Abstract
For social animals, group size discrimination may play a major role in setting the trade-off between the costs and benefits of membership. Several anuran tadpoles show different degrees of social aggregation when exposed to the risk of predation. Despite the importance of aggregative behaviour as an anti-predatory response, the mechanism underlying tadpole choice of the group to join to has not been sufficiently investigated. To establish whether visual cues provide sufficient information to enable tadpoles to choose between aggregations differing in size, we explored the abilities of the larvae of two anuran species (green toad Bufotes balearicus and edible frog Pelophylax esculentus) to discriminate among four numerical combinations of conspecific tadpoles (1 vs. 4, 3 vs. 4, 4 vs. 6 and 4 vs. 8), either in the presence or absence of predatory cues. Our results suggest that in anuran larvae the capacity to discriminate between quantities is limited to small numbers (1 vs. 4 for B. balearicus and both 1 vs. 4 and 3 vs. 4 for P. esculentus). Predator-exposed toad tadpoles stayed longer close to the larger group, supporting aggregation as a major anti-predator behaviour in bufonids, while frog tadpoles showed a preference for the smaller groups, though in predator-free trials only, probably associated with lower intra-specific competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms use information collected in the environment to survive, find food and reproduce. A common challenge for animals is the visual representation of entities (e.g. predators, refuges, food items, conspecifics) that may differ in number, size or both. In the last two decades accumulating evidence has revealed that several taxa are able to discriminate between different quantities using an Approximate Number System (ANS, sometimes also dubbed Analogue Magnitude System) that would operate in an imprecise manner and would be ratio-dependent according to Weber’s law, which means that the accuracy in the discrimination between two stimuli would be a function of the ratio between their numerousness (Gallistel and Gelman 2000; Feigenson et al. 2004; Kruske et al. 2010; reviews in Vallortigara 2014, 2017, 2018). This has been reported for mammals (Vonk and Beran 2012; Vonk et al. 2014), birds (Pepperberg 2006; Bogale et al. 2014; Bertamini et al. 2018; Rugani et al. 2008, 2009, 2010, 2011, 2013a, b, 2014, 2016), amphibians (Uller et al. 2003; Stancher et al. 2015; Lucon-Xiccato et al. 2018), reptiles (Miletto Petrazzini et al. 2017, 2018; Gazzola et al. 2018a, b), fish (Buckingham et al. 2007; Agrillo et al. 2008, 2011; Stancher et al. 2013; Potrich et al. 2015; Forsatkar et al. 2016), and invertebrates (Dacke and Srinivasan 2008; Carazo et al. 2009; Nelson and Jackson 2012; Howard et al. 2018).
The ability to properly evaluate differences among either discrete or continuous quantities is considered a fitness-related trait which can potentially benefit the organisms in many situations. According to optimal foraging theory (MacArthur and Pianka 1966; Stephens and Krebs 1986) animals tend to maximize their net energy intake, implying that, to choose the most profitable food source, they should be able to discriminate the number or size of food items (Cresswell and Quinn 2004; Kruske et al. 2010; Bogale et al. 2014; Lucon-Xiccato et al. 2015; Stancher et al. 2015; Cross and Jackson 2017). Furthermore, the ability of discriminating between quantities may allow to lower resource competition by choosing food patches hosting less conspecifics (Forsman et al. 2008). American coots (Fulica americana) have been demonstrated to count their own eggs to decide when to stop further development of maturing egg follicles (Lyon 2003), while tree frogs (Hyla intermedia) can discriminate the height and number of grass clumps when choosing for microhabitats offering greater protection and more resources (Lucon-Xiccato et al. 2018). Finally, social groups may assess numbers of potential opponents before engaging in aggressive interactions (Benson-Amram et al. 2011).
For animals living in groups, group size discrimination may play a major role in setting the trade-off between the costs and benefits of membership. Although costs, such as competition for resources and risk of being parasitized, tend to increase with group size (Krause and Ruxton 2002), aggregation has been reported to improve the efficiency of foraging, social learning and vigilance against predators (Pitcher and Parrish 1993; Krause and Ruxton 2002). Avoidance of predation is probably the major benefit of group living: as both dilution of the probability of getting caught and vigilance increase with group size, the choice of which group to join is expected to have major effects on the fitness of individuals (Cresswell 1994). In many group living species, attraction towards conspecifics is enhanced by predation risk (Lima and Dill 1990; Pitcher and Parrish 1993; Krause and Ruxton 2002). A large amount of studies is available for fish, which tend to aggregate under a perceived threat (Magurran and Pitcher 1987; Krause et al. 1998; Nordell 1998; Hoare et al. 2004; Gómez-Laplaza and Gerlai 2011), using both numerical information and overall density or activity to choose the largest available shoal (Agrillo et al. 2008; Bisazza et al. 2010).
