Abstract
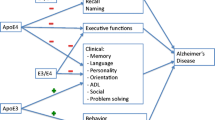
The study aimed to characterize neuropsychiatric symptomatology in Alzheimer’s disease (AD) and investigate the role of APOE genotype and other clinical variables in the onset of neuropsychiatric disorders. Moreover, an attempt to study the evolution of behavioral and psychiatric symptoms was made. Fifty-three consecutive outpatients with AD were enrolled. Twenty-four were followed longitudinally for 1 year. MMSE was used to evaluate cognitive functions. The neuropsychiatric inventory (NPI) was administered to assess behavioral and psychiatric symptoms. Genotyping was determined through laboratory testing. At baseline, no specific neuropsychiatric disorder was significantly associated with ApoE genotype, but associated with a peculiar neuropsychiatric profile. Patients with ε 4 allele showed a wider range of neuropsychiatric disturbances when compared to non-carriers and higher scores for hallucinations and aberrant motor behaviors. The longitudinal results suggest different trends in both groups: over time, ε 4 carriers showed an increase/delayed onset in some symptoms and a parallel decrease in others, while non-carriers presented an undifferentiated worsening of symptomatology. Clear relations with other clinical and demographic variables were also found. APOE ε 4 allele is associated to a peculiar neuropsychiatric profile characterizing the onset and evolution of Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to neuropsychological deficits, Alzheimer’s disease (AD) is associated with many behavioral and neuropsychiatric symptoms which are as devastating as the cognitive deficits and often lead to relevant coping problems in patient’s management.
Psychotic symptoms, such as delusions and hallucinations, have an incidence of 75% [1]. Increased apathy and depression are very common (70 and 50%, respectively) [2, 3]. Other behavioral disturbances, such as agitation, aggressiveness and wandering are also reported and appear in about 60% of patients, especially at the advanced stage of disease [2]. These changes are one of the main sources of caregiver distress and often lead to institutionalization of the patient.
As well as the behavioral and psychological symptoms of dementia, weight loss is another very common problem [4]. It increases the risk of infection and skin ulcers and decreases the quality of life in Alzheimer patients. The severity of weight loss follows disease progression and is considered a predictor of patient mortality. Significant correlations were found between the under-nourished status and MMSE, behavioral disturbances and caregiver distress [5].
Recently, many studies have investigated the role of potentially relevant factors in Alzheimer’s disease. In addition to age, which represents the most important risk factor [6], more recently a genetic component has also been evaluated. Subjects who have a relative with AD have a three to seven times greater risk in developing the disease than those with no familiarity. Therefore, the role of Apolipoprotein E (ApoE) ε 4 allele in AD etiology was investigated. It is the main cerebral lipoprotein and has three common alleles, apoE ε 2, apoE ε 3 and apoE ε 4, with allele frequencies in the general population of approximately 8, 78 and 14%, respectively. The ApoE ε 4 allele is over-represented in AD and is considered a genetic risk factor. This allele modifies risk and age at onset of AD [7, 8].
Some studies have also investigated the influence of the APOE genotype in the appearance of psychiatric symptoms. The outcomes are not univocal. In a recent study, 87 patients with AD were examined to determine whether APOE genotype predicts the incidence of psychiatric symptoms [9]. The authors found a relation between the presence of the ε 4 allele and the development of delusions: in particular, the presence of one or two ε 4 allele led to a 2.5 and 5.6 greater risk, respectively, in developing delusions. The APOE genotype did not influence the incidence of other symptoms such as illusions, behavioral disturbances or depression. However, other studies failed to find any relation between the APOE genotype and neuropsychiatric symptoms [7, 10, 11].
In the present study, we evaluated the relations between the APOE genotype and behavioral and psychiatric disorders. The influence of other demographic and clinically relevant variables was also considered. Moreover, a subgroup of patients was followed longitudinally for 1 year, with follow-up for every 6 months. The study aimed to characterize neuropsychiatric symptoms in AD using NPI, disease evolution and the role of potentially relevant variables.
Materials and methods
Subjects
This is multi-center study that included all patients consecutively admitted to two Alzheimer’s disease units in Southern Italy between 2001 and 2003. Only patients with a diagnosis of probable AD according to NINCDS-ADRDA [12] were included. Exclusion criteria were a history of head injury, substance abuse or dependence and a history of psychiatric disturbances prior to the onset of dementia.
