Abstract
Saitohin (STH) is an intronless gene nested within the human tau gene, which contains a single nucleotide polymorphism (A/G), suggested to be involved in the physiopathology and clinical course of several neurodegenerative and neuropsychiatric diseases. Recently, an association between this polymorphism and frontal hypoperfusion and clinical prognosis in frontotemporal dementia was reported. The present study sought to evaluate the possible role of the STH polymorphism as a concurring factor of cognitive decline in schizophrenia, a disease sharing both early psychotic manifestations, a core deficit of executive functions and hypofrontality with frontotemporal lobe dementia. 220 clinically stabilized patients with schizophrenia were assessed with the Wisconsin Card Sorting Test (WCST) for evaluation of executive functions and compared for STH allele frequency with 48 patients affected by frontotemporal dementia and 47 healthy subjects. There was no significant difference in allelic distribution between the healthy controls and all other groups, while we observed a significantly greater frequency of G allele among both patients with frontotemporal dementia (p = 0.037) and schizophrenia patients with poor performances of WCST (p = 0.044), compared to schizophrenia patients with best WCST performances. Among the patients with schizophrenia, stratified for age and gender, the STH polymorphism resulted in a significant predictor of WCST performance (p = 0.007). These results suggest a possible contribution of STH gene products on the heterogeneity of core frontal executive functions deterioration, probably through complex interactions with mechanism involved in neurodevelopment and neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saitohin (STH) is an intronless gene encoding for a protein consisting of 128 amino acids, located in intron 9 of the human tau gene, that contains a single nucleotide polymorphism (A/G), the A allele being most frequent in humans, which changes glutamine residue 7 to arginine (Q7R) [1]. This polymorphism is in complete linkage disequilibrium with the extended haplotype that covers the entire human tau gene: the A allele of STH with H1 tau haplotype, the G allele with H2 [1, 2]. The two tau haplotypes differ in transcriptional activity, H1 being more efficient in driving tau gene expression compared to H2 [3]. Additionally, the G allele of STH was reported to increase the inclusion of exon 10 of the tau gene much more effectively than the A allele in patients with Alzheimer disease (AD) [4]. It is known that aberrant microtubule-associated tau protein is a feature of several neurodegenerative diseases; however, the functional and clinical correlation of tau haplotype is still lacking in literature. It is still unknown whether the tau haplotype by itself modulates the function of the tau protein, or if the haplotype is in linkage disequilibrium with a causal mutation in tau or a neighboring gene, and how this haplotype may influence the spectrum of tau-related disorders, thus tuning the neuropathological process, and consequently determining a peculiar clinical endophenotype.
The location and the expression pattern of STH gene warranted research aiming to evaluate the possible role of the STH polymorphism in several neurodegenerative disorders. Recent studies focused mainly on dementia processes, both Alzheimer disease and frontotemporal dementia, but other neurodegenerative diseases, such as Parkinson disease, progressive nuclear palsy and Huntington chorea, were investigated too [5–7]. Preliminary studies suggested an association between the GG genotype of STH and higher risk for late-onset Alzheimer disease [8] and a trend for the AA genotype and frontotemporal dementia [2]; however, further studies failed to replicate this result in larger and multi-ethnic populations [9–11]. In an Italian sample of patients affected by sporadic dementia, Lorenzi et al. [12] observed a significant interaction between STH and 5-HTTLPR, as a potential susceptibility factor for neurodegenerative diseases. More recent studies focused on the possible role of STH gene polymorphism on clinical phenotype and the course of the disease. Borroni et al. [13] evaluated, by means of SPECT, the correlation between the frontotemporal lobar degeneration (FTLD) cerebral functional patterns and tau haplotype, observing a greater anterior brain hypoperfusion in carriers of the H2 haplotype, in linkage disequilibrium with the STH G allele. Moreover, the same haplotype was also associated to a worse prognosis in patients affected by frontotemporal dementia [14]. Besides, hyperphosphorylated tau is a pathological finding in Alzheimer disease and frontotemporal dementia [15, 16], neurofibrillary tangles density, even below the threshold for a neuropathological diagnosis of AD; this correlated with increased dementia severity in elderly schizophrenia patients [17]. Therefore, factors regulating the phosphorylation of tau protein are of interest, as they are suggested to be involved also in major psychiatric disorders [18, 19]. Tau is phosphorylated at specific sites and its phosphorylation occurs after a highly orchestrated cascade of intracellular reactions mediated largely by non-receptor tyrosine kinases and regulated by multiple signaling proteins [20]. Among the regulators of intracellular tau phosphorylation, reelin, a high-affinity ligand of integrin receptors involved in neurodevelopment, resulted to inhibit tau phosphorylation [21]. Importantly, diminished expression of reelin has been reported in schizophrenia and other psychosis, raising the possibility that decreases the inhibition of tau phosphorylation which occurs in psychotic disorders and it could be a contributing pathogenic mechanism. In addition, data from animal studies suggest that hyperphosphorylated tau may contribute to executive dysfunction and hypofrontality [21, 22]. Given these evidence, it appears worth of interest to study the possible role of the STH gene polymorphism in schizophrenia, a disease sharing both early psychotic manifestations, both a core deficit of executive functions and hypofrontality with frontotemporal lobe dementia. Moreover it has recently been suggested by a study on first-degree relatives of patients with FTLD that the latter may share a common etiology with schizophrenia [23].
