Abstract
Sporadic inclusion-body myositis (s-IBM) is a chronic progressive inflammatory myopathy leading to severe disability. It has been suggested that statins may benefit s-IBM patients based on their pleiotropic effects on autoimmunity and possible adverse influence of increased cholesterol on muscle pathological changes. We carried out a pilot, open-label trial to evaluate safety and tolerability of oral simvastatin in s-IBM patients. Fourteen patients were treated with 40 mg of simvastatin over 12 months. Primary outcome measures included the assessment tools proposed by International Myositis Outcome Assessment Collaborative Study group and the IBM-Functional Rating Scale. As additional data, we report the results obtained from muscle MRI, biopsy and oropharyngeal scintigraphy. Ten patients completed the trial and the treatment appeared safe and well tolerated. None of the patients showed a significant clinical improvement. Outcome measures used in this study proved to be valuable tools for global assessment of s-IBM patients. At present, we cannot recommend simvastatin as a treatment for s-IBM though our data may warrant a placebo-controlled study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Statins (HMG-CoA reductase inhibitors) are potent cholesterol-lowering drugs extensively used in medical practice for primary and secondary prevention of cardiovascular events due to atherosclerosis [1, 2]. Statins also exert a number of the so-called pleiotropic actions that include inhibition of inflammatory responses and immunomodulatory effects [3, 4], improvement of endothelial function [5], regulation of progenitor cells, antioxidant and neuroprotective properties [6, 7]. Statins have a good safety profile with occasional hepatotoxicity (<3%), myopathy (<0.2%) [8] and a very rare occurrence of rhabdomyolysis (<0.05) [9], and may be effective in treating several neurological disease, such as Alzheimer disease [10], MS and stroke [5, 11, 12], as indicated by both the animal studies and observational clinical studies. Statins interfere with autoimmune attack of target tissues by inhibiting the multiple arms of immune response. Since these anti-inflammatory effects would be of possible therapeutic value for sporadic inclusion-body myositis (s-IBM), it has been suggested that statins may benefit s-IBM patients [13]. S-IBM is actually a chronic progressive form of inflammatory myopathy with muscle infiltrates mainly composed of cytotoxic CD8+ lymphocytes but negligible regeneration, poor response to treatment with steroids or immunosuppressive drugs and slow progression, leading to severe disability with loss of autonomous deambulation and often severe dysphagia [14–16]. In addition to the putative effects on autoimmunity, the reduction of cholesterol levels obtained with oral statins would possibly be desirable “per se”. Askanas and Engel [17–19] have proposed that abnormal deposition of cholesterol together with caveolin-1 at sites of beta-amyloid accumulation and processing in vacuolated s-IBM muscle fibers, may induce amyloid-beta misfolding and aggregation, adversely influencing the degenerative features of this muscle pathology. Furthermore, in an experimental model, the increased ingestion of high levels of dietary cholesterol in rabbits resulted in skeletal muscle pathological features that resemble s-IBM [20]. Although attention is recommended for the risk of rhabdomyolysis development (1 case in 22,727 after 1-year treatment) [9], muscle disorders are not among the established contraindications to statin therapy. It is also possible, statins being a very common treatment among general the population, that some of the s-IBM patient may have taken statins as cholesterol-lowering drug; however, it is not known whether the drug is well tolerated and has any effects on the progression of the muscle disease. Thus we proposed a pilot study, in order to evaluate the safety and tolerability of oral simvastatin treatment in s-IBM patients and to point out any possible benefit of this treatment on disease progression.
Patients and methods
This was a 12 month, open-label trial with oral simvastatin treatment in s-IBM patients. The trial verified the effects of simvastatin therapy on clinical and health-related quality of life measures. The principal aim of the study was to evaluate the safety and tolerability of oral simvastatin treatment in s-IBM patients. The study also intended to validate, in a trial specifically dedicated to s-IBM patients, the assessment tools and outcome measures proposed by the International Myositis Outcome Assessment Collaborative Study (IMACS) group [21–23] and the IBM Functional Rating Scale [24]. The trial was approved by the local Ethical Committees at Catholic University and Istituto Besta and all the patients signed a written informed consent prior to their participation in the study. Fourteen patients with s-IBM were enrolled in the study. Clinical characteristics of patients are summarized in Table 1. Diagnosis of definite s-IBM in all the patients was based, according to the established criteria [25], on clinical and muscle biopsy studies. Patients with very severe disease (more than 10 years duration, severe dysphagia), pregnant women, patients assuming immunosuppressant drugs and patients with high risk of rhabdomyolysis (severe hepatic or renal dysfunction, hypothyroidism, medications predisposing to muscle toxicity, CK levels greater than five times the normal value) were excluded from the study. Continuation of steroid therapy was allowed in the patients taking oral prednisone at stable or tapering dosages, while no immunosuppressive or immunomodulatory drugs have been administered along with simvastatin.