In contrast to most fish, adult amphibians show little evidence of a true shoaling behaviour and salamander larvae are often aggressive or cannibalistic toward other larvae (Wells 2007). However, several anuran tadpoles show different degrees of social aggregation, sociality being apparently based on kinship (Blaustein and O’Hara 1986; Blaustein and Walls 1995). While kin recognition is mainly mediated by chemical cues (Blaustein and O’Hara 1982), to our knowledge experimental studies on the role played by visual cues in group formation are still lacking. Under perceived predation risk, tadpoles usually react by changing the level of activity according to the ratio between the amount of predatory cue and conspecific density (Van Buskirk et al. 2011; Gazzola et al. 2018a, b), but there is also some evidence that larval amphibians may aggregate in response to predation. As an example, high predation risk induces tadpoles of both Aglyptodactylus securifer (Mantellidae) and Dyscophus insularis (Microhylidae) to form groups (Glos et al. 2007). Aggregation is induced by either mechanical stimuli (e.g. movements of aquatic predators) or chemical alarm cues from conspecifics (Spieler and Linsenmair 1999) and may be effective in lowering the number of attacks suffered by individual tadpoles by predators (Watt et al. 1997).
Here we explored tadpoles’ ability to discriminate among different numerical quantities (as opposed to non-numerical continuous traits, e.g. total surface area) either in the presence or absence of predatory cues. We applied a 4 × 2 factorial design, combining each of four numerical combinations of conspecific tadpoles with either chemical cues or well water. As interspecific variation in tendencies to aggregate may result in different numerical (quantitative) abilities, that is more social tadpoles may have evolved more precise discriminations of quantities, tests were carried out on both green toad (Bufotes balearicus) and edible frog (Pelophylax esculentus) tadpoles. As other bufonids (Waldman 1991; Blaustein and Waldman 1992), the green toad forms large and persistent social groups and tend to aggregate more under predation risk (Stav et al. 2007). In contrast, most ranid (Ranidae) tadpoles are solitary or form temporary aggregations with little evidence of social behaviour or spatial organization (O’Hara and Blaustein 1988; Griffiths and Foster 1998; Wells 2007). Thus, we expected green toads to select the larger groups, particularly when tadpoles were exposed to predatory cues.
Materials and methods
Subjects
We collected three green toad clutches and ca. 300 edible frog tadpoles from two different ponds inside the campus of the University of Pavia (Lombardy region, Northern Italy). Both species were caught by a hand net on the same day. Animals were immediately transported to the laboratory and maintained in 60 l opaque plastic containers. We provided all containers with aged tap water and aerators. Eggs and tadpoles were kept on a natural photoperiod at 19 ± 1.5 °C, and every day all containers were provided with the same amount of rabbit food throughout the study period. Both green toad and edible frog tadpoles were selected to obtain, for each species, a focal-group and a stimulus-group formed by individuals of similar size. Gosner’s developmental stage ranged between 26 and 28 for green toads and 27–32 for edible frogs, respectively. During the experiments, edible frog tadpoles were considerably larger than those of green toads (mean total length ± SEM: 26.2 ± 0.8 mm vs. 14.2 ± 0.5 mm, N = 20).
Predators, namely eight dragonfly Anax imperator larvae, were collected in a small water body in the University campus. Dragonfly larvae were individually maintained in 0.8 l tubs, filled with 0.6 l of aged tap water, and provided with leaves to offer cover and a substrate to cling to. One hour before the onset of each daily session of the experiments, each predator was fed with tadpoles of the species that was tested at that stage. The experiment was performed in May–June 2018. Edible frog tadpoles were maintained in the laboratory for 48 h before the onset of the experiment.
Experimental design and apparatus
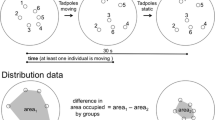
Our experimental apparatus consisted of an opaque plastic container (30 × 20 × 5 cm), filled with 1 l of aged tap water, and two transparent plastic boxes (9.4 × 6.4 × 4.8 cm), containing visual stimuli. The arena was divided into three equal zones (10 × 20 × 5 cm; Fig. 1) and the two small plastic boxes were positioned along the short sides of the arena in a symmetrical position, paying attention to make them properly adhere to the walls (Fig. 1). In this way, the two zones surrounding the visual stimuli were separated by a central, “neutral” zone. After the positioning of the plastic boxes, water depth in the arena was 2.5 cm.
