Abstract
We report a study examining interspecies emotion transfer via body odors (chemosignals). Do human body odors (chemosignals) produced under emotional conditions of happiness and fear provide information that is detectable by pet dogs (Labrador and Golden retrievers)? The odor samples were collected from the axilla of male donors not involved in the main experiment. The experimental setup involved the co-presence of the dog’s owner, a stranger and the odor dispenser in a space where the dogs could move freely. There were three odor conditions [fear, happiness, and control (no sweat)] to which the dogs were assigned randomly. The dependent variables were the relevant behaviors of the dogs (e.g., approaching, interacting and gazing) directed to the three targets (owner, stranger, sweat dispenser) aside from the dogs’ stress and heart rate indicators. The results indicated with high accuracy that the dogs manifested the predicted behaviors in the three conditions. There were fewer and shorter owner directed behaviors and more stranger directed behaviors when they were in the “happy odor condition” compared to the fear odor and control conditions. In the fear odor condition, they displayed more stressful behaviors. The heart rate data in the control and happy conditions were significantly lower than in the fear condition. Our findings suggest that interspecies emotional communication is facilitated by chemosignals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body odors constitute chemical signals that have evolved for species-specific communication (e.g., McClintock 2000; Stevenson 2009; Wyatt 2015). Research has shown that in humans, chemosignals can carry compound information ranging from genetic relatedness (Jacob et al. 2002), gender (Penn et al. 2007), to emotional states (e.g., de Groot et al. 2012; Mujica-Parodi et al. 2009; Prehn et al. 2006; Zhou and Chen 2009; Mutic et al. 2015) and more (see de Groot et al. 2017). The transmission of olfactory information related to emotional states occurs without the requirement of communicative intent (Semin and de Groot 2013) and is below the threshold of consciousness (Pause 2012). Nevertheless, such transmission induces in the receiver a partial affective, behavioral, perceptual, and neural reproduction of the state of the sender (Semin 2007). The question we addressed here was about interspecies transmission of emotional information. To this end, we employed an experimental paradigm used in our previous research (e.g., de Groot et al. 2012), whereby the signal was human body odor that was produced while the donors were experiencing experimentally induced emotional states (i.e., happy, fear). The receivers of the human chemosignals were pet dogs (Labrador retrievers and Golden retrievers). Thus, the communication paradigm we employed exposed pet dogs to chemosignals produced by humans and analyzed the dogs’ reactions. In the following, we provide an overview of the relevant research to date with dogs and then outline the current study.
Dogs have an acute sensitivity to human gestures, which are chosen as landmarks when contrasted with other signals, such as verbal commands (D’Aniello et al. 2016a; Scandurra et al. 2017). There are two accounts regarding the origins of these abilities. One of them is the “Domestication Hypothesis.” According to this view, dogs have evolved genetic predispositions allowing them to develop skills shared with humans (Hare et al. 2002; Hare and Tomasello 2005; Topál et al. 2009; Miklósi and Topál 2013). According to the other view, namely the “Two-Stage Hypothesis” (Udell and Wynne 2008, 2010; Wynne et al. 2008) the capacity to interact with humans is acquired after people are accepted as companions in early ontogeny. This close proximity provides the opportunity to learn from humans during ontogenesis and thus shape (Scandurra et al. 2015; D’Aniello et al. 2015) and improve social communicative skills (D’Aniello and Scandurra 2016; D’Aniello et al. 2017). The two theories do not necessarily contradict each other. Indeed, the synergistic hypothesis suggests that the sensitivity to human gestural cues emerges both at the evolutionary and developmental level (Gácsi et al. 2009). In any case, dogs and humans went through a convergent evolution, whereby one of the most important consequences is that the two species have become social partners (Udell et al. 2010). In such a context, the reciprocal reading of the emotional status would be a very useful tool in many situations. The ability to recognize and respond appropriately to emotional messages has biological fitness benefits for both signaler and the receiver. Particularly, reading others emotions is very important for facilitating group cohesion (Racca et al. 2012) and it allows observers to use others’ emotions to cope flexibly with events in the environment (Nelson and Russell 2013). It has been shown that dogs can discriminate between smiling and neutral human faces (Nagasawa et al. 2011), between their owner’s facial expression of sadness and happiness (Morisaki et al. 2009) and a range of emotional facial expressions (anger, joy, disgust and fear) when compared with neutral ones (Deputte and Doll 2011). Furthermore, other findings show that dogs can extract and integrate bimodal sensory-emotional information. Indeed, dogs looked significantly longer at the expression of human faces (happy/playful vs. angry/aggressive) that were congruent in expressive valence to either positive or negative vocalizations (Albuquerque et al. 2016). Moreover, there are indications that dogs, aside from recognizing human emotions, adjust their behavior according to the expressed emotion (Merola et al. 2012, 2013). Dogs are also found to be sensitive and respond accordingly to differences in the emotional content of a voice (gentle vs. harsh) used by humans in obedience tasks (Fukuzawa et al. 2005).
