PVDF membranes and the nonspecific binding of antibodies was blocked with 5% non-fat dry milk in PBS. Membranes were then probed with Notch 1 rabbit polyclonal antibody (1:300, Abcam Biotechnology), NF-κB P65 rabbit polyclonal antibody (1:100, Santa Cruz Biotechnology) overnight at 4°C. Membranes loaded with primary antibodies were washed with 0.1% tween-20 Tris-buffered saline and then were incubated with fluorescent labeling second antibodies (goat anti-rabbit, 1:8,000, Rockland, Gilbertsville, PA, USA) for 1 h at room temperature. An imaging densitometer (LI-COR Bioscience) was used to analyze the relative density of each band. Anti-rat β-actin (1:500, Zhongshan Biotechnology) was used as internal control.
Immunofluorescence
First, nylon monofilaments inserted into ICA were pulled out, and brains were perfused transcardially with saline quickly followed by 4% paraformaldehyde. Frozen coronal sections (30-μm-thick) were prepared at −20°C. After soaking in 0.3% Triton X-100 for 10 min and blocking with 10% normal horse serum for half an hour, brain sections were incubated with Notch 1 rabbit polyclonal antibody (1:300, Abcam Biotechnology), NF-κB P65 rabbit polyclonal antibody (1:100, Santa Cruz Biotechnology) at 4°C overnight. On the second day, slices were incubated with secondary antibody (anti-rabbit FITC, 1:100 dilution, Beijing) and nuclear marker Hoechst 33342 for 1 h and then were observed under 20× Laser Scanning Confocal Microscope (Olympus FV10-ASW, Japan).
Statistical analysis
All data were analyzed using SPSS 13.0 software. Results were expressed as mean ± SD deviation using one-way analysis of variance. Data were analyzed with ANOVA and followed by SNK and LSD tests for intergroup comparisons. Mann–Whitney U test was used for comparisons of neurological deficit between two groups. Differences were considered significant if P < 0.05.
Results
Notch 1 was up-regulated in the acute phase of cerebral ischemia
Immunohistochemistry and Western blot were used to detect the dynamic expression of Notch 1 in brain tissue at normal, 3, 6, 12, 24, 48 and 72 h after permanent occlusion of the middle cerebral artery (MCAO) (Figs. 1, 2). Compared with normal-control group, Notch 1 was up-regulated beginning at 3 h (P < 0.05), getting to high values at 24 h, peaking at 48 h and maintaining high values at 72 h after MCAO (P < 0.05) in protein levels. All the results of immunohistochemistry and Western blot showed that, compared with 3, 6 and 12 h after permanently MCAO, the expression of Notch 1 at 24 h was significantly increased (P < 0.05), but slightly lower than peak values.
The dynamic expression of Notch 1 after MCAO. a Representative photographs of immunohistochemistry for Notch 1 (400×). a 1 Normal group. a 2 24 h group. a 3 48 h group. b Bar graph of immunohistochemistry illustrating the dynamic expression of Notch 1. ★ P < 0.05 versus normal group. ♦ P < 0.05 versus normal group, 3 h. ▲ P < 0.05 versus normal group, 3, 6, 12 h. *P < 0.05 versus normal group, 3, 6, 12, 24, 72 h
The effect of DAPT on neurological deficit scores
Neurological deficit was examined and scored on a 6-point scale at 24 and 48 h after MCAO, and then Mann–Whitney U test was conducted. Although there were significant differences in neurological deficit scores between DAPT group and MCAO group at 48 h (P < 0.05, Fig. 3b), there was no significant reduction at 24 h (P > 0.05, Fig. 3a).
DAPT reduced the brain water content
We observed brain water content at 48 h after the operation using the standard wet–dry method [17]. DAPT could reduce the brain water content of ipsilateral hemispheres. In the sham-operated group, water content was 78.83 ± 0.35%. In DAPT group, brain water content was reduced compared with MCAO group (80.89 ± 0.51 vs. 83.84 ± 0.75%, P < 0.05). These data indicated that DAPT protected brain against brain ischemia damage at the early stage of cerebral ischemia (Fig. 4a).