The dosage of simvastatin was increased by 10 mg every 4 weeks up to 40 mg/day according to the individual patients’ tolerance (monitored by blood test for CK, lipids, liver and renal functions); we selected this in vivo regimen, as further increase of the dose would augment the risk of myotoxic effects, probably with no additional desired effects as in vitro studies on various biological systems show that low simvastatin concentration improve angiogenesis and endothelial function, while higher doses may induce cell apoptosis [26]. In addition to muscle biopsy, before starting therapy, basal evaluation included muscle MRI imaging of lower limbs, complete blood tests including muscle enzymes, lipid panel, liver, thyroid and renal function assays. CK was assessed at baseline after 1 month (before increasing dosage from the starting dose of 10 to 20 mg), then every 2 months (concomitantly with clinical evaluation) and every time unexplained myalgias or additional muscle weakness developed over several days.
Primary endpoint: clinical data
Primary outcome measures included the disease activity core set IMACS, including the manual muscle test (MMT or IMACS 4) [22], and all the patients were also evaluated, at study entry and after 12 months, with the IBM Functional Rating Scale (FRS) [24]. Patients were clinically evaluated every 2 months, and the same neurologist performed all muscle function tests without the knowledge of laboratory or biopsy data. Clinical improvement was defined according to the proposal of IMACS as >20% improvement in three or more of the core set parameters and no more than two worsened by >25% excluding MMT. Worsening was defined by >30% reduction in any three of six variables of the IMACS core set disease activity measure. A core set of disease damage measures has been developed to assess persistent changes resulting from previously active disease and from complications of therapy or other events. These include: the myositis damage index (MDI—IMACS 8), physician and patient/parent global assessment of disease damage (IMACS 9 and IMACS 10).
The patient-reported outcomes also included a generic health-related quality of life assessment using the Medical Outcomes Study 36-item Short Form (SF-36) health survey.
Four untreated s-IBM patients who underwent the same clinical assessment schedule served as a small non-randomized control group regarding clinical outcome.
Muscle MRI imaging and biopsy, oropharyngeal scintigraphy
As secondary outcome measure, muscle imaging by MRI after 12 months of therapy was performed. As additional data in the subgroups of patients, we also examined a repeated biopsy after 12 months of therapy, and in patients presenting significant dysphagia, a oropharyngeal scintigraphy was performed at baseline and end of treatment.
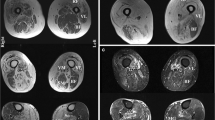
MRI muscle imaging included examination of pelvic and lower limb muscles on a Philips 1.5 tesla MR scanner at study entry and after 12 months. MRI imaging was performed with T1-W SE images (TR/TE = 500/35 ms) and T2-W STIR images (T1 = 1/50 ms). Axial slices were obtained from psoas to distal foot muscles. Both the degree of muscle degeneration (hypotrophy, fibrosis, adipose tissue substitution) and inflammatory signs were monitored and compared during follow-up examination. The changes were classified as minor, moderate or extensive (arbitrary scale).
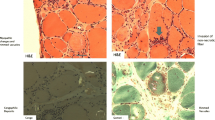
All the patients included in the study underwent a muscle biopsy at the diagnosis, and in five patients who gave informed consent, an additional needle biopsy of quadriceps muscle was performed after 12 months of simvastatin treatment. Routine histological and histochemical reactions including Congo red and immunohistochemistry with the following antibodies were performed, as described, in all diagnostic biopsies: SMI 31 (Sternberger Inc.), anti-human CD8, CD4, MHC-I, MHC-II, CD68, C5bC9, CD20 (all from Dako), and anti-human CD138 (Serotec).
Statistical analysis
The Wilcoxon signed rank test was used to compare the data before and after simvastatin treatment. In particular, we evaluated scores obtained from IMACS core set at different times during treatment. Adjustment for multiple comparisons was made, when necessary, by the use of the Bonferroni correction method; p values ≤0.05 were considered as statistically significant.
Results
Primary endpoint: clinical results
Ten patients completed the trial; three patients dropped out for personal reasons of no medical significance and not related to the study, and one patient was withdrawn from the study because of an asymptomatic significant CK increase.
