Abstract
The objective is to evaluate the prognosis of subjective cognitive complaints (SCC) patients during 4-year follow-up. A prospective study on 92 SCC patients investigating their cognitive, affective and behavioural aspects. SCC patients were classified as having no objective cognitive impairment (NOCI), mild cognitive impairment (MCI), or subtypes of MCI. Results: 43 patients were found to have NOCI and 49 MCI. During the follow-up, 45.5% of NOCI patients remained unchanged, 13.9% were diagnosed as MCI and only one progressed to dementia. Of the MCI patients, 32.3% remained stable, 18.4% became demented and 4% reverted to NOCI. Visual attention, behavioural memory, long-term verbal memory, apathy and caregiver distress, provided independent predictors of progression to dementia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subjective cognitive complaints (SCC) are of increasing interest both in clinical and research based investigations [1, 27–29], mainly due to the possibility that they are risk factor for cognitive impairment or even dementia. Nevertheless, previous studies were not conclusive. Some authors [9, 21, 25] found no evidence of objective deficits in neuropsychological performance, and therefore considered that SCC patients are affected by health or psycho-affective disorders. However, Vestberg et al. [41] on comparing patients with only SCC and patients showing objective memory deficits did not find any differences in personality factors or affective status. Others [18, 32, 35, 39] found that SCC can predict cognitive decline and even dementia, identifying a positive relationship with APOE-ε4 allele carriage [40], white-matter lesions [26], medial temporal lobe atrophy [42] and Alzheimer’s disease pathology [5].
In our previous study [15], we examined 92 consecutive SCC patients without dementia. We observed that about half of them showed an objective cognitive impairment, meeting the criteria for MCI diagnosis. The others subjects did not present any objective cognitive deficits, i.e. they had no cognitive impairment (NOCI).
In this study, we followed up the same initial patients annually for 4 years and evaluated the cognitive, affective and behavioural evolution to investigate the predictors for dementia.
Methods
We enrolled 92 outpatients out of an original 133 SCC patients without dementia consecutively referred over a 9-month period by their relatives and physicians or who spontaneously presented themselves to the University Hospital of the Department of Neurological Sciences of Bologna. All subjects gave their informed consent according to the Declaration of Helsinki. The remain 41 of 133 patients did not enter into the study because they presented one or more exclusion criteria elements (see below) or they did not accept, being not interested.
Inclusion criteria for no cognitive impairment (NOCI) diagnosis
Inclusion criteria for classification of NOCI were [15, 31]: (1) SCC self-reported by the subject; (2) MMSE [14] corrected for age and education equal to or higher than a value of 23.8, according to an Italian standardization [24]; (3) absence of impaired tasks on our neuropsychological examination considering a 1.5-SD cutoff using normative Italian data; (4) absence of the criteria for dementia diagnosis according to DSM-IV [2]; (5) normal functioning in the activities of daily living (ADL) and in Instrumental ADL (IADL) [22].
Inclusion criteria for MCI diagnosis
In addition to criteria 2 and 4 for NOCI, we also included these criteria [31]: (1) self-reported SCC confirmed by an informant; (2) an objective impairment in at least one cognitive domain detected by neuropsychological tests with 1.5-SD cutoff derived by the same normative data; (3) normal ADL, with absent or minimal impairment on IADL.
Diagnosis of MCI type
Diagnosis of MCI subtypes [31], i.e. amnesic MCI (MCI-a), single non-amnesic domain MCI (MCI-sd) and multiple domain MCI (MCI-md), were based, respectively on objective impairment in at least one task in the neuropsychological domains of memory, single non-memory test domain (i.e. attention, language, executive, visuospatial functions, etc.) and multiple impairment in at least two different cognitive domains (MCI-md), including or not including memory.
Normal controls
Sixty normal volunteers matched for sex, age and education were selected as the control group. These subjects did not have any past or present neurological, psychiatric or general diseases. They were selected mainly from the relatives of the patients. The vital parameters of this group are shown in Table 1.
Exclusion criteria
Exclusion criteria were: current or previous neurological, psychiatric and systemic diseases, alcoholism or other substance abuse, use of neuroleptics or other antipsychotics and tricyclic antidepressants considering their possible negative effects on cognition. Low doses (i.e. 1 mg of lorazepam) of a stable therapy of benzodiazepines or selective serotonin or norepinephrine reuptake inhibitors antidepressants were allowed.