DAPT reduced the infarct volume
The protective effects of DAPT were also evaluated by measuring infarct volumes at 48 h after ischemia. We examined infarct sizes at 48 h after operation using vital staining with 2, 3, 5-triphenyltetrazolium chloride (TTC). No infarction was observed in the sham-operated group. Extensive lesion was developed in both striatum and lateral cortex in MCAO group. In DAPT group, the infarct volume was decreased significantly from 52.41 ± 1.41 to 41.50 ± 1.93% (P < 0.05, Fig. 4b, c).
DAPT suppressed the expression of Notch 1 and NF-κB
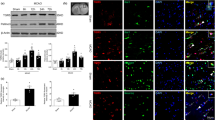
The expression of positive cells of Notch 1 and NF-κB were observed in ischemic cortex around infarct regions at 48 h post-ischemia with treatment of DAPT. Immunohistochemistry showed that the number of positive cells of Notch 1 and NF-κB dramatically increased in ischemic cortex in MCAO group, while the DAPT group showed a much lower number (P < 0.05, Fig. 5). Western blot analyses also showed a significant decrease of Notch 1 and NF-κB expression in DAPT group (P < 0.05 vs. MCAO group Fig. 6), which was consistent with the result of immunohistochemistry. We next examined the expression of Notch 1 and NF-κB at 48 h after MCAO with or without DAPT using confocal microscope in order to confirm the inhibition of Notch 1 by DAPT. Immunofluorescent intensity showed a significant increase of Notch 1 and NF-κB immunoreactivity after MCAO, while the DAPT group again showed a lower intensity (Fig. 7).
a Representative photographs of Western blot showing the effect of DAPT on Notch 1 protein levels. b Representative photographs of Western blot showing the effect of DAPT on NF-κB protein levels. c Bar graph showing the effect of DAPT on Notch 1 protein levels. d Bar graph showing the effect of DAPT on NF-κB protein levels. ★ P < 0.05 versus MCAO group
Discussion
Our study showed that Notch 1 was up-regulated at early stage after ischemia, beginning at 3 h, peaking at 48 h and maintaining high levels till 72 h when we ceased to observe. These results indicated that Notch 1 pathway participated in the pathologic process of cerebral ischemia at early time. Notch 1 is a double-edged sword in cerebral ischemia according to earlier studies. It is reported that Notch 1 stimulated regenerative responses in low-oxygen conditions by maintaining NSCs, promoting proliferation of neural progenitors, but inhibiting differentiation into neurons in vitro and in vivo experiments [19–24]. On the other hand, Notch 1 inhibits neural progenitor differentiation into neurons in vitro and in vivo experiments and participates in various aspects of inflammatory reactions by modulating the development and activation of inflammatory cells, such as T cell, lymphocyte and microglia [25–30]. The balance of Notch 1 pathway may influence the degree of brain injury after cerebral ischemia. DAPT, the inhibitor of Notch 1 pathway, was used in our study to investigate the final function of Notch 1 pathway in cerebral ischemia. Neurological deficit, brain water content and infarct sizes were measured 48 h after MCAO. Data showed that DAPT reduced brain water content and infarct volumes and improved the functional outcome after cerebral ischemia. Notch 1 might be benefit for neural regeneration; however, DAPT inhibiting Notch 1 protected brain damage from ischemic stroke. It implicated that Notch 1 participating in inflammatory processes may play a major role in cerebral ischemia or DAPT may regulate other signaling pathway.