A total of 53 outpatients were enrolled in the study. For the longitudinal study, 24 patients were followed-up for 1 year, with follow-up for every 6 months. All patients underwent mental status examination, neurological and neuroradiological examinations and laboratory testing for the determination of the APOE genotype.
Patients consuming psychotropic drugs were not excluded, but all the prescribed medications were noted and analyzed for between group differences. In particular, at the enrolling, 38 out of 53 patients (71%) were consuming psychotropic drugs, most of which were anticholinesterases drugs (55%), but also antipsychotics (5%) and antidepressants (3%) and anxiolytics (3%) have been recorded, and a combination of the above drugs as well (34%).
The control group was formed of 61 healthy subjects, recruited out of hospital, and matched with AD patients for sex and age. Exclusion criteria included prior neurological or psychiatric disorders, recent reduction of cognitive efficiency and the use of antidepressant or tranquilizing therapy. All cases were submitted to a careful psychiatric history and neuropsychiatric evaluation by a neurologist.
For all participants, written informed consent was obtained.
Cognitive and psychiatric evaluation
Cognitive function was examined using the mini-mental state examination (MMSE) [13, 14] and neuropsychiatric symptoms by the neuropsychiatric inventory (NPI) [15]. It assesses 12 behavioral domains: delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, aberrant motor activity, night-time behavioral disturbances and appetite/eating disorders.
The caregiver must rate the severity of the neuropsychiatric disturbances on a scale from 1 to 3, and the frequency from 1 to 4.
For each behavioral domain, the score is the product of the frequency and severity. Total score is the sum of the subscale scores.
The NPI has a good content and concurrent validity, as well as adequate test–retest, between rater reliability and internal consistency [15]. The NPI also shows changes at the different phases of illness in patients [16].
The Italian version of the NPI was validated by Binetti et al. [17] in AD patients and has demonstrated comparable psychometric properties.
Nutritive condition evaluation
Mini-nutritional assessment (MNA) was administered to assess the nutritive condition at the initial visit [18].
The MNA is an inventory used to identify geriatric (>65 years) patients at risk of malnutrition. It consists of 18 items about anthropometric measurements (BMI, mid-arm and calf circumference and weight loss), dietary intake (number of meals consumed, food and fluid intake and feeding autonomy), a global assessment (lifestyle, medication, mobility, presence of acute stress and presence of dementia or depression) and a self-assessment (self-perception of health and nutrition). An MNA score of <17 indicates a status of malnutrition, <23.5 indicates a risk of malnutrition and a score of >23.5 denotes a good nutritional status.
The MNA is well validated and correlates highly with clinical assessment and objective indicators of nutritional status.
Data analysis
Differences between demographic and clinical data of patients with and without ε 4 allele were analyzed with the Student’s t test and Chi-square analyses as appropriate.
Due to the ordinal nature of the scale, the neuropsychiatric data were analyzed using non-parametric tests. The analysis consisted of a preliminary description of NPI score distribution in the pathological sample and estimation of symptom incidence.
The bootstrap analysis [19] was adopted to compare NPI mean between groups. The bootstrap is a non-parametric re-sampling technique that allows a probability function based on the data-set actually obtained in the population. It combines the scores of the samples studied into a single pool of data, extracts a number of groups equal to the original number and calculates the mean differences from the randomly constituted groups. The re-sampling and mean difference computation are repeated on the data-set 3,000 times to produce a distribution of the possible mean difference for each NPI sub-scale. For each possible value obtained, the probability is then computed by the frequency distribution. If the observed difference is greater than 95% of the expected difference from random re-sampling, it is judged to be significant at 0.05 level.
A logistic regression analysis (forward stepwise) was used to evaluate the role of demographic and clinical variables predicting the onset of neuropsychiatric symptoms. These variables, considered as independent variables, were coded as 1 or 0 according to the presence or absence of the feature. The demographic variables were age (coded as 0, <75 and 1, ≥75) and sex (coded as 0 male and 1 female); the clinical variables were the APOE genotype (coded as 1 presence of ε 4 and 0 absence of allele), onset of disease (coded in months), MMSE score (coded as 1, <24 and 0, ≥24) and MNA score (coded as 1, <25 and 0, ≥25).