The experiment joined a visual stimulus, represented by four numerical combinations (1 vs. 4, 4 vs. 8, 4 vs. 6, 3 vs. 4), with an olfactory stimulus (chemical cues vs. well water). The numerical combinations corresponded to a range of increasing ratios, 0.25, 0.5, 0.67, 0.75, respectively, with intermediate ratios testing tadpole ability to discriminate large quantities (> 4). For each trial, the chemical stimulus was obtained by collecting a total of 20 ml of conditioned water from four randomly selected tubs hosting tadpole-fed predators, and thus presumably consisted of a mixture of predator kairomones, prey-borne cues and digestion-released cues (Hettyey et al. 2015). These cues have been demonstrated to trigger behavioural anti-predator defenses in tadpoles (Chivers and Smith 1998; Relyea 2001; Van Buskirk 2001; Kishida and Nishimura 2005). Generally, the time an individual spends active is inversely proportional to the amount of cue provided (Van Buskirk and Arioli 2002; Bennett et al. 2013; Gazzola et al. 2018a, b). Focal tadpoles were allowed to perceive only the predatory cue, while the diffusion of cues released by conspecifics was prevented by the use of plastic boxes.
After having randomly confined the required number of stimulus tadpoles inside the transparent plastic boxes, the focal tadpole was positioned in the center of the arena within a cylindrical fiberglass net (5 cm in diameter). After 5 min of acclimation, we added either predatory cues or well water in two opposite corners of the arena (10 ml for each corner; Fig. 1), to provide symmetrical chemical stimuli with respect to the visual ones. After further 5 min, the fiberglass net was gently raised and the focal tadpole released. Group preference was then recorded over a 10-min period by a digital camera (Canon Legria) hanged up 1 m above a group of four arenas (one per each numerical combination, in randomized order) arranged as to easily cover a 60 × 40 cm large area. We inserted a 1 m high cardboard barrier all around the arenas to prevent disturbance during the recordings. After each trial, the experimental arenas and all plastic boxes were carefully washed before being re-filled with tap water. Both the position of the visual stimuli and the corners where chemical stimulus were added varied randomly during the experiment.
All trials were carried out from 10:00 a.m. to 02:00 p.m., when tadpoles are more active and gregarious (Bisazza et al. 2002). As cues of predation have been observed to still trigger strong behavioral responses after 36–48 h of aging in well water (Van Buskirk et al. 2014), we were confident that the duration of each session did not affect the strength of the stimulus. Each focal tadpole was tested only once and none of the tadpoles used as visual stimulus was used as focal individual. Overall, we tested 20 tadpoles for each combination “visual × chemical stimuli”, for a total of 160 focal tadpoles for each species.
Behavioural responses and statistical procedures
The total amount of time the focal tadpole spent inside each zone was quantified over a period of 10 min. A preference index was calculated as the proportion of time each tadpole spent close to the larger group with respect to the total time spent close to any of the two groups (i.e. within the two zones containing the visual stimuli). Tadpole was considered to have chosen a specific visual stimulus when its whole body had crossed the line which delimited the corresponding zone. As data were not normally distributed, a non-parametric one-sample signed-rank Wilcoxon test was used to compare this proportion against a chance value of 0.5 (i.e. random choice).
To uncover the effect of predatory cues on the level of activity of focal tadpoles, we applied a generalized linear model (GLM) with negative binomial distribution, with the total number of crossed lines (i.e. the number of movements from one zone to a neighboring one over a 10 min period) as response variable and the chemical treatment (water vs. predatory cues) as fixed factor. We then run a GLM for each species, with treatment as a single fixed factor. We also explored the effect of perceived predation risk on the average length of the time intervals spent inside the zone containing either the larger or the smaller group (hereafter permanence time) before moving out. Permanence time was used as the response variable in a general linear model (GLM) with a negative binomial distribution. Before the analyses, we removed all trials during which focal tadpoles did not move for the whole duration of the test (600 s). Statistics were performed using the R 3.5.1 package (R-Development-Core-Team 2018). We used two-tailed probabilities with alpha levels of 0.05 and, unless otherwise stated, results are reported as means ± SEM.
Results
Green toad tadpoles showed a significant preference for the groups composed of 4 individuals over a single conspecific, both with (V = 126, P = 0.020) and without (V = 99, P = 0.028; Fig. 2) predatory cue. No significant preference was found for all the other numerical contrasts, either with or without predatory cues (highest difference: V = 108, P = 0.14). Edible frog tadpoles showed a significant preference for the smaller group in the combinations 1 vs. 4 and 3 vs. 4 without predatory cue (V = 58, P = 0.047 and V = 27, P = 0.003, respectively), but they did not show any preference when exposed to chemical cues (V = 93, P = 0.760 and V = 97, P = 0.78, respectively; Fig. 2). No significant preference was found for contrasts with quantities larger than 4, either with or without predatory cues (highest difference: V = 101, P = 0.267).