No rhabdomyolysis cases have been observed in any patient, and CK values remained unchanged or slightly increased (one- to twofold increase of basal level) only during the first weeks of therapy. Only one patient has shown a significant CK increase (5 fold the basal level), thus suspended simvastatin treatment, was strictly monitored by monthly clinical and laboratory studies and did not present muscle symptoms suggestive for a simvastatin-induced myopathy, such as myalgias, acute worsening of muscle weakness and further rapid increase of CK.
No other significant side effects were observed in any patient during simvastatin treatment; in particular liver and renal function did not show any abnormality and none of the treated patients developed infections or metabolic complications. Clinical results are summarized in Table 2. None of the patients showed a significant clinical improving or worsening according to the IMACS definition. Considering both IBM-FRT and IMACS report data, in four patients we have observed a slight improvement of muscle strength by MMT, while two patients reported a subjective improvement of general conditions and in managing common daily activities. Two patients presented a slight worsening of clinical status, while two patients remained substantially unchanged. In four untreated patients, undergoing the same evaluation protocol, we observed (Fig. 1) a slight worsening that, although not reaching statistical significance (p = 0.07), indicates a trend of clinical progression not paralleled in the treated patients.
Overview panels of FRS and IMACS4 scores. a Changes of functional rating scale (FRS) score between first and fifth clinical examination, both in simvastatin treated s-IBM patients (no significant changes, p = 0,349) and in untreated s-IBM patients (slight worsening, p = 0,147). b Changes of IMACS 4 score between first and fifth examination, both in simvastatin treated s-IBM patients (unchanged score or slight improvement not statistically significant, p = 0,202), and in untreated s-IBM patients (trend of slight worsening, p = 0,071). c Changes in mean IMACS 4 score in simvastatin treated and untreated s-IBM patients
Additional data: MRI, muscle biopsy, oropharyngeal scintigraphy
At the basal MRI, all the patients showed various degrees of inflammatory changes (areas of focal/diffuse hyperintensity on T2-W STIR) and signs of muscle degeneration (hypotrophy, fibrosis, adipose tissue substitution). In our experience with s-IBM patients, MRI examination correlates with the level of functional impairment, but sensitivity in detecting the extent and the severity of muscle involvement is higher than that of clinical examination. MRI after 12 months showed no significant changes in inflammatory or degenerative abnormalities (not shown). Similarly, the needle muscle biopsy performed in five patients did not show major histopathological changes when compared with the biopsy at the diagnosis (not shown). Interestingly, we did notice that a characteristic pattern of muscle involvement was maintained in each patient. In fact, both the relative proportion of inflammatory changes and vacuolated muscle fibers containing either Congo red or SMI31-positive inclusions, as well as the percentage of main lymphomonocytic subpopulations (CD8+, CD4+, CD20+, CD68+, CD138+) were maintained even if the second biopsy was performed on the different muscle (quadriceps vs. deltoid), suggesting that every patient may present an individual “pathologic muscle signature” relatively constant at least within the time frame of this study. None of the four patients who underwent oropharyngeal scintigraphy showed a significant worsening of the dysphagia.
Discussion
Simvastatin treatment in s-IBM appeared to be safe, well tolerated, and without significant collateral effects both as monotherapy and in association with corticosteroids. Even though it was not primarily a trial of clinical efficacy, this was a safety study designed to investigate also possible effects on disease progression. Indeed, primary endpoint was represented by clinical evaluation of patients performed through the IMACS core set and the IBM Functional Rating Scale. Although in four patients we observed a slight improvement of muscle strength by MMT, simvastatin apparently did not significantly modify the patients’ clinical impairment. However, based on the comparative evaluations at baseline and end of treatment time points, we noted an apparent stability of clinical condition in the majority of treated patients, in contrast with the trend of clinical worsening observed in the four non-simvastatin treated s-IBM patients. In fact, after 1 year of simvastatin treatment, only two treated patients presented a slight worsening of clinical condition, while 80% of our treated patients did not show a significant clinical progression. Nevertheless, none of the patients showed either a clinical improvement or worsening fulfilling the IMACS definition.
Regarding secondary outcome measures muscle MRI evaluation showed no significant changes in terms of inflammatory or degenerative abnormalities, and the specific distribution of muscle involvement observed at baseline was unchanged in every patient. Additional instrumental data obtained only in subgroups of patients cannot be considered as effective secondary outcome measures but, as a corollary to clinical and MRI data, provide additional information useful for the overall evaluation of patients. Either oropharyngeal scintigraphy or evaluation of repeated muscle biopsies showed no significant changes after simvastatin treatment. As a corollary, this highlights that pathologic characteristics, such as degree of inflammation, amount of vacuolated fibers and fibrotic changes in affected muscles (also examining an affected muscle different from the previously biopsied one) tend to remain rather constant in individual s-IBM patients at least within a 1 year time-frame.