Throughout this research on the social communicative interactions between dogs and humans, the focus has been on the visual and acoustic systems as they mediate emotional responses. The contribution that the olfactory system may have has barely been studied. Dogs have an extraordinary ability to detect airborne odors and not surprisingly their olfactory system is a significant contributor to the regulation of their social relations (Thesen et al. 1993; Miklósi 2007). For instance, male dogs can recognize kin, probably to avoid inbreeding (Hamilton and Vonk 2015). They can discriminate odors from different parts of the body of the same person or from human twins (Hepper 1988; Schoon and De Bruin 1994). Social smells have been shown to activate specific brain areas differentially in dogs, such as the caudate nucleus (Berns et al. 2015), which is involved in positive expectations in many species (Montague and Berns 2002; Schultz et al. 1997; Knutson et al. 2001; Berns et al. 2012, 2013), including social rewards (Rilling et al. 2002; Izuma et al. 2008). A dog’s caudate nucleus is activated more strongly when it is exposed to the body odor of a familiar human compared to odors from a familiar or a strange dog, an unfamiliar human and even the dog’s own odor (Berns et al. 2015) suggesting a positive emotional response to the odor of a familiar human (Panksepp 2004; Bekoff 2007).
One of the early olfactory transfer of emotion studies (Siniscalchi et al. 2011) showed that a veterinarian’s sweat increased a dog’s arousal. While this finding does not demonstrate the ability of dogs to perceive olfactory emotional messages from humans, a later study by the same research group (Siniscalchi et al. 2016) examined asymmetries in nostril use while the dogs were sniffing different emotive stimuli. The emotive stimuli were human (i.e., axillary sweat samples) and canine (i.e., perianal, interdigital and salivary secretions) odors produced under different emotional (fear, joy, stress) conditions. This research revealed that nostril use during sniffing of canine versus human odors varied systematically. Particularly, dogs consistently used their right nostril (right hemisphere) to sniff conspecific odors collected during a stressful situation. On the other hand, they preferred to use the left nostril to sniff human odors (left hemisphere) collected during fearful situations.
Siniscalchi et al. (2016) found that human fear chemosignals induced higher cardiac activation in dogs than neutral odors. Samples collected in humans during the induced state of joy emotion did not trigger a different cardiac effect than control condition. Furthermore, dogs appeared more stressed when they sniffed human chemosignals in the emotional fear condition than those obtained in the neutral or joy conditions.
The research we report here was designed to examine a new perspective, namely the transmission of emotional states from humans to dogs via human body odors produced during happiness and fear. The experimental setup for this examination was in a space in which the dogs could move and were free to manifest any behavior. Moreover, both the owner and a stranger were present in the room in which the examination took place. This interpersonal context was created to examine if dogs, when exposed to odors of happiness and fear, would display systematic differences in their social interest behavior toward their owner and a stranger. An important question is: do dogs manifest attachment behavior when exposed to fear odors? Dogs are known to form an affective bond with their owners fulfilling all attachment criteria described between parents and offspring in humans (Ainsworth and Bell 1970). This is expressed behaviorally through a preference for the attachment figure over other individuals and through behaviors aimed at obtaining and maintaining proximity to the caregiver during worrisome or dangerous situations (Palestrini et al. 2005; Palmer and Custance 2008; Prato-Previde et al. 2003; Rehn et al. 2013; Topál et al. 1998; Scandurra et al. 2016). We expected that the attachment system of dogs would be activated when they are exposed to fear chemosignals and lead dogs to look more to their owner as a “secure base”. In contrast, we expected happiness chemosignals to make dogs more confident in the environment and toward the stranger.