Chemical treatment (χ2 = 34.90, P < 0.0001) and species (χ2 = 4.90, P = 0.026) had a significant effect on the activity level of focal tadpoles, while no relevant interaction was detected (treatment × species: χ2 = 1.51, P = 0.21). Both species responded to predatory cues by significantly reducing the number of movements from zone to zone in comparison to controls (green toad: estimate = − 0.55 ± 0.13, z = − 4.19, P < 0.0001; edible frog: estimate = − 0.35 ± 0.08, z = − 4.11, P < 0.0001; Fig. 3). Predator-exposed green toad tadpoles showed a significantly longer permanence time close to the larger group in comparison to controls for the numerical contrast 1 vs. 4 (estimate = 0.74 ± 0.34, z = 2.16, P = 0.03), while no significant effect of treatment or numerical combination was recorded for the permanence time of edible frog tadpoles close to the smaller group (Fig. 4).
Discussion
Group formation in tadpoles occurs in at least ten, distantly related families (Blaustein and Walls 1995; Wells 2007). Tadpoles likely use visual cues to locate conspecifics (Wassersug and Hessler 1971; Foster and McDiarmid 1982), nevertheless, to our knowledge, till now there has been no attempt to assess whether visual cues provide sufficient information to enable tadpoles to choose between aggregations differing in size. Our results suggest that in anuran larvae the capacity to discriminate between quantities may be limited to small numbers. Edible frog tadpoles showed to be more precise in group discrimination than green toad tadpoles, being able to discriminate among both 1 vs. 4 and 3 vs. 4 combinations. In contrast, tadpoles did not appear to discriminate more favourable ratios, such as 4 vs. 8 and 4 vs. 6. This appears puzzling in terms of the ANS which is supposed to be constrained by ratio of the numerosity to be discriminated. However, it has been hypothesized that number discrimination can be obtained, indirectly, by another, quite different mechanism, the Objects File System, which is not devoted to estimate quantity but is instead based on attention. In contrast to the ANS, this OFS would be precise but limited to sets in the range of one to about three–four items, because this is the limit of the number of objects that can be individuated and stored in working memory (Trick and Pylyshyn 1994; Uller et al. 1999; Feigenson et al. 2002). The existence of an OFS in non-human animals is controversial, though some evidence has been reported for both birds (Rugani et al. 2008) and fish (Agrillo et al. 2012).
As edible frog tadpoles had reached a later Gosner’s stage, the difference in species’ performances may depend on a mechanism that gradually develops during ontogeny, allowing more precise choices. Accordingly, adult frogs, although of different species (Bombina variegata and B. orientalis), are able to distinguish between larger groups (3 vs. 6 and 4 vs. 8; see Stancher et al. 2015; Vallortigara 2017), while forty-days-old guppies (Poecilia reticulata) can discriminate larger quantities than younger conspecifics (Bisazza et al. 2010), suggesting that the ANS may undergo some development during ontogeny and metamorphosis. The inability of tadpoles to discriminate between groups larger than four individuals is consistent with the apparently negligible effect of thinning on the responses of predator-exposed, large groups (N = 20) of tadpoles (Gazzola et al. 2018a, b).
Green toad tadpoles selected for the largest groups even in the absence of predator cues, which is consistent with the tendency to aggregate that has been widely reported for bufonids (Wells 2007). According to previous studies, the tadpoles of both species would markedly reduce their level of activity when exposed to predatory cues. However, toad tadpoles would also stay for longer close to the larger group, supporting aggregation as a major anti-predator behaviour in bufonids (see also Stav et al. 2007). Unexpectedly, frog tadpoles showed a preference for the smaller groups, though in predator-free trials only. Their preference may be explained considering how resource competition affects several fitness-related traits in ranids. For example, the impact of conspecific density on both the mass and development rate of Rana temporaria is more than double that of heterospecific density, while in Bufo bufo these impacts are similar (Gazzola and Van Buskirk 2015). Under predation risk, frog tadpoles were possibly exposed to contrasting urges—avoidance of competitors vs. anti-predatory aggregation—resulting in repeated switching between the two groups. Moreover, vulnerability to predation is size-dependent (Semlitsch 1990; Leu et al. 2013), tadpoles generally becoming abler to avoid predators, including cannibalistic conspecifics, as they grow up (Brown and Taylor 1995), supporting the adoption by edible frog tadpoles, which were much larger than green toad larvae, of a more swaggering behaviour with respect to predation risk. We made no attempt to unveil the mechanism underlying group discrimination. Several physical variables, such as total volume or surface area, covary with numerosity and may dominate the choice of focal tadpoles (Gómez-Laplaza and Gerlai 2013; Stancher et al. 2015). A recent work suggests that continuous physical variables rather than number underlies shoal size discrimination in fish (Xiong et al. 2018).