Development of new therapies for s-IBM has been hampered by the rarity of disease, the limited understanding of its pathogenesis and the paucity of randomized clinical trials [27–31] which are extremely difficult to be set and evaluated in a chronic myopathy characterized by slow progression. Our trial had a recommended minimum duration of 1 year and also avoided comparison with other inflammatory myopathies which could hamper data analysis.
To the best of our knowledge, this is the first clinical trial specifically dedicated to s-IBM patients based on IMACS group assessment tools and proposed outcome measures [21–23], together with the IBM Functional Rating Scale [24]. This core set, correlated with information derived from muscle pathology and MRI imaging, proved to be useful for a comprehensive assessment of s-IBM patients. It will be applicable also in upcoming studies with different pharmacological or cellular therapies in s-IBM and may be further developed to better standardize outcome measures, thus improving therapeutic trials design in this specific population of elderly myositis patients.
We found out a good accordance between IBM-FRS and IMACS scales that were also consistent with the patient’s subjective evaluations. The efficacy of tools capable to explore functional ability in common daily activities, in addition to muscle strength evaluation, has to be underscored. Such a global assessment can be more suitable to detect even slight differences of clinical condition in a chronic progressive disease like s-IBM. Core sets measures IMACS 7 and IMACS 8 which evaluate mainly extramuscular involvement, usually scarce in s-IBM, are of less interest than in other inflammatory myopathies, such as polymyositis and dermatomyositis. More importantly, IMACS definition of improvement and worsening should be tailored for s-IBM allowing small but significant clinical changes to be definitely considered. In fact, while acute or subacute clinical evolution of PM and DM consents to evaluate response to treatment in a short period of time, in a chronic slowly progressive disease, such as s-IBM, it is necessary to also assess the minimal changes in the clinical course. Although the clinical outcome measures did not evidence a statistically significant improvement, and MRI and muscle biopsies showed stability of pathologic changes, a possible positive effect of simvastatin administration indicated by the slowing of clinical progression could have been missed by our investigation.
Nearly, all the pharmacological therapies proposed for s-IBM patients are expensive and/or weighted by important side effects, their use is often empiric and not validated in controlled studies, and for most of all no clinically relevant effects have been indeed demonstrated, since claim of efficacy have been reported anecdotally or in small uncontrolled study. In s-IBM the main goal of treatment is, at least, to halt disease progression and stabilize patient’s clinical conditions. Therefore, achieving such a result using a low cost oral agent, with an acceptable tolerability and toxicity profile, could imply a significant benefit in both clinical and pharmacoeconomics terms. We are also obviously aware of limitations of this study that was arguably small, not blind and placebo-controlled, thus not designed to unequivocally demonstrate clinical efficacy. Assessment bias and placebo effect are important factors unavoidable in uncontrolled studies, thus, at the present we cannot recommend simvastatin as a treatment for s-IBM. However, our data on safety and preliminary clinical observation may warrant a double-blind placebo-controlled study to verify whether s-IBM patients may indeed benefit from chronic simvastatin treatment.