Results
Table 1 shows the basic demographic, clinical and neurological features of the entire sample studied. Twenty-five patients were APOE ε 3–ε 4 genotype (3 have ε 4–ε 4) and 28 were APOE ε 3–ε 3. The two groups were comparable for all variables considered with the exception of gender (21 of 25 patients were female in the APOE ε 3–ε 4 subgroup vs. 15 of 28 in the other subgroup). Groups were also comparable for number of patients under pharmacological treatments (19 out of 25 patients in the APOE ε 3–ε 4 subgroup vs. 19 out of 28 in the other subgroup, X 2 = 0.43, n.s.).
Table 2 reports the distribution of composite NPI scores and the incidence of neuropsychiatric disturbances in the entire sample of AD patients. The most frequent neuropsychiatric symptom was depression, shown in 79% of all patients, followed by apathy (77%), anxiety (75%), irritability (68%), agitation (62%), aberrant motor behavior (40%), night-time disorders (28%), appetite/eating disorders (21%) and disinhibition, hallucination, delusion and euphoria were less than 20%.
Table 3 shows the mean composite NPI scores for the two groups of patients. Both groups differed from controls in various neuropsychiatric dimensions; however, patients with ε 4 allele showed a wider range of neuropsychiatric symptoms. Non-carriers differed from the controls for depression, anxiety, apathy, aberrant motor behavior (P < 0.001 in all comparisons), irritability and night-time disturbances (P < 0.05). Patients with ε 4 allele, in addition to these symptoms, showed significant delusions, hallucinations, agitation (P < 0.001 in all comparisons) and euphoria (P < 0.05). Moreover, they exhibited higher scores for hallucinations (P < 0.001) and aberrant motor behavior (P < 0.01) when compared to non-carriers.
Relations with other clinical or demographic features
Multiple logistic regression showed that depression and anxiety were associated with gender. Female patients had a higher risk of developing depressive symptoms (OR = 8.667, 85% CI = 1.85–40.6, accuracy in prediction 75.6%, significance of model X 2 = 8.58, P = 0.003) and anxiety (OR = 17.357, 95% CI = 3.04–99.2, accuracy in prediction 80%, significance of model X 2 = 13.57, P = 0.000) than males.
With regard to irritability, the age of patients was important. Elderly subjects (under 75 years) had a five times greater risk of becoming irritable when compared to younger patients (OR = 4.872, 95% CI = 1.12–21.20, accuracy in prediction 71.1%, significance of model X 2 = 5.09, P = 0.02).
Hallucinations and aberrant motor activity were significantly linked to the disease onset. Patients assessed in a more advanced phase had a higher risk of developing hallucinations (OR = 1.047, 95% confidence interval (CI): 1.00–1.1, accuracy in prediction 86.7%, significance of model X 2 = 4.26, P = 0.03) and aberrant motor behavior (OR = 1.07, 95% CI = 1.02–1.12, accuracy in prediction 64.2, significance of model X 2 = 9.93, P = 0.002) than others.
The onset of AD was an important variable in predicting the occurrence of appetite/eating disorders (OR = 1.065, 95% CI = 1.02–1.11, accuracy in prediction 80%, significance of model X 2 = 9.59, P = 0.002).
Apathy was linked with the MNA score. Patients with lower MNA values had a higher risk of developing apathy than the well-nourished patients (OR = 4.472, 95% CI = 0.98–20.49, accuracy in prediction 77.8%, significance of model X 2 = 4.07, P = 0.04).
No specific neuropsychiatric disorder was significantly associated with APOE genotype.
Results from the second multiple logistic regression analysis, evaluating the role of pharmacological variables, showed that no pharmacological therapy was significantly linked with any of the NPI symptoms.
Follow-up
Of the 53 patients, a subgroup of 24 was followed for 1 year with an assessment for every 3 months. Fourteen patients had a ε 3–ε 4 genotype and 10 had a ε 3–ε 3 genotype (Table 4).
The neuropsychiatric inventory and the mini-mental state examination were administered at all follow-ups.