Materials and methods
Odor collection
Odor donors were heterosexual males (see de Groot et al. 2012, 2015). They watched fear or happiness-inducing videos in two sessions separated by 1 week. They followed a strict protocol. Two days prior to the donation, odorous food, alcohol, smoking, and excessive exercise was prohibited. They were provided with scent-free personal care products and detergents. The sweat was collected with sterile absorbent compresses (Cutisorb, BSN Medical, Hamburg, Germany) from both armpits. Donors, who were students at ISPA University, Lisbon, with an average age of 21 watched 25-min videos. Before and after the videos, donors completed Spielberger’s State-Trait Anxiety Inventory (Van der Ploeg 1980). Afterward, sweat pads were removed and stored at − 22 °C. The sweat pads were transferred to the Italian laboratory in dry ice. The odors were sent in two tranches: the first were from 4 Caucasian males, in Spring 2016; the second from a further 4 Caucasian males, in Spring 2017. To rule out interindividual differences in body odor, the pads of four different individuals were cut in four pieces and matched in a glass tube, thereby creating a pooled sample (see Mitro et al. 2012).
Subjects
The subjects were 40 pet dogs (17 males and 23 females; 31 Labrador retrievers and 9 Golden retrievers mean age in months 43.7 ± 5.0), recruited through personal contacts and advertisements in public places, veterinary surgeons and through the internet. All dogs lived in a household with at least two people. Dogs were randomly allocated to one of the three odor conditions: Happiness (7 males and 8 females; 10 Labrador retrievers and 5 Golden retrievers, mean age in months 45.3 ± 9.7), Fear (6 males and 9 females; 12 Labrador retrievers and 3 Golden retrievers, mean age in months 40.1 ± 7.9) and Empty (i.e., unused sweat pads) (4 males and 6 females; 9 Labrador retrievers and 1 Golden retrievers). The overall mean age for the pet dogs was 46.7 ± 7.6 months.
Apparatus and procedure
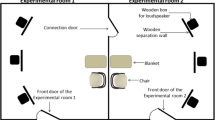
The study was conducted at the University of Naples “Federico II” (Naples) in a 4 × 3 m room that was new to the dogs. The room contained a water bowl in a corner and two chairs in two opposite corners (one for the owner and the other for a researcher (E1), unknown to dogs). The chairs were equidistant from the apparatus placed in the center of the room. The apparatus consisted of a wooden board of 39.5 × 30 cm with a semitransparent plastic container fixed at center. The vial, without a cap, containing the samples was inserted in the container. The lid in the upper part of the container had a circular hole (diameter 3 cm), which allowed the dogs to engage in the olfactory exploration of the contents while preventing the dog from contaminating the substances by direct contact.
Before starting, the owner had been informed about the testing procedure, while the dogs were free to move in a space outside of the room where the trials took place. Subsequently, a heart rate monitor was attached to the dog (Polar RS800CX) and the dog was free again for about 10 min before the experimental procedure started. Generally, the dogs showed some anxious behavior when the heart rate monitor was applied, but they adapted to the device after a while. At the end of this procedure, the owner entered the experimental room with the dog, where the E1 was already present. At this stage, the owner was free to interact with the dog while E1 ignored both, reading a magazine. After 5 min, in which the dog had finished exploration and its arousal due to the new environment was lowered, the owner was asked to hold the dog, while a second researcher (E2), entered the room to fix the experimental apparatus in the center of the room. When E2 left the room, the owner released the dog and the trial started. From this moment on, the owner and E1 did not interact with each other or the dog, and did not respond to any eventual solicitation by the dog. During this phase, the owner also begins to read a magazine to avoid looking at the dog or the experimenter. Both people in the room were not aware of the condition provided to the dog, so as not to influence accidentally their behavior. Each dog was allocated randomly to only one condition which lasted 2 min.
At the end of each trial, the bowl, the apparatus and the room were cleaned with a non-toxic disinfectant. This was done to eliminate the odors of dogs that had previously performed the trial and the vials with the samples were frozen again. Each sample was not used more than 4 times. The trial was recorded with a camera (HDR-PJ260VE) that was placed at a height of 220 cm in a corner of the room.
Behavioral parameters
All behaviors that were related to the apparatus and the people, such as approaching, interacting and gazing were grouped and categorized as apparatus, owner and stranger directed behaviors. All the stressful signals have been summarized and categorized as stressful behaviors (see Table 1 for the ethogram adopted). The frequency of all stressful behaviors was recorded, as well as the duration of the stressful behaviors. When two or more stressful behaviors co-occurred, we recorded the one that lasted longer.