Objective and instrumental examinations
Patients were investigated by a general and neurological examination, blood tests (haemachrome, thyroid function, vitamin B12, folates, cholesterol, triglycerides, syphilis serology, and homocysteine). CT or MRI scans were performed to exclude space occupying and neoplastic lesions or major atrophic and vascular lesions, according to exclusion criteria and to avoid an excessive variability of the sample.
Neuropsychological, affective and neuropsychiatric evaluation
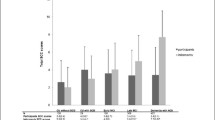
The details of the neuropsychological tests have been previously described [15]. These tests (listed in Table 1) were standardized for age, years of education and sex in the Italian population [7, 8, 12, 16, 17, 30, 37]. We evaluated global cognition by mini mental status examination (MMSE) [14, 24], mental deterioration battery (MDB) [8] and brief mental deterioration battery (BMDB) [16, 17]. MDB consists of eight verbal and visuospatial tasks, which are scored using a method of equivalent points. Pathological tasks (PT) are those in which the subject’s performance is below the lower limit of the tolerance interval of 95% for a confidence level of 95%. BMDB is derived from MDB by discriminant function analysis’s procedures, allowing inclusion of the smallest tasks with the highest correct classification and with a “Final Result” (FR) allowing a classification for each subject with respect to the threshold value of zero with negative scores considered as pathological [16, 17]. We also investigated learning, verbal, visual and behavioural memory using the Rivermead behavioural memory test (RBMT) with standardized (std) and screening (scr) scores [43] of memory, attention, language, constructional praxis, abstract and concrete thinking. We evaluated depression with the Beck Depression Inventory (BDI) [6], anxiety by the State-Trait Anxiety Inventory—Form Y (STAI) [36]; neuropsychiatric symptoms by Neuropsychiatric Inventory (NPI) [11] in which 12 behavioural domains (listed in Table 2) are explored, considering their frequency (range 0–4) and gravity (range 1–3) for each domain (total range 0–12); the symptoms are present if the score is comprised between 0 and 4; the symptoms are considered “significant” when they are superior to 4; the NPI total score is the sum of the value of each domain (range 0–144); the caregiver distress is scored for each domain (range 0–5) (total range: 0–60). Subjective memory complaints were evaluated using the memory assessment clinics-questionnaire (MAC-Q) [10].
Times of examinations
All SCC patients who fulfilled the above-mentioned inclusion criteria were evaluated at basal time (T0) and annually for the following 4 years (i.e. T1, T2, T3 and T4).
Statistical analysis
Data were analysed using the SPSS statistical analysis software, version 16.0. We performed a descriptive analysis of the various parameters of the patient groups; the comparisons among groups were obtained by chi-square for nominal variables; for quantitative variables the multivariate general linear model with Bonferroni’s correction was employed with the significance level set at p = 0.05 and sex, age, education as covariates. In addition, multiple logistic regression analyses with forward variable selection were used to investigate predictors of conversion to dementia. Furthermore, we applied an analysis of the principal component using subsequent varimax rotation to illustrate the structure of the employed scales.
Results
Sample characteristics, clinical and cognitive findings at T0
According to the classification criteria, we classified 43 patients as having NOCI and 49 as MCI. Subsequently, MCI patients were divided into the following subgroups: 28 (57.1%) patients belonged to MCI-md, 12 (24.5%) to MCI-sd and 9 patients (18.4%) to the MCI-a group.
Table 1 lists the demographic parameters and neuropsychological performances at T0 of the groups’ controls and the NOCI, MCI, MCI subgroups. There were no significant differences with regard to gender among all groups. NOCI patients did not differ from the controls with respect to age, education, and in neuropsychological performances, even showing some better results.
The entire MCI group with respect to the controls had significantly higher age, worse FR of BMDB and worse results in many tests as did the MCI subgroups. MCI with respect to NOCI had significantly higher age, lower education and worse general cognitive indexes (except for MMSE corrected for age and education) and RBMT indexes; no significant difference emerged between MCI and NOCI groups regarding subjective evaluation of memory (MAC-Q) (MCI = 24.06, SD 2.8; NOCI = 23.16, SD 2.79). To avoid circular aspects, no comparisons were made between MCI and NOCI groups in their single neuropsychological test performances which were employed to classify these two subgroups.
With respect to the cognitive domains in the 49 MCI patients, attention was impaired in 34 patients, memory in 30 (isolated in the 9 MCI-a patients), language in 11, executive functions in 8 and visuospatial abilities in 7. Nineteen patients had no memory impairment. The most compromised tests were: the selective visual attention test (Stroop test) (24 patients), associate words learning (21 patients), visual search attention test (barrage) in 18 patients, followed by the other tasks.