After 6 months, all patients were consuming just anticholinesterases, 5 patients were also taking antidepressants (2 vs. 3 in the ε 3–ε 4 and ε 3–ε 3 subgroups, respectively), and five antipsychotic drugs (1 vs. 4 in the ε 3–ε 4 and ε 3–ε 3 subgroups, respectively).
After 1 year, all patients kept consuming anticholinesterases, 4 patients were also taking antidepressants (2 in each group) and 7 antipsychotics. (4 vs. 3 in the ε 3–ε 4 and ε 3–ε 3 subgroups, respectively). No significant differences were seen between groups.
Results
Tables 5 and 6 show the mean NPI composite scores in the two groups of patients at baseline and at 6 and 12 months follow-up.
Patients differed from controls in various neuropsychiatric dimensions, at baseline and at the two follows-up. However, a different trend in the progression of neuropsychiatric disturbances in ε 4 carriers was also observed when compared to non-carriers.
Non-carriers showed a general worsening of symptomatology at 6 and/or 12 months from baseline. Four out of five symptoms already manifested at baseline, such as apathy, irritability, aberrant motor behavior (P < 0.001), and night-time disturbances (P < 0.01) increased with respect to baseline, anxiety and depression remained constantly present (P < 0.001 vs. controls), while hallucinations and delusions (P < 0.001 vs. controls and vs. baseline) had a delayed onset (hallucination since the 6 months of follow-up, delusion at the 1-year evaluation).
Patients with ε 4 allele, progressing in the disease, exhibited an increase of apathy (P < 0.05 and P < 0.001 vs. baseline, respectively at 6 and 12 months), irritability (P < 0.001 vs. baseline), and delusion (P < 0.01 vs. baseline), delayed onset of night-time disturbances (P < 0.001 vs. controls), with anxiety and depression constantly present (P < 0.001 vs. controls), while hallucination, aberrant motor behavior (P < 0.001 vs. baseline), agitation and euphoria (P < 0.001 and P < 0.05, respectively, vs. baseline) decreased.
Discussion
Present data suggest that the ApoE ε 4 allele is associated to the onset of a wider and more severe neuropsychiatric profile in AD patients. At the baseline, ε 4 carriers manifested nine out of the 12 investigated neuropsychiatric symptoms (75%), while non-carriers mainly suffered from depression, anxiety, apathy, irritability, aberrant motor behavior and night-time disturbances. Moreover, scores of higher intensity were recorded for hallucinations and aberrant motor behavior in carriers when compared to non-carriers.
Previous studies have examined the relationship of APOE genotype and neuropsychiatric disturbances in AD. Some authors have found an association between the ε 4 allele and apathy [20] or delusions [21, 22], others failed to find any relationship between this genotype and psychiatric symptoms. Lopez et al. [7] reported no association between APOE genotype and major depression, delusions or hallucinations. The variety of the results could be due to the different measurement used for assessing psychiatric and behavioral symptoms. In many cases, structured interviews were used (e.g. SCID) or a monosymptomatic scale such as the Hamilton Depression Rating Scale or Cornell Depression Scale evaluated specific disturbances. Variability in study design is another relevant factor to be considered. Very often studies are not comparable for sample size, selection criteria (e.g. patients with two ε 4 alleles vs. one ε 4 allele) or stage of dementia examined.
Our results suggest that it is necessary to use a neuropsychiatric measure such as NPI to have a wide behavioral and psychiatric assessment of AD patients.
Moreover, the results showed that other clinical variables influenced the onset of neuropsychiatric symptoms.
Time between the onset of cognitive symptoms of AD and the first neuropsychiatric evaluation was an important predictor for the occurrence of hallucinations, aberrant motor behavior and appetite\eating disorders. A similar pattern was reported in other studies evaluating dementia patients. Piccininni et al. [23], for example, found an effect of the duration of disease on the probability of developing hallucinations and aberrant motor behavior.
The nutritional state resulted as an important risk factor for apathy (although we cannot exclude an inverse relationship, i.e. apathy may lead to undernourishment). However, also in other studies, undernourishment was found to be more frequently associated to low MMSE, high behavioral disturbances and high distress of caregivers [5].
