Abstract
There is no effective alternative to surgery in the treatment of the symptomatic cases of chiari malformation. Nonetheless, in literature there is no unanimous consensus about what is the surgical “gold standard” and which are the candidates for surgery. No doubt that intracranial hypertension and ventricular dilatation have to be investigated and treated before considering decompression. It is also very important to keep in mind that a surgery does not guarantee a complete recovery from every symptoms. We report our experience about who are the candidates for surgery, which is the most appropriate surgical technique and when is the correct time for surgery along the natural history of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgery is the treatment of choice in symptomatic chiari malformation (CM) patients. Once hydrocephalus is ruled out, the degree of tonsillar ectopia and the presence of hydromyelia are evaluated, than the treatment may be performed, based on the indication of clinical symptoms.
Well-timed intervention is the most important factor for a favourable result, notably in patients with a proved medullary compromise.
Despite being a relatively safe procedure, craniovertebral decompression (CVD) is not completely devoid of risks of complications, such as bleedings, damage to neural structures, CSF fistulas, meningitis, pseudomeningocele and occipito-cervical instability. Acute post-operative hydrocephalus and anterior compression of the brain stem by retroflexion of odontoid have been seldom described [4]. Moreover, the cerebellar “slump” due to a highly extended CVD, especially by an occipital bone lateral or superior opening and a big duroplasty, is a complication that needs a complex correction by cranioplasty.
Patients and methods
Between January 1999 and June 2007, in the Clinic of Neurosurgery of the University Federico II in Naples, 36 patients with CMI have been treated (17 males; 19 females, average age 36 years). Twenty-five had also an associated syringomyelia (70%).
At admission, the whole group reported a suboccipital headache worsened by Valsalva manoeuvres, while neurological examination was altered in 30 patients (83%).
On 22 patients, a pure bone decompression (osseous CVD) was performed by a suboccipital craniectomy and a C1 laminectomy. On the remaining 14 patients, a duraplasty was associated, using a synthetic dural substitute or a pericranial graft.
In the same period, eight CMII patients were treated in our clinic (3 males; 5 females, average age 10 years). A ventriculo-peritoneal shunt was implanted on each CMII patient at birth, when their spinal cleft had been closed. All the patients presented a spinal syrinx of protean length and diameter. Clinical examination at admission showed occipito-cervical headache, variable degree of scoliosis, cerebellar signs and lower cervical nerves injuries. Therefore, duraplasty after bone decompression was considered to be appropriate.
The follow up was between 2 and 8 years (average 5 years), evaluating the clinical history, pre- and postoperative neurological examination, radiological imaging and the type of surgical procedure.
Neuroradiological assessment
Magnetic resonance (MR) in CM patients should be extended on the whole brain and spinal cord, to allow the evaluation of tonsillar ectopia degree, size of the ventricular cavities, presence and length of syrinx and craniovertebral junction and spinal associated dysmorphysms. Post-operative MRI of brain and spinal cord were performed at least 1 year after surgery.
Surgical technique
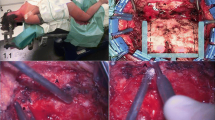
The patients were prone positioned, with head flexed and fixed by Mayfield head frame with no rotation. A middle linear skin incision was carried out from inion to spinous process of C2 and the muscolo-fascial plane was opened. The occipital squama and the posterior arch of atlas were exposed; after a musculoskeletal dissection, a middle suboccipital craniectomy was performed and the posterior arch of atlas was removed. Then dural layer was removed at the level of atlo-occipital junction, or, if necessary, the dura mater was opened and grafted (Fig. 1).
Results
75% of CMI patients and 60% of CMII showed clinical improvement at post-operative examination, with reversal of headache in 100% and disappearance of neurological deficits in 40% of the whole series of CMI and 25% of CMII. Clinical stability was observed in the 60% of CMI and in the 75% of CMII (Table 1).
Clinical examination was carried out 6 and 18 months after surgery, showing a further clinical improvement in 5% of CMI and only in 2% of CMII patients. No change was observed at further controls.
Neuroradiological examination 1 year after surgery revealed a decreasing diameter of syrinx in 20% and no change in the remaining cases. The whole group showed restoration of CSF dynamic in subarachnoidal spaces of PCF. No change in following examinations. About post-operative complications, three cases had a pseudomeningocele and two had an aseptic meningitis out of the 36 CMI cases; one aseptic meningitis occurred in the CMII series.
Discussion
Chiari malformation has been historically considered a rare disease, however, wide application of MRI examination on the population showed an unexpectedly higher prevalence. Clinical presentation may happen between 6 and 60 years (average age at diagnosis, 40 years) and may be earlier in patients with syrinx [7]. Surgical treatment of CM patients presents three main questions.
-
1.
Which patients to treat? There is consensus that indication to surgery should be based on occurrence of clinical symptoms rather than on radiological findings. But the clinic may vary from a complete absence of symptoms to headache, episodes of pseudotumor cerebri, Meniér-like syndrome and spinal cord disturbances [3, 5, 6, 8]. The presence of a syrinx is responsible for a cohort of specific signs and symptoms that range from dysesthetic sensations with classical algothermal dissociation to spasticity and paresis.