References
Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J (2001) Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 103(7):926–933
Notarbartolo A, Davì G, Averna M, Barbagallo CM et al (1995) Inhibition of thromboxane biosynthesis and platelet function by simvastatin in type IIa hypercholesterolemia. Arterioscler Thromb Vasc Biol 15(2):247–251
Weitz-Schmidt G, Welzenbach K, Brinkmann V et al (2001) Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 7(6):687–692. doi:10.1038/89058
Bellosta S, Via D, Canavesi M et al (1998) HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol 18(11):1671–1678
Endres M, Laufs U, Huang Z et al (1998) Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 95(15):8880–8885. doi:10.1073/pnas.95.15.8880
Zacco A, Togo J, Spence K et al (2003) 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci 23(35):11104–11111
Chen J, Zhang ZG, Li Y et al (2003) Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol 53(6):743–751. doi:10.1002/ana.10555
Ballantyne CM, Corsini A, Davidson MH et al (2003) Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med 163(5):553–564. (Review). doi:10.1001/archinte.163.5.553
Graham DJ, Staffa JA, Shatin D et al (2004) Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 292(21):2585–2590
Fassbender K, Simons M, Bergmann C et al (2001) Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ 42 and Aβ 40 in vitro and in vivo. Proc Natl Acad Sci USA 98(10):5856–5861. doi:10.1073/pnas.081620098
Vollmer T, Key L, Durkalski V et al (2004) Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 363(9421):1607–1608. doi:10.1016/S0140-6736(04)16205-3
LaRosa JC, He J, Vupputuri S (1999) Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA 282:2340–2346
Steinman L (2006) Controlling autoimmunity in sporadic inclusion-body myositis. Neurology 66(2 Suppl 1):S56–58. (Review)
Askanas V, Engel WK (2008) Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol 116(6):583–595. doi:10.1007/s00401-008-0449-0
Dalakas MC (2006) Sporadic inclusion body myositis—diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol 2(8):437–447. doi:10.1038/ncpneuro0261
Garlepp MJ, Mastaglia FL (2008) Inclusion body myositis: new insights into pathogenesis. Curr Opin Rheumatol 20(6):662–668. doi:10.1097/BOR.0b013e328313644c
Askanas V, Engel WK (2003) Proposed pathogenic cascade of inclusion-body myositis: importance of amyloid-β, misfolded proteins, predisposing genes, and aging. Curr Opin Rheumatol 15:737–744. doi:10.1097/00002281-200311000-00009
Kefi M, Vattemi G, Engel WK, Askanas V (2002) Abnormal accumulation of caveolin-1 and its colocalization with cholesterol, amyloid-β and phosphorylated tau in inclusion-body myositis (IBM) muscle. Neurology 58:391
McFerrin J, Engel WK, Leclerc N, Askanas V (2002) Combined influence of amyloid-β-precursor protein (AβPP) gene transfer and cholesterol excess on cultured normal human muscle fibers. Neurology 58:489
Chen X, Ghribi O, Geiger JD (2008) Rabbits fed cholesterol-enriched diets exhibit pathological features of inclusion body myositis. Am J Physiol Regul Integr Comp Physiol 294(3):R829–R835. doi:10.1152/ajpregu.00639.2007
Oddis CV, Rider LG, Reed AM, International Myositis Assessment Clinical Studies Group et al (2005) International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis Rheum 52(9):2607–2615. doi:10.1002/art.21291
Miller FW, Rider LG, Chung YL, International Myositis Outcome Assessment Collaborative Study Group et al (2001) Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology (Oxford) 40:1262–1273. doi:10.1093/rheumatology/40.11.1262
Rider LG, Giannini EH, Brunner HI, International Myositis Assessment and Clinical Studies Group et al (2004) International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthr Rheum 50:2281–2290. doi:10.1002/art.20349
Jackson CE, Barohn RJ, Gronseth G, Pandya S, Herbelin L, Group MuscleStudy (2008) Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve 37(4):473–476. doi:10.1002/mus.20958
Griggs RC, Askanas V, DiMauro S et al (1995) Inclusion body myositis and myopathies. Ann Neurol 38(5):705–713. doi:10.1002/ana.410380504
Yokota K, Miyoshi F, Miyazaki T et al (2008) High concentration simvastatin induces apoptosis in fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Rheumatol 35(2):193–200
Lindberg C, Trysberg E, Tarkowski A, Oldfors A (2003) Anti-T-lymphocyte globulin treatment in inclusion body myositis: a randomized pilot study. Neurology 61(2):260–262
Barohn RJ, Herbelin L, Kissel JT et al (2006) Pilot trial of etanercept in the treatment of inclusion-body myositis. Neurology 66(2 Suppl 1):S123–S124
Dastmalchi M, Grundtman C, Alexanderson H et al (2008) A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis 67(12):1670–1677. doi:10.1136/ard.2007.077974
Sultan SM, Ng KP, Edwards JC, Isenberg DA, Cambridge G (2008) Clinical outcome following B cell depletion therapy in eight patients with refractory idiopathic inflammatory myopathy. Clin Exp Rheumatol 26(5):887–893
Dalakas MC, Rakocevic G, Schmidt J et al (2009) Effect of Alemtuzumab (CAMPATH 1-H) in patients with inclusion-body myositis. Brain 132(Pt 6):1536–1544. doi:10.1093/brain/awp104
Acknowledgments
This study was supported by grants from Istituto Superiore di Sanità (“Rare Diseases Italy-USA program” ISS 526D/15 grant), Don Carlo Gnocchi ONLUS Foundation, and Catholic University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sancricca, C., Mora, M., Ricci, E. et al. Pilot trial of simvastatin in the treatment of sporadic inclusion-body myositis. Neurol Sci 32, 841–847 (2011). https://doi.org/10.1007/s10072-011-0657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0657-6