Finally, age was found to be relevant for irritability and gender for the development of depression and anxiety. The presence of gender differences is in agreement with other studies where women showed a higher probability of suffering from depressive and anxiety symptoms both in normal older population studies [24] as well as in neurological patients [25, 26].
As far as the possible role of pharmacological treatment is concerned, the regression analysis did not point out any relationship between drugs and the various NPI symptoms.
Overall, data are likely to support the hypothesis that the behavioral symptoms are related to patients’ APOE genotype and the other clinical variable investigated.
So far the role of apolipoprotein E in neuropsychiatric symptom development is still a matter of debate. Some studies reported that the APOE genotype is not related to the development of non-cognitive symptomatology in the disease [29].
However, the presence of APOE ε 4 allele has been associated to an increase in β-amyloid senile plaques and neuritic plaques [27] and to more profound deficits in cholinergic neurons [28]. The development of neuropsychiatric symptoms in AD may be related to specific neurotransmitter imbalances, notably acetylcholine. Also, Borroni et al. [29] found that other genes are involved in behavioral disturbances in AD. For example, the catechol-O-methyltransferase (COMT) was related to psychotic symptoms.
A second relevant contribution of the present study is its focus on the evolution of neuropsychiatric disturbances. Results suggest a peculiar trend in ε 4 carriers when compared to non-carriers; over time, the symptomatology became generally more severe in ε 4 non-carriers, while ε 4 carriers displayed an increase of some symptoms and a parallel decrease of others (although two of them remained clinically relevant when compared to controls).
Overall, the data suggest that, as the disease advanced, non-carriers presented an undifferentiated worsening of the neuropsychiatric profile with symptoms becoming clinically relevant or more intense, while ε 4 carriers seemed to show a change of polarity. In these patients, in fact, it seems that symptoms linked to a dimension of withdrawal [1], such as apathy and scarce compliance, become prevalent, while all productive symptoms, with the exception of delusion, decreased over time.
In literature, there are few longitudinal studies dealing with the association of neuropsychiatric symptoms and APOE genotype. Chang et al. [22] found that psychiatric symptoms were more frequent in AD patients with ApoE ε 4 allele, and that those with an ApoE ε 4 allele (14 patients with one ε 4 allele and 4 with two) had a greater tendency to develop delusions and hallucinations over time. However, another study found that the presence of two ε 4 alleles was associated with a reduced risk for developing hallucinations [9]. In our study, AD patients with one ε 4 allele, assessed 1 year after diagnosis, showed a significant decrease in hallucinations and a moderate, but significant, increase in delusions. Therefore, our data partially overlap the results reported by Chang et al. [22] (most, though not all, of their patients had the APOE genotype that is comparable to our sample). Nevertheless, caution is also needed due to the small size of our longitudinal samples.
In conclusion, neuropsychiatric disturbances constitute an important part of co-morbidity in patients suffering from Alzheimer’s disease. Further research is needed to better characterize the various symptoms and their evolution in patients with and without APOE ε 4 genotype and the role of one versus two ε 4 alleles.
References
Drevets WC, Rubin EH (1989) Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry 25:39–48
Padovani A, Rozzini L, Vicini Chilovi B et al (2002) I disturbi comportamentali nella malattia di Alzheimer: aspetti clinici ed epidemiologici. Neurol Sci 23:S523–S525
Craig D, Hart DJ, McLlory SP, Passmore P (2004) Apolipoprotein E ε 4 allele influences aggressive behaviour in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 75:1327–1330
Rivière S, Gillette-Guyonnet S, Nourhashemi F, Vellas B (1999) Nutrition and Alzheimer’s disease. Nutr Rev 57(12):363–367
Broker P, Benhamidat T, Benoit M et al (2003) Nutritional status and Alzheimer’s disease: preliminary results of the REAL.FR study. Rev Med Intern 24(Suppl 3):314s–318s
