Abstract
This study investigated the possible involvement of matrix metalloproteinase 9 (MMP-9) in early brain injury (EBI) of subarachnoid hemorrhage (SAH) in rats. MMP-9 activities in hippocampus were examined at 6, 12, 24, 48 and 72 h after SAH. Laminin was detected by immunohistochemistry. Apoptosis of neurons in hippocampus was observed by TUNEL. Brain water content was also examined. MMP-9 activity and the number of apoptotic neurons increased from 12 to 72 h with a peak at 24 h. Laminin was found to decrease at 12 h, reached minimum at 24 h and began to increase from 48 h, which had a negative correlation with apoptotic neurons. The changes of brain water content were found to be coincidence with that of neuronal apoptosis. Our findings suggest that MMP-9 is probably involved in the pathophysiological events of EBI after SAH, through degrading laminin which leads to neuronal anoikis of hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) is an important cause of death and disability worldwide. In the past, researches have concentrated primarily on cerebral vasospasm after SAH. To date, there is still not a definitive treatment to absolutely prevent brain injury after SAH. Early brain injury (EBI) has been pointed to be the primary cause of mortality in SAH patients [1]. Apoptosis occurred in neuronal tissues, particularly in the hippocampus after SAH, is involved in the pathological process of EBI. Neuronal apoptosis contributes to cytotoxic edema, which usually develops early after brain injury [2, 3].
Matrix metalloproteinase 9(MMP-9), which belongs to a large family of endopeptidases that are able to cleave extracellular matrix proteins especially laminin, has been suggested mediating apoptosis which called anoikis after neurological injury [4]. In the present study, we examined the time course of MMP-9 expression, its substrate laminin and the occurrence of apoptosis in hippocampus after the initial bleeding. In addition, the brain water content was also observed.
Materials and methods
This protocol was evaluated and approved by the Animal Care and Use Committee at Chongqing Medical University in Chongqing, China.
Experimental groups
One hundred and eight Sprague-Dawley male rats weighing between 250 and 300 g were randomly assigned to two groups: sham group and experimental SAH group. According to the time points of sacrificed animals, the experimental SAH group was divided into five subgroups, including 6, 12, 24, 48 and 72 h groups after SAH. Sham group and each subgroup had 18 rats in which 6 were used for examining the activity of MMP-9, 6 were used for observing the expression of laminin and the apoptosis in hippocampus and 6 were used for examining brain water content.
SAH rat model
Subarachnoid hemorrhage induction was performed in experimental SAH group as reported previously with slight modifications [5]. Briefly, rats were anesthetized with chloral hydrate (40 mg/kg IP). Animals were intubated, and respiration was maintained with a small animal respirator (Harvard Apparatus). Rectal temperature was maintained at 37°C with a heating pad. At either side of the skull, 3 mm from the midline and 5 mm anteriorly from the bregma, hole was drilled through the skull bone down to dura mater without perforation. Finally, a PE 10 canula was introduced about 10 mm from the bone hole, and 250 μl blood was withdrawn from the femoral artery and injected intracranially through the canula at a pressure equal to the mean arterial blood pressure (80–100 mmHg). Subsequently, canula was removed and incisions closed.
In sham group rats were treated by the same protocol as described above except that no blood was injected into the subarachnoid space.
Antibodies and reagents
Gelatin was purchased from Sigma. Rabbit polyclonal antibody against laminin was purchased from Lab Vision Corporation. TUNEL apoptosis assay kit was purchased from Roche Diagnostics.
Brain water content
Rat brains were removed at 6, 12, 24, 48 and 72 h (n = 6 per time point) after SAH. Immediately, the entire brain was weighed after removal (wet weight) and again after drying in an oven at 105°C for 24 h (dry weight) as described by Xi [6]. Sham-operated control rats (n = 6) were killed at 72 h. The percentage of water content was calculated as [(wet weight − dry weight)/wet weight]/100%.
Preparation of tissue extracts
At 6, 12, 24 48 and 72 h after SAH, rats (n = 6 per time point) were deeply anesthetized with halothane and then the brains were removed quickly and hippocampuses were dissected and frozen immediately in liquid nitrogen, and stored at −80°C. Sham-operated rats (n = 6) were killed at 72 h. Brain tissue extracts were prepared as previously described [7]. Briefly, brain samples were homogenized in lysis buffer on ice. After centrifugation, supernatant was collected, and total protein concentrations were determined using the Coomassie Brilliant Blue method.