The level of activity, that is the amount of movements of group members, has been also reported as a visual stimulus steering group choice. In particular, adult salamanders of the genus Plethodon seem to respond on the basis of movement cues (Uller et al. 2003; Kruske et al. 2010). Anuran tadpoles change their level of activity according to the number of conspecifics, increasing their movements as groups get larger (Griffiths and Foster 1998; Cresswell et al. 2000; McClure et al. 2009). Thus, we cannot exclude a role of movement cues in group choice by focal tadpoles. On the other hand, using plastic containers we prevented focal tadpoles from assessing the numerosity of their conspecifics by water-borne chemical cues, which seem to play a major role in kin recognition and aggregation (Blaustein and O’Hara 1982). While visual cues proved sufficient to discriminate small groups, conspecifics’ cues may be involved in the choice between larger groups, a hypothesis that needs further research.
References
Agrillo C, Dadda M, Serena G, Bisazza A (2008) Do fish count? Spontaneous discrimination of quantity in female mosquitofish. Anim Cogn 11:495–503. https://doi.org/10.1007/s10071-008-0140-9
Agrillo C, Piffer L, Bisazza A (2011) Number versus continuous quantity in numerosity judgments by fish. Cognition 119:281–287. https://doi.org/10.1016/j.cognition.2010.10.022
Agrillo C, Piffer L, Bisazza A, Butterworth B (2012) Evidence for two numerical systems that are similar in humans and guppies. PLoS One 7(2):e31923. https://doi.org/10.1371/journal.pone.0031923
Bennett AM, Pereira D, Murray DL (2013) Investment into defensive traits by anuran prey (Lithobates pipiens) is mediated by the starvation-predation risk trade-off. PLoS One 8(12):e82344
Benson-Amram S, Heinen VK, Dryer SL, Holekamp KE (2011) Numerical assessment and individual call discrimination by wild spotted hyaenas, Crocuta crocuta. Anim Behav 82:743–752. https://doi.org/10.1016/j.anbehav.2011.07.004
Bertamini M, Guest M, Vallortigara G, Rugani R, Regolin L (2018) The effect of clustering on perceived quantity in humans (Homo sapiens) and in chicks (Gallus gallus). J Comp Psychol 132:280–293
Bisazza A, De Santi A, Bonso S, Sovrano VA (2002) Frogs and toads in front of a mirror: lateralisation of response to social stimuli in tadpoles of five anuran species. Behav Brain Res 134:417–424
Bisazza A, Piffer L, Serena G, Agrillo C (2010) Ontogeny of numerical abilities in fish. PLoS One 5(11):e15516
Blaustein AR, O’Hara RK (1982) Kin recognition cues in Rana cascadae tadpoles. Behav Neural Biol 36:77–87
Blaustein AR, O’Hara RK (1986) Kin recognition in tadpoles. Sci Am 254:108–116
Blaustein AR, Waldman B (1992) Kin recognition in anuran amphibians. Anim Behav 44:207–221
Blaustein AR, Walls SC (1995) Aggregation and kin recognition. In: Heatwole H, Sullivan BK (eds) Amphibian biology, Social behaviour, vol 2. Surrey Beatty and Sons, New South Wales, pp 568–602
Bogale BA, Aoyama M, Sugita S (2014) Spontaneous discrimination of food quantities in the jungle crow, Corvus macrorhynchos. Anim Behav 94:73e78
Brown RM, Taylor DH (1995) Performance and maneuvering behavior through larval ontogeny of the wood frog, Rana sylvatica. Copeia 1:1–7
Buckingham JN, Wong BBM, Rosenthal GG (2007) Shoaling decisions in female swordtails: how do fish gauge group size? Behaviour 144:1333–1346
Carazo P, Font E, Forteza-Behrendt E, Desfilis E (2009) Quantity discrimination in Tenebrio molitor: evidence of numerosity discrimination in an invertebrate? Anim Cogn 12:463–470. https://doi.org/10.1007/s10071-008-0207-7
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator/prey interactions: a review and prospectus. Écoscience 5:338–352
Cresswell W (1994) Flocking is an effective anti-predation strategy in redshanks, Tringa totanus. Anim Behav 47:433–442
Cresswell W, Quinn JL (2004) Faced with a choice, sparrow hawks more often attack the more vulnerable prey group. Oikos 104:71–76
Cresswell W, Hilton GM, Ruxton GD (2000) Evidence for a rule governing the avoidance of superfluous escape flights. Proc R Soc Lond B 267:733–737