Activated NF-κB is transferred to the nucleus, where it combines with target genes to promote proinflammatory cytokine expression which results in leukocyte adherence and migration, as well as expanded inflammatory reaction. NF-κB is a key factor contributing to the brain damage after ischemic stroke [12]. Numerous reports have described that Notch 1 regulated NF-κB and vice versa. NF-κB could modulate and integrate into Notch 1 pathway through Notch ligands Jagged-1 and intracellular Notch modulators N-CoR. Notch 1 regulated NF-κB via Jagged-1 and NF-κB modulators IκBα [31–33]. Several lines of evidences supported a consonance of the Notch and NF-κB signaling pathways in activation and function [13, 14], and both of them are positively activated by ischemia. Our observations showed that DAPT significantly inhibited the expression of NF-κB at protein level. Other researchers provided parallel results about γ-secretase inhibitors [34].
Previous studies have demonstrated that DAPT may present an effective and novel treatment for autoimmune and lymphoproliferative diseases, degenerative disease and cancers [27, 35–37]. The neuroprotection of DAPT in cerebral ischemia by decreasing the apoptotic cascades in neurons, restraining the activation of microglias and repressing the infiltration of proinflammatory leukocytes has been demonstrated [30].
In summary, the results showed that the regularity of the time course expression of Notch 1 upregulated at very early date after cerebral ischemia. Systemic administration of DAPT decreased the infarct size and the brain edema. These results may indicate that the downregulation of Notch 1–NF-κB pathway after ischemia by administration of DAPT is a potential mechanism for its protection.
References
Artavanis–Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398:518–522
Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson–Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE (2001) Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem 76:173–181
Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C (2001) Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep 2:835–841
Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C (2002) A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep 3:688–694
El Mouedden M, Vandermeeren M, Meert T, Mercken M (2006) Reduction of Abeta levels in the Sprague Dawley rat after oral administration of the functional gamma-secretase inhibitor, DAPT: a novel non-transgenic model for Abeta production inhibitors. Curr Pharm Des 12:671–676
Roberson ED, Mucke L (2006) 100 years and counting: prospects for defeating Alzheimer’s disease. Science 314:781–784
Oya S, Yoshikawa G, Takai K, Tanaka JI, Higashiyama S, Saito N, Kirino T, Kawahara N (2009) Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. Neuroscience 158:683–692
Hellström M, Phngm LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela–Arispe ML, Kalén M, Gerhardt H, Betsholtz C (2007) Dll4 signalling through Notch 1 regulates formation of tip cells during angiogenesis. Nature 445:776–780
Campbell KJ, Perkins ND (2006) Regulation of NF-kappa B function. Biochem Soc Symp 73(2006):165–180
Perkins ND (2007) Integrating cell-signalling pathways with NF-kappaB and IKK function. Natl Rev Mol Cell Biol 8:49–62
Liu Y, Zhang XJ, Yang CH, Fan HG (2009) Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-κB expression. Brain Res 1268:174–180
Ang HL, Tergaonkar V (2007) Notch and NF kappa B signaling pathways: do they collaborate in normal vertebrate brain development and function? Bioessays 29:1039–1047
Osipo C, Golde TE, Osborne BA, Miele LA (2008) Off the beaten pathway: the complex cross talk between Notch and NF-kappaB. Lab Invest 88(2008):11–17
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Yang CH, Zhang XJ, Fan HG, Liu Y (2009) Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 1282:133–141
Hatashita S, Hoff JT, Salamat SM (1988) Ischemic brain edema and the osmotic gradient between blood and brain. J Cereb Blood Flow Metab 8:552–559
Tatlisumak T, Carano RA, Takano K, Opgenorth TJ, Sotak CH, Fisher M (1998) A novel endothelin antagonist, A-127722, attenuates ischemic lesion size in rats with temporary middle cerebral artery occlusion: a diffusion and perfusion MRI study. Stroke 29(1998):850–857