Gelatin zymography
Prepared protein samples were loaded and separated by 10% Tris-glycine gel with 0.1% gelatin as substrate. After separation by electrophoresis, the gel was renatured and then incubated with developing buffer at 37°C for 24 h. After developing, the gel was stained with 0.5% Coomassie Blue R-250 for 30 min and then destained appropriately.
Immunohistochemistry
To assess the changes of laminin in hippocampus after SAH, rats were intracardially perfused with ice-cold PBS, pH 7.4, followed with ice-cold 4% paraformaldehyde in PBS, pH 7.4, at 6, 12, 24, 48 and 72 h after the induction of SAH (n = 6, per time point). Sham-operated rats (n = 6) were killed at 72 h. The brains were removed, immersed with 4% paraformaldehyde in PBS overnight at 4°C. Coronal sections (5 μm thick) were prepared using a microtome. After quenching endogenous peroxidase in 0.3% H2O2 in PBS and blocking with 5% normal goat serum, sections were incubated overnight at 4°C with the laminin rabbit polyclonal antibody (1:100). The sections were washed with PBS, incubated with antirabbit IgG secondary antibody at 1:5,000 dilution for 1 h and followed by 1 h of incubation with an avidin–horseradish peroxidase complex. Peroxidase was visualized by incubation with diaminobenzidine (DAB) substrate. Negative control sections received identical treatment except for the primary antibody.
TUNEL
To assess the neuronal apoptosis in hippocampus after SAH, brains were harvested and thereafter sectioned at 5 μm according to the above method. Sections at the level of the hippocampus were stained according to the manual of TUNEL kit. Briefly, brain sections were deparaffinaged, permeabilized, treated with 0.3% H2O2, and incubated with 150 U/ml terminal transferase and 2 ml biotin-16-dUTP for 1 h at 37°C. DNA degradation was visualized using DAB.
Statistics
Data were expressed as mean ± SD. Statistical differences between individual groups were analyzed using one-way ANOVA and the correlation between expression of laminin and apoptotic neurons was examined using correlation analysis. P value of <0.05 was considered statistically significant.
Result
Brain water content
No difference was detected in rats at 6 h compared with sham (P > 0.05 versus sham). Significant increases (P < 0.01, ANOVA; Fig. 1) in brain water content were detected in rat brains at 12 h, reached maximum at 24 h and began to decrease from 48 to 72 h, but the brain water content at 72 h after SAH was still higher than that of sham-operated group(P < 0.01).
Gelatin activity of MMP-9
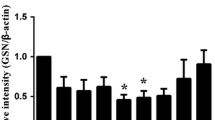
The time course of MMP-9 protein activity after SAH was assessed in rat hippocampus (Fig. 2a). The proteins extracted from the tissues were evaluated by Coomassie Blue method before further analysis. MMP-9 protein activity was evaluated by zymography. The results demonstrated that the activity of MMP-9 was detected in the hippocampus at 12 h after SAH, reached maximum at 24 h, stayed at a higher level at 48 h and was still higher at 72 h than that of sham-operated group (P < 0.01, ANOVA; Fig. 2b). According the gelatin zymogram, the gelatinase activity of MMP-9 was not detected in the sham-operated animals and 6 h group after SAH (P > 0.05).
a The activity of MMP-9 at each group in hippocampus of rats by zymography. 1 Sham group, 2 6 h after SAH group, 3 12 h after SAH group, 4 24 h after SAH group, 5 48 h after SAH group, 6 72 h after SAH group. b Quantitative analysis for active 97 kDa MMP-9 in relative optical density (mean + SD; n = 6). According the gelatin zymogram, the gelatinase activity of MMP-9 was clearly observed in the hippocampus. The activity of MMP-9 began to increased in the hippocampus at 12 h after SAH (**P < 0.01 versus sham), reached maximum at 24 h after SAH, maintained at a level at 48 h and was still higher than that of sham-operated group (**P < 0.01) at hour 72
Immunohistochemistry
Immunohistochemistry analysis of laminin expression revealed the changes of laminin staining in hippocampus of rats after SAH (Fig. 3a). Quantitative analysis of laminin staining was performed in each group. The mean optical density decreased from 12 to 72 h groups (P < 0.01 versus sham, ANOVA; Fig. 3b) and reached minimum at 24 h group.