Cross FR, Jackson RR (2017) Representation of different exact numbers of prey by a spider-eating predator. Interf Focus 7(3):20160035
Dacke M, Srinivasan MV (2008) Evidence for counting in insects. Anim Cogn 11:683–689. https://doi.org/10.1007/s10071-008-0159-y
Feigenson L, Carey S, Hauser M (2002) The representations underlying infants’ choice of more: object files versus analog magnitudes. Psychol Sci 13(2):150–156
Feigenson L, Dehaene S, Spelke E (2004) Core systems of number. Trends Cogn Sci 8(7):307–314
Forsatkar MN, Nematollahi MA, Bisazza A (2016) Quantity discrimination in parental fish: female convict cichlid discriminate fry shoals of different sizes. Anim Cogn 19:959–964
Forsman JT, Hjernquist MB, Taipale J, Gustafsson L (2008) Competitor density cues for habitat quality facilitating habitat selection and investment decisions. Behav Ecol 19:539–545. https://doi.org/10.1093/beheco/arn005
Foster MS, McDiarmid RW (1982) Study of aggregative behavior of Rhinophrynus dorsalis tadpoles: design and analysis. Herpetologica 38:395–404
Gallistel CR, Gelman R (2000) Non-verbal numerical cognition: from reals to integers. Trends Cogn Sci 4(2):59–65
Gazzola A, Van Buskirk J (2015) Isocline analysis of competition predicts stable coexistence of two amphibians. Oecologia 178:153–159. https://doi.org/10.1007/s00442-015-3273-y
Gazzola A, Sacchi R, Ghitti M, Balestrieri A (2018a) The effect of thinning and cue: density ratio on risk perception by Rana dalmatina tadpoles. Hydrobiologia 813:75–83
Gazzola A, Vallortigara G, Pellitteri-Rosa D (2018b) Continuous and discrete quantity discrimination in tortoises. Biol Lett 14:20180649. https://doi.org/10.1098/rsbl.2018.0649
Glos J, Erdmann G, Dausmann KH, Linsenmair KE (2007) A comparative study of predator-induced social aggregation of tadpoles in two anuran species from western Madagascar. Herpetol J 17:261–268
Gómez-Laplaza LM, Gerlai R (2011) Spontaneous discrimination of small quantities: shoaling preferences in angelfish (Pterophyllum scalare). Anim Cogn 14:565–574. https://doi.org/10.1007/s10071-011-0392-7
Gómez-Laplaza LM, Gerlai R (2013) Quantification abilities in angelfish (Pterophyllum scalare): the influence of continuous variables. Anim Cogn 16:373–383
Griffiths RA, Foster JP (1998) The effect of social interactions on tadpole activity and growth in the British anuran amphibians (Bufo bufo, B. calamita, and Rana temporaria). J Zool (London) 245:431–437
Hettyey A, Zoltán T, Thonhauser EK, Frommen JG, Penn DJ, Van Buskirk J (2015) The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 79:699–710
Hoare DJ, Couzin ID, Godin JGJ, Krause J (2004) Context-dependent group size choice in fish. Anim Behav 67:155–164. https://doi.org/10.1016/j.anbehav.2003.04.004
Howard S, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG (2018) Numerical ordering of zero in honeybees. Science 360:1124–1126. https://doi.org/10.1126/science.aar4975
Irie-Sugimoto N, Kobayashi T, Sato T, Hasegawa T (2009) Relative quantity judgment by Asian elephants (Elephas maximus). Anim Cogn 12(1):193–199
Kishida O, Nishimura K (2005) Multiple inducible defences against multiple predators in the anuran tadpoles, Rana pirica. Evol Ecol Res 7:619–631
Kloke JD, McKean JW (2012) Rfit: rank-based estimation for linear models. The R Journal 4(2):57–64
Krause J, Ruxton GD (2002) Living in groups. Oxford series in ecology and evolution. Oxford University Press, Oxford
Krause J, Ruxton GD, Rubenstein D (1998) Is there always an influence of shoal size on predator hunting success? J Fish Biol 52:494–501
Kruske P, Uller C, Dicke U (2010) Quantity discrimination in salamanders. J Exp Biol 213:1822–1828
Leu ST, Whiting MJ, Mahony MJ (2013) Making friends: social attraction in larval green and golden bell frogs, Litoria aurea. PLoS One 8(2):e56460. https://doi.org/10.1371/journal.pone.0056460
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Canad J Zool 68:619–640