de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA (1997) Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124:1139–1148
Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D (2002) Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 16(2002):846–858
Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M (2005) Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9:617–628
Yoon K, Gaiano N (2005) Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci 8:709–715
Androutsellis–Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD (2006) Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442:823–826
Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N (2007) Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449:351–356
Wolfer A, Wilson A, Nemir M, MacDonald HR, Radtke F (2002) Inactivation of Notch 1 impairs VDJbeta rearrangement and allows pre-TCR-independent survival of early alpha beta Lineage Thymocytes. Immunity 16:869–879
Maillard I, Tu L, Sambandam A, Yashiro-Ohtani Y, Millholland J, Keeshan K, Shestova O, Xu L, Bhandoola A, Pear WS (2006) The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J Exp Med 203b:2239–2245
Teachey DT, Seif AE, Brown M, Bruno M, Bunte RM, Chang YJ, Choi JK, Fish JD, Hall J, Reid GS, Ryan T, Sheen C, Zweidler-McKay P, Grupp SA (2008) Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood 111:705–714
Weng AP, Ferrando AA, Lee W, Morris JP 4th, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC (2004) Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306:269–271
Fung E, Tang SM, Canner JP, Morishige K, Arboleda-Velasquez JF, Cardoso AA, Carlesso N, Aster JC, Aikawa M (2007) Delta-Like 4 Induces notch signaling in macrophages: implications for inflammation. Circulation 115:2948–2956
Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP (2006) Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med 12:621–623
Bash J, Zong WX, Banga S, Rivera A, Ballard DW, Ron Y, Gelinas C (1999) Rel/NF-kappaB can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. EMBO J 18:2803–2811
Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L (2002) Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ 9:842–855
Espinosa L, Ingles-Esteve J, Robert-Moreno A, Bigas A (2003) IkappaBalpha and p65 regulate the cytoplasmic shuttling of nuclear corepressors: cross-talk between Notch and NFkappaB pathways. Mol Biol Cell 14:491–502
Yao J, Duan L, Fan M, Wu X (2007) Gamma-secretase inhibitors exerts antitumor activity via down–regulation of Notch and Nuclear factor kappa B in human tongue carcinoma cells. Oral Dis 13:555–563
Mayer SC, Kreft AF, Harrison B, Abou-Gharbia M, Antane M, Aschmies S, Atchison K, Chlenov M, Cole DC, Comery T, Diamantidis G, Ellingboe J, Fan K, Galante R, Gonzales C, Ho DM, Hoke ME, Hu Y, Huryn D, Jain U, Jin M, Kremer K, Kubrak D, Lin M, Lu P, Magolda R, Martone R, Moore W, Oganesian A, Pangalos MN, Porte A, Reinhart P, Resnick L, Riddell DR, Sonnenberg-Reines J, Stock JR, Sun SC, Wagner E, Wang T, Woller K, Xu Z, Zaleska MM, Zeldis J, Zhang M, Zhou H, Jacobsen JS (2008) Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer’s disease. J Med Chem 51:7348–7351
Tammam J, Ware C, Efferson C, O’Neil J, Rao S, Qu X, Gorenstein J, Angagaw M, Kim H, Kenific C, Kunii K, Leach KJ, Nikov G, Zhao J, Dai X, Hardwick J, Scott M, Winter C, Bristow L, Elbi C, Reilly JF, Look T, Draetta G, Van der Ploeg L, Kohl NE, Strack PR, Majumder PK (2009) Down-regulation of the Notch pathway mediated by a gamma-secretase inhibitor induces anti-tumour effects in mouse models of T-cell leukaemia. Br J Pharmacol 158:1183–1195
Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE, Buser C, Yeatman T, Coppola D, Winter C, Clark EA, Draetta GF, Strack PR, Majumder PK (2010) Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res 70:2476–2484
Acknowledgments
This work was funded by Hebei Province, No: C2010000564 and No: 10276104D and a Grant from the Major State Basic Research Development Program of China (No. 2009CB521905).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Zhang, X., Wang, Y. et al. DAPT protects brain against cerebral ischemia by down-regulating the expression of Notch 1 and Nuclear factor kappa B in rats. Neurol Sci 33, 1257–1264 (2012). https://doi.org/10.1007/s10072-012-0948-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-012-0948-6