To test the hypothesis of a possible role of STH polymorphism as a concurring factor of cognitive decline among schizophrenic patients, we first compared STH genotype frequencies between the patients affected by schizophrenia, patients affected by frontotemporal dementia and a control sample of healthy subjects, and then analyzed the relationship between STH genotype and executive performance among the patients affected by schizophrenia.
Materials and methods
Sample
The study included: 220 biologically unrelated outpatients meeting DSM-IV criteria for schizophrenia. All patients with schizophrenia had to be treated with a stable dose of the same antipsychotic in monotherapy for at least 3 months and to be responders (good response was defined as a reduction of 30% or more in Positive and Negative Syndrome Scale (PANSS) total score after 3 months of treatment).
48 patients (29 M, 19 F), meeting Lund Manchester (1994) diagnostic criteria for frontotemporal dementia, with mean age and onset, respectively, 65.64 ± 8.99 and 61.50 ± 8.00.
47 healthy subjects (25 M, 22 F), with mean age of 81.61 ± 10.97 were used as a control sample.
All subjects provided informed consent to a protocol approved by the local Ethical Committee and following the principles of the Declaration of Helsinki.
Genotyping
A polymerase chain reaction (PCR) was performed with the following primers: 5′-CCC TGT AAA CTC TGA CCA CAC-3′ and 5′-ACA GGG AAG CTA CTT CCC ATG-3′. The PCR reaction was carried out by ABI 9700 PCR thermal-cycler (Applied Biosystems, APPLERA) as follows: after a first step at 94°C for 3 min, steps of 94°C for 30 s, 60°C for 30 s, and 70°C for 30 s for 35 cycles. Then, a final extension step at 70°C for 6 min was added. PCR product was digested using HinfI (New England Biolabs, England, UK) at 37°C overnight; fragments were separated in 3% Seakem agarose gel with ethidium bromide. The cleaved bands were visualized by ultraviolet light. Depending on the presence of one or two restriction HinfI sites, either two fragments 171 + 55 bp (allele A or allele Q) or three fragments 97 + 74 + 55 bp (allele G or allele R) were produced.
Assessment
Only patients affected by schizophrenia were assessed.
Psychopathology was assessed by means of the PANSS for schizophrenia [24] administered by a trained psychiatrist.
Neuropsychological performances were assessed with computerized Wisconsin Card Sorting Test (WCST), for the evaluation of cognitive flexibility [25] administered by a trained psychologist.
Basic clinical and demographic data were collected from clinical reports.
Data analysis
Analysis of variance (ANOVA) was performed among patients affected by schizophrenia, to examine genotype group effects, with demographic characteristics and psychopathological evaluations as dependent variables and STH genotype as independent factor.