Von Strauss E, Viitanen M, De Ronchi D et al (1999) Aging and occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol 56:587–592
Lopez OL, Kamboh MI, Becker JT et al (1997) The apolipoprotein E ε 4 allele is not associated with psychiatric symptoms or extrapyramidal signs in probable Alzheimer’s disease. Neurology 49:794–797
Parasuraman R, Greenwood P, Sunderland T (2002) The Apolipoprotein E gene, attention and brain function. Neuropsychology 16(2):254–274
Scarmeas N, Brandt J, Albert M et al (2002) Association between the APOE genotype and psychopathologic symptoms in Alzheimer’s disease. Neurology 58:1182–1188
Holmes C, Levy R, McLoughlin DM et al (1996) Apolipotrotein E: non-cognitive symptoms and cognitive decline in late onset Alzheimer’s disease. J Neurol Neurosurg Psychiatry 61:580–583
Levy ML, Cummings JL, Fairbanks LA et al (1999) Apolipoprotein E genotype and noncognitive symptoms in Alzheimer’s disease. Biol Psychiatry 45:422–425
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRA Working Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Magni E, Binetti G, Padovani A et al (1996) The mini-mental state examination in Alzheimer’s disease and multi-infarct dementia. Int Psychogeriatr 8:127–134
Cummings JL, Mega M, Gray K et al (1994) The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314
Mega MS, Cummings JL, Fiorello T, Gornbein J (1996) The spectrum of behavioral changes in Alzheimer’s disease. Neurology 46:130–135
Binetti G, Mega MS, Magni E et al (1998) Behavioral disorders in Alzheimer’s disease: a transcultural perspective. Arch Neurol 55:539–544
Guigoz Y, Lauque S, Vellas BJ (2002) Identifying the elderly at risk for malnutrition: the mini nutritional assessment. Clin Geriatr Med 18(4):737–757
Angelelli P, Paolucci S, Bivona U et al (2004) Development of neuropsychiatric symptoms in poststroke patients: a cross-sectional study. Acta Psychiatr Scand 109:1–9
Monastero R, Mariani E, Camarda C et al (2006) Association between apolipoprotein E ε 4 allele and apathy in probable Alzheimer’s disease. Acta Psychiatr Scand 113:59–63
Spalletta G, Bernardini S, Bellincampi L et al (2006) Delusion symptoms are associated with APOE ε 4 allelic variant at the early stage of Alzheimer’s disease with late onset. Eur J Neurol 13:176–182
Chang JB, Wang PN, Chen WT et al (2004) Apoe ε 4 allele is associated with incidental hallucinations and delusions in patients with AD. Neurolgy 63:1105–1107
Piccininni M, DiCarlo A, Baldereschi M et al (2005) Behavioral and psychological symptoms in Alzheimer’s disease frequency and relationship with duration and severity of the disease. Dement Geriatr Cogn Disord 19:276–281
Murrel SA, Himmelfarb S, Wright K (1983) Prevalence of depression and its correlates in older adults. Am J Epidemiol 117:173–185
Andersen G, Vestergaard K, Ingemann-Nielsen M, Lauritzen L (1995) Risk factors for post-stroke depression. Acta Psychiatr Scand 92:193–198
Kotila M, Numminen H, Waltimo O, Kaste M (1998) Depression after stroke: results of the FINNSTROKE Study. Stroke 29:368–372
Sparks DL, Scheff SW, Liu H et al (1996) Increased density of senile plaques (SP), but not neurofibrillary tangles (NFT), in non-demented individuals with the apolipoprotein E4 allele: comparison to confirmed Alzheimer’s disease patients. J Neurol Sci 138:97–104
Allen SJ, MacGowan SH, Tyler et al (1997) Reduced cholinergic function in normal and Alzheimer’s disease brain is associated with apolipoprotein E4 genotype. Neurosci Lett 239:33–36
Borroni B, Grassi M, Agosti C et al (2006) Genetic correlates of behavioral endophenotypes in Alzheimer disease: role of COMT, 5-HTTLPR and APOE polymorphisms. Neurobiol Aging 27:1595–1603
Author information
Authors and Affiliations
Corresponding author
Additional information
Note added in proof
It should be noted that the irritability subscale includes items such as rapid mood changes, impatience and intolerance that may be signs of poor compliance and interaction with the environment.
The study was sponsored by the Italian Ministry of Health.
Rights and permissions
About this article
Cite this article
Del Prete, M., Spaccavento, S., Craca, A. et al. Neuropsychiatric symptoms and the APOE genotype in Alzheimer’s disease. Neurol Sci 30, 367–373 (2009). https://doi.org/10.1007/s10072-009-0116-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-009-0116-9