Clinical and cognitive findings during the follow-up
Figure 1 lists the algorithm of the evolution of diagnoses of all SCC patients from T0 to T4. Twenty NOCI subjects (45.51% of initial NOCI patients) and 16 (32.35% of initial MCI patients) MCI patients remained stable. Six (13.95%) NOCI subjects were subsequently diagnosed as having MCI (3 MCI-sd, 1 MCI-a and 2 MCI-md); and only 1 NOCI patient progressed to dementia (between T3 and T4). Of the initial MCI patients, 9 (18.4%) developed dementia between T0 and T4 (2 at T1); 5 at T2 and 3 at T3, and none at T4; 2 MCI (4.0%) patients did not show any deficits after 4 years, therefore being classified as NOCI subjects. These two patients belonged to the two MCI-sd group. The MCI-md group at T0 showed the highest conversion to dementia [8/10 (28.6% of the MCI-md group and 16.3% of the entire MCI group)]. Only one patient in the MCI-sd group progressed to dementia. It is noteworthy that none of the MCI-a patients became demented. All demented patients (except the NOCI patient who converted to dementia) demonstrated at T0 impairment in the attention domain alone or in association with other task impairments, in particular memory, which was observed in six patients.
At T4, the remaining NOCI subjects were younger (67.95 years, SD 9.30) than the MCI patients. (76.45, SD 6.28, p = 0.01) and had a higher education level (10.77, SD 4.23 years with respect to MCI patients: 6.24, SD 3.20, p = 0.001). Controls were younger (66.97, SD 10.69) than MCI patients (p = 0.001) with a higher education level (9.77, SD 4.00, p = 0.002). No differences were detected between the controls and NOCI.
Types of dementia
Of the ten patients diagnosed as having dementia according to DSM-IV [2] criteria, only one patient was classified as frontotemporal dementia [20], three subjects were categorized as degenerative dementia with parkinsonism and six individuals were affected by probable Alzheimer’s disease [23].
Converting and nonconverting patients
We compared cognitive, mood and behavioural aspects of the 10 (1 NOCI and 9 MCI patients) who converted to dementia (“converters”) with the 44 patients who remained free from dementia between T0 and T4 (“nonconverters”) (i.e. 20 NOCI patients and 16 stable MCI patients; 6 NOCI converting to MCI and 2 MCI converting to NOCI) (Table 2). Chi-square analysis was used for comparisons of nominal variables and univariate analysis of variance for quantitative variables. Significant differences in gender, education, subjective complaints (Mac-Q), depression (BDI) and anxiety (STAI) scales were not detected (Table 2). Notably, (Table 2) nonconverters patients had, with respect to converters, lower age, better general cognitive indexes (except PT of MDB) and were better on neuropsychological tasks (especially visual attention, semantic fluency, verbal learning and memory, including behavioural as RBMT). Furthermore, converters had higher total scores of NPI, caregiver distress, and many NPI domains such as depression, anxiety and apathy. All these variables, which are associated with a significant risk of conversion to dementia, were included as covariates in the binary logistic regression with the dependent variable constituted by the conversion to dementia during the 4-year follow-up period. By forward variable selection, the model includes in five steps, as independent predictors, the variables of the RBMT standardized score, NPI caregiver distress (NPIcd), barrage (a test of visual searching), Rey 15ltc and NPI apathy.
The sensitivity and specificity appeared to increase from step 1 to 5 (Table 3). RBMT std alone explains a high sensitivity but low specificity which increases adding NPIcd. With the third step, which includes barrage, it is possible to reach 90.7% of the final correct global classification. Including Rey long-term score at step 4 and NPI apathy at step 5, we reached 100% of the global correct classification.
Dropouts
Thirty-eight (16 NOCI patients and 22 MCI patients) (41.3% of the entire SCC group) (Fig. 1) dropped out during the study due to refusal or personal reasons (relocation, general illnesses, etc.).
Principal component analysis
To understand the correlations among the variables, we performed an analysis of the principal components by varimax rotation obtaining ten components that explain the 78.44% of experimental variance. The description of the ten obtained components is beyond the scope of this work and we herein report only the collocation of the scales STAI and BDI and anxiety and depression domains of NPI. The STAI and BDI belong to a unique component which is orthogonal in comparison with another component which includes NPI anxiety and NPI depression, NPI total score and NPI apathy.