The Chi squared test was first used to compare allele frequency among patients with diagnosis of schizophrenia, frontotemporal dementia and healthy controls. We divided the sample of patients affected by schizophrenia in two subgroups, according to WCST performances, and compared the “best performance” group (n = 44) with the “poor performance” group (n = 176), and also each schizophrenia subgroup with both the frontotemporal dementia group and the controls. Groups were divided by means of a cut-off which separated the most impaired patients from all the others, already used in previous studies [26], and corresponding to 75th percentile of the healthy subject’s performances distribution analyzed in a previous work [27].
A multiple logistic regression was performed to further analyze the STH genotype effect on executive functions deficit in schizophrenia patients, taking into account possible confounding factors such as age and gender too.
Results
Descriptive analysis
DNA analysis showed the following allelic distribution:
-
136 subjects A/A, 75 A/G and 9 G/G for patients with schizophrenia;
-
20 subjects A/A, 26 A/G and 2 G/G for patients with frontotemporal dementia;
-
29 subjects A/A, 14 A/G and 4 G/G for healthy controls.
Because of the rarity of the G/G genotype, for analysis we grouped subjects in A/A homozygous and G carriers, as used in previous studies [1].
Table 1 shows demographic and clinical variables of the patients with schizophrenia according to genotype groups. No differences were found for any of the variables considered between groups.
Comparison analysis
We didn’t observe any significant difference in allelic distribution between the healthy controls and all other groups.
Among the sample of schizophrenia patients, we found a significant difference in allelic distribution between the “best performance” group and the “poor performance” group (p = 0.044), with a higher frequency of G allele in the “poor performance” group.
We found a significant difference also comparing the “best performance” subgroup of patients with schizophrenia with the sample of patients with frontotemporal dementia (p = 0.037) with a higher frequency of G allele among the latter group.
There were no differences in frequency between patients with frontotemporal dementia and the subgroup of schizophrenia patients with a more deficitary performance.
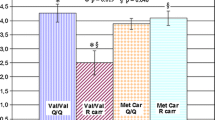
The percentage genotype frequencies among the two schizophrenia subgroups, the sample of patients with frontotemporal dementia and the healthy controls group are shown in Fig. 1.
STH genotype frequencies. Percentage of STH genotype frequencies among the two schizophrenia subgroups (BP “best performances group”, PP “poor performances group”), the sample of patients with frontotemporal dementia (FTD) and the healthy controls group (HC). Significant differences were observed between BP patients with schizophrenia and both PP patients (p = 0.044) and FTD patients (p = 0.037)
Logistic regression
Among patients with SKZ, the logistic regression showed a significant effect of STH genotype on executive functions (p = 0.007), the G allele being associated with higher risk of having more deficitary performances. The odds ratio adjusted for age and gender was 4.09.
Discussion
To our knowledge, this is the first study to evaluate the effect of STH polymorphism in a sample of patients affected by schizophrenia.
Our results showed a significantly different distribution of STH genotype between the “best performance” group of patients with schizophrenia and both the “poor performance” group of schizophrenia patients and the sample of patients with frontotemporal dementia. Compared to the “best performance” group of patients with schizophrenia, the G allele of STH resulted more frequently among both the sample of patients with frontotemporal dementia and the group of patients affected by schizophrenia with more impaired performance of WCST, these latter groups showing a similar genotype distribution. Finally, the allelic frequencies observed in the healthy controls group were not significantly different from any other group. Although it is to notice that the sample size is too small to draw conclusions, this last finding supports previous studies that failed to report a significant association between the STH genotype and frontotemporal lobe dementia risk [2, 28], suggesting that the potential role on disease susceptibility could be of minor relevance. However, the G allele of STH gene was reported to be associated to worse prognosis and more severe hypofrontality [13, 14], a pathological feature common to both frontotemporal dementia and schizophrenia. This evidence and our results lead to hypothesize that the STH polymorphism may directly influence cognitive functions across diagnosis, rather than being associated with a specific disease.
Regarding the STH effect on executive functions, we observed that among schizophrenia patients, the presence of the G allele was a significant predictor of having more deficitary performances of executive functions, when stratified for age and gender too.