Lucon-Xiccato T, Miletto Petrazzini ME, Agrillo C, Bisazza A (2015) Guppies discriminate between two quantities of food items but prioritize item size over total amount. Anim Behav 107:183e191
Lucon-Xiccato T, Gatto E, Bisazza A (2018) Quantity discrimination by treefrogs. Anim Behav 139:61–69. https://doi.org/10.1016/j.anbehav.2018.03.005
Lyon BE (2003) Egg recognition and counting reduce costs of avian conspecific brood parasitism. Nature 422:495–499
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609. https://doi.org/10.1086/282454
Magurran AE, Pitcher TJ (1987) Provenance, shoal size and the sociobiology of predator-evasion behaviour in minnow shoals. Proc R Soc Lond B 229:439–465
McClure KV, Mora JW, Smith GR (2009) Effects of light and group size on the activity of wood frog tadpoles Rana sylvatica. Acta Herp 4(1):103–107
Miletto Petrazzini ME, Fraccaroli I, Gariboldi F, Agrillo C, Bisazza A, Bertolucci C, Foa A (2017) Quantitative abilities in a reptile (Podarcis sicula). Biol Lett 13:20160899. https://doi.org/10.1098/rsbl.2016.089915
Miletto Petrazzini ME, Bertolucci C, Foa A (2018) Quantity discrimination in trained lizards (Podarcis sicula). Front Psychol 9:274. https://doi.org/10.3389/fpsyg.2018.00274
Nelson XJ, Jackson RR (2012) The role of numerical competence in a specialized predatory strategy of an araneophagic spider. Anim Cogn 15:699–710. https://doi.org/10.1007/s10071-012-0498-6
Nordell SE (1998) The response of female guppies, Poecilia reticulata, to chemical stimuli from injured conspecifics. Envir Biol Fishes 51:331–338
O’Hara RK, Blaustein AR (1988) Hyla regilla and Rana pretiosa tadpoles fail to display kin recognition behaviour. Anim Behav 36:946–948
Pepperberg IM (2006) Grey parrot numerical competence: a review. Anim Cogn 9:377–391. https://doi.org/10.1007/s10071-006-0034-7
Pitcher TJ, Parrish JK (1993) Functions of shoaling behaviour in teleosts. In: Pitcher TJ (ed) The behavior of teleost fishes, 2nd edn. Chapman and Hall, New York, pp 363–439
Potrich D, Sovrano VA, Stancher G, Vallortigara G (2015) Quantity discrimination by zebrafish (Danio rerio). J Comp Psychol 129:388–393
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Rugani R, Regolin L, Vallortigara G (2008) Discrimination of small numerosities in young chicks. J Exp Psychol Anim Behav Proc 34:388–399
Rugani R, Fontanari L, Simoni E, Regolin L, Vallortigara G (2009) Arithmetic in newborn chicks. Proc R Soc Lond B 276:2451–2460
Rugani R, Regolin L, Vallortigara G (2010) Imprinted numbers: newborn chicks’ sensitivity to number vs. continuous extent of objects they have been reared with. Dev Sci 13:790–797
Rugani R, Regolin L, Vallortigara G (2011) Summation of large numerousness by newborn chicks. Front Psychol 2:179. https://doi.org/10.3389/fpsyg.2011.00179
Rugani R, Cavazzana A, Vallortigara G, Regolin L (2013a) One, two, three, four, or is there something more? Numerical discrimination in day-old domestic chicks. Anim Cogn 16:557–564
Rugani R, Vallortigara G, Regolin L (2013b) Numerical abstraction in young domestic chicks (Gallus gallus). PLoS One 8(6):e65262. https://doi.org/10.1371/journal.pone.0065262
Rugani R, Vallortigara G, Regolin G (2014) From small to large: numerical discrimination by young domestic chicks (Gallus gallus). J Comp Psychol 128:163–171
Rugani R, McCrink K, de Hevia M-D, Vallortigara G, Regolin L (2016) Ratio abstraction over discrete magnitudes by newly hatched domestic chicks (Gallus gallus). Sci Rep 6:30114. https://doi.org/10.1038/srep30114
Semlitsch RD (1990) Effects of body size, sibship, and tail injury on the susceptibility of tadpoles to dragonfly predation. Can J Zool 68:1027–1030
Spieler M, Linsenmair KE (1999) Aggregation behaviour of Bufo maculatus tadpoles as an antipredator mechanism. Ethology 105:665–686. https://doi.org/10.1046/j.1439-0310.1999.00446.x
Stancher G, Sovrano VA, Potrich D, Vallortigara G (2013) Discrimination of small quantities by fish (redtail splitfin, Xenotoca eiseni). Anim Cogn 16:307–312
Stancher G, Rugani R, Regolin L, Vallortigara G (2015) Numerical discrimination by frogs (Bombina orientalis). Anim Cogn 18:219–229. https://doi.org/10.1007/s10071-014-0791-7