a The expressions of laminin in hippocampuses of rats were observed in each group by immunohistochemistry. A Sham group, B 6 h after SAH group, C 12 h after SAH group, D 24 h after SAH group, E 48 h after SAH group, F 72 h after SAH group. b Quantitative analysis of mean optical density of laminin in hippocampus of rats (mean + SD; n = 6). The mean optical density decreased from 12 to 72 h (**P < 0.01 versus sham)
TUNEL staining
Apoptotic neurons with condensed brown DAB precipitates were observed in hippocampus of rats. TUNEL-positive neurons with apoptotic bodies were found after 12 h after SAH in the neurons of hippocampus (Fig. 4a). The number of apoptotic neurons per ten fields in experimental SAH groups was compared with that of sham-operated rats. In hippocampus, the number of apoptotic neurons was significantly elevated from 12 to 72 h with a peak at 24 h after SAH (P < 0.01 versus sham, ANOVA; Fig. 4b).
a The apoptosis of neurons in hippocampuses of rats were examined in each group by TUNEL staining (mean + SD; n = 6). A Sham group, B 6 h after SAH group, C 12 h after SAH group, D 24 h after SAH group, E 48 h after SAH group, F 72 h after SAH group. b Quantitative analysis of apoptotic neurons in hippocampus of rats (mean + SD; n = 6). According TUNEL staining, the number of apoptotic neurons in hippocampus was significantly elevated from 12 to 72 h with a peak at 24 h after SAH (**P < 0.01 versus sham)
The correlation between expression of laminin and apoptotic neurons of hippocampus
There was a negative correlation between the expression of laminin and apoptotic neurons in hippocampus from 12 to 72 h after SAH (r = −0.892, P < 0.01). It revealed that laminin degradation was associated with the apoptotic neurons in hippocampus during early phase after SAH. Increased MMP-9 gelatinolytic activity is related to apoptotic neurons by degrading laminin.
Discussion
Subarachnoid hemorrhage is a devastating and complicated disease which has a high rate of morbidity and mortality. About 10/100,000 people suffer from an aneurysmal SAH. Although there are some advances in treatment for SAH [8, 9], the rates of morbidity and mortality have not changed in recent years [10]. Many researches have been designed to reveal the pathophysiological mechanisms and improve outcome of SAH. But to date, there is not a definitive treatment modality to prevent or ameliorate brain injury after SAH. More and more researchers have pointed out that EBI is the primary cause of mortality in SAH patients [1, 11], suggesting that EBI should be considered as a primary target for future research. The term EBI has recently been coined and refers to the immediate injury to the brain as a whole, within the first 72 h of the ictus, secondary to a SAH [12]. Therefore, EBI refers to the events that occur in the brain tissue before cerebral vasospasm, although the etiology of vasospasm may be related to that of EBI, because they certainly share many of the same characteristics.
Apoptosis has been extensively studied in diseases of central nervous system and has been shown to be the important form of cell death [13–15]. And during early phase after SAH, apoptosis has been shown to be widespread in the brain, especially in hippocampus, as a result of the global ischemic injury, secondary to raised ICP and decreased CBF [1, 12]. There are a number of apoptotic pathways that are believed to play a role in SAH: the death receptor pathway, caspase-dependent and -independent pathways, as well as the mitochondrial pathway [16, 17]. Recent study has found that anoikis may play an important role in apoptosis of hippocampal neurons in transient cerebral ischemia [4].
Previously, it had been demonstrated that extracellular matrix proteins such as laminin are important for cell survival and prevention of anoikis, in which cells detach from their matrix [18]. MMP-9 belongs to a large family of endopeptidases that are able to cleave extracellular matrix proteins, especially laminin [19]. It has been reported that neuronal nitric oxide synthase could increase the activity of MMP-9 by S-nitrosylation which could lead to laminin cleavage [4]. Recently, the presence or activity of MMP-9 has been implicated as a negative prognostic factor in human cerebral vascular disease [20–22].
In the present study, we evaluated the time course of gelatin activity of MMP-9 and the association between MMP-9 and apoptosis in rat hippocampus after SAH. It has been demonstrated that after SAH, the activity of MMP-9 and its substrate, laminin, are significantly altered in hippocampus of SAH rats at different time points. According to the immunohistochemistry, expression of laminin was not significantly different with sham-operated rats at 6 h after SAH, but began to decrease at 12 h and decreased to the lowest level at 24 h. At 48 h after SAH, expression of laminin began to increase, but was still lower than that of sham group at 72 h. The decreased expression of laminin accompanied the increased activity of MMP-9. According to the TUNEL staining, TUNEL positive neurons were detected in hippocampus at 12 h after SAH. The number of TUNEL-positive neurons reached a peak at 24 h after SAH in hippocampus. The association between the expression of laminin and apoptotic neurons in hippocampus from 12 to 72 h after SAH showed a negative correlation. The degradation of laminin led to the detachment of hippocampal neurons from extracellular matrix, which called anoikis. Therefore the expression and activity of MMP-9 may be linked to the anoikis in hippocampus after SAH. Neuronal cell death contributes to cytotoxic edema, which usually develops early after brain injury [11]. In the study, we found that the time course of brain edema was coincidence with that of anoikis of neurons in hippocampus after SAH. Thus, MMP-9 seems to have some relationship with apoptosis in hippocampus after SAH.