These results confirm the hypothesis of a possible contribution of STH gene products on the heterogeneity of core frontal executive functions deterioration. The G allele seems to condition a more deficitary syndrome, probably through complex interactions with mechanisms involved in neurodevelopment and neurodegeneration. The “hypofrontality” interpretation of our results is supported by data from Borroni et al. [14], showing an association between the tau H2 haplotype (in linkage disequilibrium with the G allele of STH) and greater anterior cerebral hypoperfusion, including at the dorsolateral frontal cortex level, a critical area known to present reduced efficiency in schizophrenia and to be related to cognitive deficit, mainly of executive functions [29].
The underlying mechanisms of this correlate are still largely unknown; however, tau phosphorylation is likely to be involved and could represent a point of convergence between prominent neurodegenerative diseases such as frontotemporal dementia, characterized by hyperphosphorylated tau finding, and more complex neurodevelopmental and partially neurodegenerative diseases, such as schizophrenia and other psychosis. The role of hyperphosphorylated tau in schizophrenia is suggested by reports of an association between cognitive deterioration and the degree of neurofibrillary tangles in elderly patients [17]. Furthermore, the hypothesis of a common etiology between schizophrenia and dementia arises from evidence of an higher morbid risk for schizophrenia in relatives of FTLD probands [23]. An involvement of tau hyperphosphorylation in schizophrenia was recently hypothesized by Costa et al. [18], who reported reduced expression of reelin among schizophrenia patients, resulting in decreased inhibition of tau phosphorylation. Moreover, the reelin knock-out mouse model shows impairments in tasks of executive functions [30], suggesting a role of reelin and, therefore, more complex balanced tau phosphorylation for correct brain development in critical areas for executive functions. In this view, it can be suggested that the STH polymorphism could tune the neuropathological process and consequently modulate, among other factors, the degree of neuropsychological deterioration in schizophrenia.
Our results are yet limited by the unbalanced sample size between the two subgroups of patients affected by schizophrenia, due to the higher frequency of patients with poor performances. This is a naturalistic sample and reflects the clinical reality of the distribution of performances at WCST (mainly poor) among subjects affected by schizophrenia [31].
Conclusions
These preliminary results suggest a possible contribution of STH gene products on the heterogeneity of core frontal executive functions deterioration, probably through complex interactions with mechanism involved in neurodevelopment and neurodegeneration. More complex and comprehensive studies, including a specific neurocognitive assessment on patients affected by dementia, are worth on larger samples, which might help in understanding the possible physiopathological and prognostic value of this polymorphism, and taking into account interactions with other genes influencing the tau phosphorylation process.
References
Conrad C, Vianna C, Schultz C, Thal DR, Ghebremedhin E, Lenz J et al (2004) Molecular evolution and genetics of the saitohin gene and tau haplotype in Alzheimer’s disease and argyrophilic grain disease. J Neurochem 89:179–188
Verpillat P, Ricard S, Hannequin D, Dubois B, Bou J, Camuzat A et al (2002) Is the saitohin gene involved in neurodegenerative diseases? Ann Neurol 52:829–832
Kwok JB, Teber ET, Loy C, Hallupp M, Nicholson G, Mellick GD et al (2004) Tau haplotypes regulate transcription and are associated with Parkinson’s disease. Ann Neurol 55:329–334
Gao L, Tse SW, Andreadis A, Conrad C (2005) Saitohin, which is nested in the tau locus and confers allele-specific susceptibility to several neurodegenerative diseases, interacts with peroxiredoxin 6. J Biol Chem 280:39268–39272
de Silva R, Hope A, Pittman A, Weale ME, Morris HR, Wood NW et al (2003) Strong association of the saitohin gene Q7 variant with progressive supranuclear palsy. Neurology 61:407–409
Jankovic N, Kecmanovic M, Dimitrijevic R, Markovic M, Dobricic V, Keckarevic D et al (2008) HD phenocopies—possible role of saitohin gene. Int J Neurosci 118:391–397
Levecque C, Elbaz A, Clavel J, Vidal JS, Amouyel P, Alpérovitch A et al (2004) Association of polymorphisms in the tau and saitohin genes with Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:478–480