Depression
No significant difference was detected in BDI between the NOCI and MCI groups at T0 (p = 0.87). Overall, no differences were detected between the SCC (NOCI and MCI together) 30 depressed and the 62 not depressed subjects regarding age, education, neuropsychological tasks or RBMT scores. No differences were found between the number of depressed patients converters and nonconverters (3/10 vs. 16/28, not significant). The BDI score was not higher (p = 0.43) in SCC patients with respect to the controls.
Anxiety
No differences in STAI trait and STAI state were detected between NOCI and MCI patients at T0. Four out of ten of the converted patients were anxious, as shown in the STAI trait while 21/49 of the nonconverters were anxious (not significant). Four out of ten patients who had progressed to dementia resulted as being anxious in the STAI state compared to 25 out of 44 nonconverters (difference not significant).
In STAI state, controls showed lower scores with respect to the overall MCI group (p = 0.001) and MCI subgroups and all other groups (MCI-sd, p = 0.001; MCI-md, p = 0.005; MCI-a, p = 0.038; NOCI, p = 0.007). The same occurred regarding the STAI trait when comparing controls to the overall MCI group (p = 0.002) and MCI subgroups (MCI-sd, p = 0.005; MCI-a, p = 0.046; MCI-md, p = 0.01; NOCI, p = 0.047)
Neuropsychiatric symptoms
At T0, the total NPI score was higher in the MCI group to the limit of significance (p = 0.05). The persons living with MCI subjects were significantly more stressed than those living with NOCI patients (p = 0.01) [15]. Comparing the symptoms present on the 12 domains (score > 0), the MCI group had more severe disturbances in depression (p = 0.03) and irritability (p = 0.02). For the clinically significant symptoms (score ≥ 4 in each NPI domain), the MCI group had significantly more depression (p = 0.04).
In the total NPI score, MCI-md had greater scores than MCI-a (p = 0.03), MCI-sd (p = 0.022) and NOCI (p = 0.002); in NPI caregiver distress, MCI-md had a higher score compared to MCI-a (p = 0.22), MCI-sd (p = 0.044) and NOCI (p = 0.002). In the depression domain, MCI-md had higher scores only with respect to NOCI (p = 0.018); in NPI anxiety, MCI-md did not have higher scores with respect to NOCI (p = 0.08). For NPI apathy, MCI-a had a higher score than MCI-sd (p = 0.043).
Scores for the irritability domain were greater in MCI-md in comparison to NOCI (p < 0.0001) and MCI-sd (p = 0.033). Sleep disturbances are significant comparing MCI-md with MCI-a (p = 0.027).
Discussion
SCC represents an interesting aspect in the spectrum between normal cognition and dementia. In many cases, this condition corresponds to an exclusive alteration of the patient’s perception of their own cognitive performance without an objective cognitive impairment detectable by neuropsychological tests (i.e. NOCI patients). However, SCC could be the beginning of a less favourable cognitive condition, representing the first symptom required for a diagnosis of MCI in which objective cognitive deficits are detectable.
In our sample, about half of the 43 NOCI subjects remained unchanged during the 4-year follow-up period, while only a minority (13.9%) was subsequently diagnosed as MCI. It is noteworthy that only one of the NOCI patients progressed to dementia, reaching this condition during the observation between the third and fourth year. Therefore, our findings suggest that the absence of objective deficits in an extensive neuropsychological battery is a quite but not absolutely favourable element of a patient’s cognitive evolution. It is reasonable to reassure the NOCI subjects, investigating their affective status and aspects of their personality, and evaluating a possible finding of depression, anxiety or a particular “cognitive style” [9, 21]. In these subjects, pharmacological and/or psychological treatment could be useful. Nevertheless, other confirmations during a prolonged follow-up would be useful.
Of our initial MCI patients, 18.4% (mean 4.6% per year) became demented and almost all these patients belonged to MCI-md group. It is also of interest that all MCI patients who converted to dementia at T0 showed attention impairment alone or in association with deficits in other domains, in particular memory.
After 4-year follow-up, only 2 out of 49 in the initial MCI group were classified as NOCI not having cognitive deficits. All these patients, at T0, were MCI-sd. In the same way, in the group of patients described as MCI-sd, there was a very small progression to dementia (only 1 out of 49 MCI patients).