In summary, we examined the time courses of gelatin activity of MMP-9, laminin and anoikis of neurons in hippocampus after SAH to show that MMP-9 contributes to neuronal anoikis in hippocampus during EBI after SAH. This is further supported by the finding that one of the pathological phenomena of EBI, such as brain edema, is associated with the neuronal anoikis. Meanwhile, we found that the activity of MMP-9 still kept at a higher level at 72 h after SAH than that of sham rats suggesting MMP-9 may also play roles in the pathophysiological process of delayed cerebral vasospasm. Therefore, we conclude that targeting MMP-9 during EBI in SAH patients is a highly promising therapeutic approach that deserves further exploration.
References
Bederson JB, Germano IM, Guarino L (1995) Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 26:1086–1091
Bazan NG, Rodriguez de Turco EB (1980) Membrane lipids in the pathogenesis of brain edema: phospholipids and arachidonic acid, the earliest membrane components changed at the onset of ischemia. Adv Neurol 28:197–205
Park S, Yamaguchi M, Zhou C et al (2004) Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke 35:2412–2417
Gu Z, Cui J, Brown S et al (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25:6401–6408
Prunell GF, Mathiesen T, Diemer NH et al (2003) Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery 52:165–175
Xi G, Hua Y, Keep RF et al (2002) Brain edema after intracerebral hemorrhage: the effects of systemic complement depletion. Acta Neurochir Suppl 81:253–256
Macdonald RL, Curry DJ, Aihara Y et al (2004) Magnesium and experimental vasospasm. J Neurosurg 100:106–110
Zhou C, Yamaguchi M, Colohan AR et al (2005) Role of p53 and apoptosis in cerebral vasospasm after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 25:572–582
Muroi C, Frei K, El Beltagy M et al (2008) Combined therapeutic hypothermia and barbiturate coma reduces interleukin-6 in the cerebrospinal fluid after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 20:193–198
Schievink WI, Riedinger M, Jhutty TK et al (2004) Racial disparities in subarachnoid hemorrhage mortality: Los Angeles County, California, 1985–1998. Neuroepidemiology 23:299–305
Cahill WJ, Calvert JH, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26:1341–1353
Kusaka G, Ishikawa M, Nanda A et al (2004) Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 24:916–925
Sugawara T, Fujimura M, Noshita N et al (2004) Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx 1:17–25
Ueda H, Fujita R (2004) Cell death mode switch from necrosis to apoptosis in brain. Biol Pharm Bull 27:950–955
Gibson RM (2001) Does apoptosis have a role in neurodegeneration? BMJ 322:1539–1540
Cahill J, Calvert JW, Marcantonio S et al (2007) p53 may play an orchestrating role in apoptotic cell death after experimental subarachnoid hemorrhage. Neurosurgery 60:531–545
Iseda K, Ono S, Onoda K et al (2007) Antivasospastic and antiinflammatory effects of caspase inhibitor in experimental subarachnoid hemorrhage. J Neurosurg 107:128–135
Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124:619–626
Cunningham LA, Wetzel M, Rosenberg GA (2005) Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 50:329–339
Copin JC, Goodyear MC, Gidday JM et al (2005) Role of matrix metalloproteinases in apoptosis after transient focal cerebral ischemia in rats and mice. Eur J Neurosci 22:1597–1608
Machado LS, Kozak A, Ergul A et al (2006) Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci 7:56
Svedin P, Hagberg H, Savman K et al (2007) Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J Neurosci 27:1511–1518
Acknowledgments
This research was supported in part by the Foundation for Excellent Doctoral Dissertation of Chongqing Medical University (0200101174). The authors are appreciative of John H. Zhang from Loma Linda University Medical Centre for his helpful discussions in conducting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Z., Sun, X., He, Z. et al. Role of matrix metalloproteinase-9 in apoptosis of hippocampal neurons in rats during early brain injury after subarachnoid hemorrhage. Neurol Sci 31, 143–149 (2010). https://doi.org/10.1007/s10072-009-0192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-009-0192-x