Conrad C, Vianna C, Freeman M, Davies P (2002) A polymorphic gene nested within an intron of the tau gene: implications for Alzheimer’s disease. Proc Natl Acad Sci USA 99:7751–7756
Clark LN, Levy G, Tang MX, Santana H, Ciappa A, Tycko B et al (2003) The saitohin ‘Q7R’ polymorphism and tau haplotype in multi-ethnic Alzheimer disease and Parkinson’s disease cohorts. Neurosci Lett 347:17–20
Cook L, Brayne CE, Easton D, Evans JG, Xuereb J, Cairns NJ et al (2002) No evidence for an association between saitohin Q7R polymorphism and Alzheimer’s disease. Ann Neurol 52:690–691
Streffer JR, Papassotiropoulos A, Kurosinski P, Signorelli A, Wollmer MA, Tsolaki M et al (2003) Saitohin gene is not associated with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 74:362–363
Lorenzi C, Marcone A, Pirovano A, Marino E, Cordici F, Cerami C, Delmonte D, Cappa SF, Bramanti P, Smeraldi E (2010) Serotonin transporter and saitohin genes in risk of Alzheimer’s disease and frontotemporal lobar dementia: preliminary findings. Neurol Sci 31:741–749
Borroni B, Paghera B, Agosti C, Anchisi D, Perani D, Archetti S et al (2008) Tau haplotype influences cerebral perfusion pattern in frontotemporal lobar degeneration and related disorders. Acta Neurol Scand 117:359–366
Borroni B, Grassi M, Agosti C, Alberici A, Premi E, Archetti S et al (2010) Establishing short-term prognosis in frontotemporal lobar degeneration spectrum: role of genetic background and clinical phenotype. Neurobiol Aging 31(2):270–279
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917
Hong M, Lee VM (1997) Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem 272:19547–19553
Rapp MA, Schnaider-Beeri M, Purhoit DP, Reichenberg A, McGurk SR, Haroutunian V, Harvey PD (2010) Cortical neuritic plaques and hippocampal neurofibrillary tangles are related to dementia severity in elderly schizophrenia patients. Schizophr Res 116:90–96
Costa E, Chen Y, Davis J, Dong E, Noh JS, Tremolizzo L et al (2002) Reelin and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv 2:47–57
Guidotti A, Auta J, Davis M, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR et al (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57:1061–1069
Bock HH, Herz J (2003) Reelin activates src family tyrosine kinases in neurons. Curr Biol 13:18–26
Rice DS, Curran T (1999) Mutant mice with scrambled brains: understanding the signaling pathways that control cell positioning in the CNS. Genes Dev 13:2758–2773
Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P et al (2001) Downregulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci USA 98:3477–3482
Schoder D, Hannequin D, Martinaud O, Opolczynski G, Guyant-Maréchal L, Le Ber I, Campion D (2010) Morbid risk for schizophrenia in first-degree relatives of people with frontotemporal dementia. Br J Psychiatry 197:28–35
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Stratta P, Arduini L, Daneluzzo E, Rinaldi O, Di Genova A, Rossi A (2004) Relationship of good and poor Wisconsin Card Sorting Test performance to illness duration in schizophrenia: a cross-sectional analysis. Psychiatry Res 121:219–227
Cavallaro R, Cavedini P, Mistretta P, Bassi T, Angelone SM, Ubbiali A et al (2003) Basal-corticofrontal circuits in schizophrenia and obsessive-compulsive disorder: a controlled, double dissociation study. Biol Psychiatry 54:437–443
Ermoli E, Anselmetti S, Bechi M, Cocchi F, Smeraldi E, Cavallaro R (2005) Assessment of psychosis in schizophrenia: neuropsychological profile of chronic schizophrenia. Clin Neuropsychiatry 2:243–249
Johansson A, Zetterberg H, Håkansson A, Nissbrandt H, Blennow K (2005) TAU haplotype and the Saitohin Q7R gene polymorphism do not influence CSF Tau in Alzheimer’s disease and are not associated with frontotemporal dementia or Parkinson’s disease. Neurodegener Dis 2:28–35
Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD (2009) Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res 108:143–150
Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA (2006) Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci 120:984–988
Gold J (1998) Schizophrenia and intellectual decline. Am J Psychiatry 155:1633–1634
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bosia, M., Buonocore, M., Guglielmino, C. et al. Saitohin polymorphism and executive dysfunction in schizophrenia. Neurol Sci 33, 1051–1056 (2012). https://doi.org/10.1007/s10072-011-0893-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0893-9