It must be underlined that in our sample the results of behavioural memory battery (RBMT) tasks, visual attention of the barrage test, long-term verbal memory and NPI caregiver distress and apathy scores resulted in independent predictors of conversion to dementia. The combination of the cognitive tasks assessment and behavioural evaluation allows a correct classification in distinguishing between converters and nonconverters to dementia (Table 3). Kazui et al. [19] previously identified very highly correct classifications of RBMT in distinguishing MCI from normal controls and Alzheimer’s disease patients. Nevertheless, in our sample, RBMT alone explains a high sensitivity but low specificity in detecting patients converting to dementia.
We emphasize the negative prognostic significance of multiple domain impairment, especially with deficits of visual attention and verbal memory. A single domain impairment (including memory), could represent a more favourable prognostic feature.
The annual conversion rate from MCI to dementia that we observed is quite lower (about 4%) than the 10–15% or even more in others studies [3], in agreement with other findings [33].
Furthermore, we found a relatively small number of our MCI-a (18.4% of our MCI patients), confirming other authors’ data [13, 34]. Anyone of our MCI-a became demented, unlike Petersen et al. [31] findings showing an increased risk of Alzheimer’s disease in the MCI-a group. Nevertheless, others have reported findings similar to ours [13, 34, 38].
Another interesting aspect is the relevance of affective and behavioural findings in our MCI patients with respect to patients without objective cognitive impairment (NOCI subjects), such as total NPI score, caregiver distress, depression and irritability. In our sample, NPI caregiver distress and apathy resulted predictors of dementia conversion. Others found similar results, confirming the relevance and negative prognostic features of the neuropsychiatric manifestations in MCI patients [4].
Regarding depression assessed by the BDI scale, it is noteworthy that it is not significantly higher in the patients that make subjective cognitive complaints (both NOCI and MCI) compared to the controls. Furthermore, MCI patients do not have an increased frequency of depression detectable by this scale compared to NOCI patients.
Anxiety, of both state and trait, detected by STAI, was increased by the belief of having a cognitive impairment, independently to the finding of an objective deficit.
Therefore, the lack of differences between NOCI and MCI by the scales requiring a prevalent subjective evaluation of depression and anxiety (BDI and STAI), suggests that MCI patients tend to underestimate their affective and behavioural symptoms. In fact, they showed relevant affective and behavioural disorders only when they were evaluated by a relative, i.e. “caregiver” by NPI.
It is likely that NOCI subjects consider their cognitive performances more serious than they actually are.
Conclusion
It is essential to appropriately evaluate patients with SCC to identify the absence or presence of objective cognitive deficits. Patients with objective cognitive deficits, especially regarding attention and multiple domains impairment as well as relevant behavioural symptoms detected by NPI have an increased risk of developing dementia. SCC can have different outcomes and adequate combination of clinical, cognitive and behaviour evaluations are required to make a suitable prognosis.
References
Abdulrab K, Heun R (2008) Subjective memory impairment a review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry 23:321–330
Association American Psychiatric (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Press, Washington, DC
Amieva H, Letenneur L, Dartigues JF, Rouch-Leroyer I, Sourgen C, Chee-Biree F, Dib M, Barberger-Gateau P, Orgogozo JM, Fabrigoule C (2004) Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dement Geriatr Cogn Disord 18:87–93
Apostolova LG, Cummings JL (2008) Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord 25:115–126
Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA (2006) Memory complaints are related to Alzheimer disease pathology in older persons. Neurology 67:1581–1585
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Caffarra P, Vezzadini G, Dieci F (2002) Una versione abbreviata del test di Stoop: dati nomativi nella popolazione italiana. Nuova Rivista di Neurologia 12:111–115
Carlesimo GA, Caltagirone C, Gainotti G (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384
Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C (2002) Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord 72:157–165
Crook TH III, Feher EP, Larrabee GJ (1992) Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatry 4:165–176
Cummings JL (1997) The neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 48:S10–S16
De Renzi E, Faglioni P, Ruggerini C (1977) Prove di Memoria verbale d’impiego clinico per la diagnosi di amnesia. Arch Psicol Neurol Psichiatr 3:303–318
Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl KH (2007) Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 68:288–291
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Gallassi R, Bisulli A, Oppi F, Poda R, Di Felice C (2008) Subjective cognitive complaints, neuropsychological performance, affective and behavioural symptoms in non-demented patients. Int J Geriatr Psychiatry 23:95–101
Gallassi R, Lenzi P, Stracciari A, Lorusso S, Ciardulli C, Morreale A, Mussuto V (1986) Neuropsychological assessment of mental deterioration: purpose of a brief battery and a probabilistic definition of “normality” and “non-normality”. Acta Psychiatr Scand 74:62–67
Gallassi R, Morreale A, Di Sarro R, Lorusso S (2002) Value of clinical data and neuropsychological measures in probable Alzheimer’s disease. Arch Gerontol Geriatrics 34:123–134
Jessen F, Wiese B, Cvetanovska G, Fuchs A, Kaduszkiewicz H, Kolsch H, Luck T, Mosch E, Pentzek M, Riedel-Heller SG, Werle J, Weyerer S, Zimmermann T, Maier W, Bickel H (2007) Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med 37:1753–1762
Kazui H, Matsuda A, Hirono N, Mori E, Miyoshi N, Ogino A, Tokunaga H, Ikejiri Y, Takeda M (2005) Everyday memory impairment of patients with mild cognitive impairment. Dement Geriatr Cogn Disord 19:331–337
Knopman DS, Boeve BF, Parisi JE, Dickson DW, Smith GE, Ivnik RJ, Josephs KA, Petersen RC (2005) Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol 57:480–488
Lautenschlager NT, Flicker L, Vasikaran S, Leedman P, Almeida OP (2005) Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. Am J Geriatr Psychiatry 13:731–734
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Measso G, Cavarzeran F, Zappala G, Lebowitz BD, Crook TH, Pirozzolo FJ, Amaducci LA, Massari D, Grigoletto F (1993) The Mini-Mental-State-Examination—normative study of an Italian random sample. Dev Neuropsychol 9:77–85
Metternich B, Schmidtke K, Hull M (2009) How are memory complaints in functional memory disorder related to measures of affect, metamemory and cognition? J Psychosom Res 66:435–444
Minett TS, Dean JL, Firbank M, English P, O’Brien JT (2005) Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry 13:665–671
Mitchell AJ (2008) Is it time to separate subjective cognitive complaints from the diagnosis of mild cognitive impairment? Age Ageing 37:497–499
Mitchell AJ (2008) The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry 23:1191–1202
Mitchell AJ (2009) A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 43:411–431
Novelli G, Papagno C, Capitani E, Laiacona M, Cappa S, Vallar G (1986) Tre test clinici di ricerca e produzione lessicale Taratura su soggetti normale. Arch Psicol Neurol Psichiatr 47:279–296
Petersen RC, Negash S (2008) Mild cognitive impairment: an overview. CNS Spectr 13:45–53
Reid LM, Maclullich AM (2006) Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 22:471–485
Ritchie K, Artero S, Touchon J (2001) Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 56:37–42
Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M, Padovani A (2007) Conversion of amnestic mild cognitive impairment to dementia of Alzheimer type is independent to memory deterioration. Int J Geriatr Psychiatry 22:1217–1222
Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y (1997) Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Am J Psychiatry 154:609–615
Spielberger CD, Vagg PR, Barker LR, Donahan GW, Westberry LG (1980) Stress and anxiety—the factor structure of the state-trait anxiety inventory. Hemisphere Publishing Corporation, Washington, DC
Spinnler H, Tognoni G (1987) Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci 6(Suppl 8):1–120
Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Zamora D, Goodkind M, Bell K, Stern Y, Devanand DP (2006) Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry 63:916–924
Tobiansky R, Blizard R, Livingston G, Mann A (1995) The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med 25:779–786
van der Flier WM, Pijnenburg YA, Schoonenboom SN, Dik MG, Blankenstein MA, Scheltens P (2008) Distribution of APOE genotypes in a memory clinic cohort. Dement Geriatr Cogn Disord 25:433–438
Vestberg S, Passant U, Risberg J, Elfgren C (2007) Personality characteristics and affective status related to cognitive test performance and gender in patients with memory complaints. J Int Neuropsychol Soc 13:911–919
von Gunten A, Ron MA (2004) Hippocampal volume and subjective memory impairment in depressed patients. Eur Psychiatry 19:438–440
Wilson BA, Cockburn J, Baddeley AD (1990) Rivermead behavioural memory test manual. Organizzazioni Speciali, Firenze
Acknowledgments
This work was partially funded by an educational grant no. 2006.3251 from the Fondazione Carisbo, Bologna, Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallassi, R., Oppi, F., Poda, R. et al. Are subjective cognitive complaints a risk factor for dementia?. Neurol Sci 31, 327–336 (2010). https://doi.org/10.1007/s10072-010-0224-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-010-0224-6