Stav G, Kotler BP, Blaustein L (2007) Direct and indirect effects of dragonfly (Anax imperator) nymphs on green toad (Bufo viridis) tadpoles. Hydrobiologia 579:85–93
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Trick LM, Pylyshyn ZW (1994) Why are small and large numbers enumerated differently? A limited-capacity preattentive stage in vision. Psychol Rev 101:80–102
Uller C, Carey S, Huntley-Fenner G, Klatt L (1999) What representations might underlie infant numerical knowledge. Cogn Dev 14:1–36
Uller C, Jaeger R, Guidry G, Martin C (2003) Salamanders (Plethodon cinereus) go for more: rudiments of number in an amphibian. Anim Cogn 6:105–112. https://doi.org/10.1007/s10071-003-0167-x
Vallortigara G (2014) Foundations of number and space representations in non-human species. In: Geary DC, Bearch DB, Mann Koepke K (eds) Evolutionary origins and early development of number processing. Elsevier, New York, pp 35–66
Vallortigara G (2017) An animal’s sense of number. In: Adams JW, Barmby P, Alex M (eds) The nature and development of mathematics. Taylor and Francis, Oxon, pp 43–65
Vallortigara G (2018) Comparative cognition of number and space: the case of geometry and of the mental number line. Philos Trans R Soc B 373:20170120. https://doi.org/10.1098/rstb.2015.0615
Van Buskirk J (2001) Specific induced responses to different predator species in anuran larvae. J Evol Biol 14:482–489
Van Buskirk J, Arioli M (2002) Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology 83:1580–1585
Van Buskirk J, Ferrari M, Kueng D, Näpflin K, Ritter N (2011) Prey risk assessment depends on conspecific density. Oikos 120:1235–1239
Van Buskirk J, Krugel A, Kunz J, Miss F, Stamm A (2014) The rate of degradation of chemical cues indicating predation risk: an experiment and review. Ethology 120:942–949
vanMarle K, Wynn K (2011) Tracking and quantifying objects and non-cohesive substances. Dev Sci 14(3):502–515
Vonk J, Beran MJ (2012) Bears “count” too: quantity estimation and comparison in black bears, Ursus americanus. Anim Behav 84:231–238. https://doi.org/10.1016/j.anbehav.2012.05.001
Vonk J, Torgerson-White L, McGuire M, Thueme M, Thomas J, Beran MJ (2014) Quantity estimation and comparison in western lowland gorillas (Gorilla gorilla gorilla). Anim Cogn 17:755–765. https://doi.org/10.1007/s10071-013-0707-y
Waldman B (1991) Kin recognition in amphibians. In: Hepper PG (ed) Kin recognition. Cambridge University Press, Cambridge, pp 162–219
Wassersug RJ, Hessler CM (1971) Tadpole behaviour: aggregation in larval Xenopus laevis. Anim Behav 19:386–389
Watt PJ, Nottingham SF, Young S (1997) Toad tadpole aggregation behaviour: evidence for a predator avoidance function. Anim Behav 54:865–872
Wells KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago
Xiong W, Yi L-C, Tang Z, Zhao X, Fu S-J (2018) Quantity discrimination in fish species: fish use non-numerical continuous quantity traits to select shoals. Anim Cogn. https://doi.org/10.1007/s10071-018-1214-y
Acknowledgements
We are grateful to Prof. Francesco Bracco and to the Botanic Garden of Pavia for providing the laboratory for behavioural experiments. In particular, we are grateful to Prof. Solveig Tosi for her enthusiastic and sincere care which allowed us to perform the experiments in a supporting environment. We also thank Paolo Cauzzi for his useful suggestions. A heartfelt thanks to Leonardo and Tommaso Pellitteri-Rosa for their nice and funny help during tadpole field collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. The permits to perform this study were obtained from the Italian Ministry of Environment, Land and Sea (0006075–23/03/2018—PNM).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balestrieri, A., Gazzola, A., Pellitteri-Rosa, D. et al. Discrimination of group numerousness under predation risk in anuran tadpoles. Anim Cogn 22, 223–230 (2019). https://doi.org/10.1007/s10071-019-01238-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-019-01238-5