The contemporary presence of CM and a syrinx is considered as an absolute indication to decompressive surgery, even if recently some authors reported some cases of asymptomatic patients with syrinx with its spontaneous disappearance [1].
Clinical findings of CMII patients are often due to lower cranial nerve deficits and to brainstem dysfunction, but caused by an associated hydrocephalus and syringomyelia [9, 10]. The clinical onset CMII in neonates frequently presents as a surgical emergency: a severe damage of brain stem can appear with rapid neurological worsening. No doubts about indication to surgery in the symptomatic infants, while it remains controversial in asymptomatic patients with a syrinx larger than 50% of transverse spinal diameter [10].
-
2.
What is the best way of treating? The treatment of choice is CVD, alone or with duraplasty. In CMI patients, limited bone decompression and outer dural layer removal is usually enough. A meta-analysis of Durham et al. has reported an improvement of syrinx size in 50% with simple suboccipital cranietcomy and in 80–100% if associated with duraplasty [2]. So the “mini-invasive” approach reduces time of hospitalization, at the price of a lower chance to solve the problem.
There is no consensus about best treatment of CMII. In our experience, CMI patients with no or small syrinx, were submitted to simple bone decompression with atlo-occipital ligament removal, while patients with a symptomatic medullary cavity underwent duraplasty as well. Clinical improvement was obtained in 75% of CMI patients, with remission of pre-operative neurological signs in 40% of them. The rate of post-operative complications in our series was 14%: we had three cases of occipital meningocele and two patients with aseptic meningitis.
CMII patients were subjected to suboccipital craniectomy, C1 laminectomy, dura mater opening, cisterna magna exploration, sub-arachnoidal adherences lysis and duraplasty. We obtained a lasting clinical improvement in 62% and a remission of neurological deficits in 20% of the cases. The only complication was an aseptical meningitis (rate of complication, 12%).
The size of syrinx was stable in 80% and decreased in 20% of the whole series, despite a valid liquoral flow through the sub-arachnoidal spaces of PCF was documented in all the cases.
Conclusions
Surgical treatment of Chiari malformation is effective and technically safe, with clinical and neurological improvement and, in severe cases, long-term stabilization. In our experience symptomatic CM patients need decompressive surgery as soon as possible in order to avoid further neurological impairment.
For what concerns the so-called “mini-invasive” technique, it is indicated in patients who have no or a small syrynx, because it has a low rate of post-operative complications, but also a less chance of obtaining syrynx shrinkage.
References
Bogdanov EI, Mendelevich EG (2002) Syrinx size and duration of symptoms predict the pace progressive mielopathy: retrospective analysis of 103 unoperated cases with craniocervical junction malformations and syringomyelia. Clin Neurol Neurosurg 104:90–97
Durham SR, Fjeld-Olenec K (2008) Comparison of posterior fossa decompression with and without duroplasty for the surgical treatment of Chiari malformation Type I in pediatric patients: a meta-anallysis. J Neurosurg Pediatr 2:42–49
Dyste GN, Menezes AH, VanGilder JC (1989) Symptomatic Chiari malformation. An analysis of presentation, management and long-term outcome. J Neurosurg 71:159–168
Elton S, Tubbs RS, Wellons JC 3rd, Blount JP, Grabb PA, Oakes WJ (2002) Acute hydrocephalus following a Chiari I decompression. Pediatr Neurosurg 36:101–104
Greenlee JD, Donovan KA, Hasan DM, Menezes AH (2002) Chiari I malformation in very young child: the spectrum of presentations and experience in 31 children under age 6 years. Pediatrics 110(6):1212–1219
Listernick R, Tomita T (1991) Persistent crying in infancy as a presentation of Chiari type 1 malformation. J Pediatr 118:567–569
Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, Speer MC (1999) CMI redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurg 44:1005–1017
Nohira V, Oakes WJ (1990) Chiari I Malformation: a review of 43 patients. Pediatr Neurosurg 16:222–227
Oakes WJ, Worley G, Spock A (1988) Surgical intervention in twenty-nine patients with symptomatic type II Chiari malformations: clinical presentation and outcome. Concepts Pediatr Neurosurg 8:76–85
Oakes WJ, Gaskill SJ (1992) Symptomatic Chiari malformation and syringohydromyelia in childhood. In: Park TS Spinal dysraphism. Blackwell Boston, pp 104–105
Pollack IF, Kinnunen D, Albright AL (1996) The effect of early craniocervical decompression on functional outcome in neonates and and young children with myelodysplasia and symptomatic Chiari II malformation: results from a prospective series. Neurosurg 38:703–710
Salomao JF, Bellas AR, Leibinger RD, Barbosa AP, Brandao MA (1998) Symptomatic Chiari type II malformation. Arq Neuropsiquiatr 56:98–106
Conflict of interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imperato, A., Seneca, V., Cioffi, V. et al. Treatment of Chiari malformation: who, when and how. Neurol Sci 32 (Suppl 3), 335–339 (2011). https://doi.org/10.1007/s10072-011-0709-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0709-y