The frequency and duration of each behavior were recorded using Solomon Coder® beta 16.06.26 (ELTE TTK, Hungary).
Heart rate monitoring
A Polar® RS800CX heart rate (HR) measuring system was used since it is an instrument scientifically validated for dogs (Jonckheer-Sheehy et al. 2012; Essner et al. 2013). Polar® heart rate monitor consisted of electrode belt and transmitter W.I.N.D. and heart rate monitor RS800CX. Following Essner et al. (2013), the coat was clipped at all electrode sites and Cogel® ECG electrode transmission gel was applied liberally to promote conductivity. We used two different electrode belts depending on the animal’s size (S–M or L–XL). These were strapped around the chest of the dogs with the transmitter placed ventrally and the electrodes on each side of the sternum. This instrument allows storing the R–R interval recordings, as well as the time data, automatically in the watch-computer for later analysis. The R–R intervals are the inter-beat intervals, and they are obtained as differences between successive R-wave occurrence times (Tarvainen et al. 2014).
The cardiac activity analysis was performed using the Polar Pro Trainer 5™ 5.40.170 software (Polar®Electro Öy, Kempele, Finland).
Data analysis
To evaluate the responses recorded during the 2 min of the trial, a point sampling approach was used. The data (the behavioral categories, i.e., owner directed behaviors; stranger directed behaviors; apparatus directed behaviors; stressful behaviors and heart rate) were recorded every 5 s, for a total of 24 sample points per dog. Each sample point contained all behavioral data displayed during the 5 s preceding the sample point. Similarly, the average heart rate during the 5 s prior to the chosen sample point was obtained. The averages of each sample point were obtained from 10 dogs exposed to the Empty condition (E), 15 the Happiness (H) and 15 the Fear (F) conditions.
To demonstrate response differences as a function of sweat sampled under different conditions, we adopted a linear discriminant analysis (LDA) to examine how well the measured variables (i.e., behavioral categories and the physiological parameter) could predict the specific odor condition in which each dog was.
The averages of the frequency and the durations of sample points obtained across the three different conditions were used to monitor the effect of the odors during the time on the behavioral parameters. The same approach was applied to the HR sample points. The temporal pattern was represented by a smoothing spline approach. The smoothing parameter was automatically selected, minimizing the residuals.
The distributions of the sample points of the behavioral parameters and of the HR were subsequently compared. If they were normally distributed, as showed by Shapiro–Wilk test, then they were analyzed with repeated measures ANOVA test with Tukey’s pairwise post hoc comparisons. In the case of not normally distributed data, a Kruskal–Wallis test was used to compare medians.
All statistical analyses were performed with IBM SPSS Statistics 22 and R (3.3.3).
Results
Linear discriminant analysis (LDA)
The LDA revealed two discriminant functions. As can be seen in Table 2, the first function is represented by variables related to fear (stress frequency and duration; heart rate) and the second function by approach-avoidance variables (owner and stranger directed behaviors). As can be seen in Table 2, these functions discriminate successfully between the three conditions (Table 3).
The first function mainly discriminated the F condition from the others. The second function highlighted the differences between H and E conditions (Fig. 1).
As can be seen in Table 4, the classification predicted by the two functions overlapped with a high degree of accuracy (87.5%) with the a priori classifications of the dogs to the odor conditions.
Temporal unfolding
In the following section, we describe the trends of the behavioral categories and the HR along the 1 min of test as a function of the sample points recorded for the frequency and the duration. At the same time, we compared the sample points across the different conditions.
Owner directed behaviors
The frequency of the owner directed behaviors in the E condition increased progressively, peaking at 35 s and decreasing 95 s. It remained stable subsequently and was constantly lower than the fear condition until the end of the trial. The F condition showed the highest initial value for the owner directed behaviors and then decreased in the first 30 s and remained practically constant until the end of the trial. The H condition had a decreasing pattern until the 70th second, and remained constant thereafter, but was always below the F condition (Fig. 2a).
The statistical analysis of the sample points representing the curves of the owner directed behaviors were significantly different (ANOVA: N E = N F = N H = 24, F = 6.94, P = 0.002), with the H condition lower than E (Tukey’s post hoc: Q = 5.18, P = 0.001) and F (Tukey’s post hoc: Q = 3.42, P = 0.037).
The duration of the owner directed behaviors follows the same pattern as in the case of the frequency measure. However, all curves converge on the same points after 90 s (Fig. 3a). The statistical comparison of sample points showed a significant difference (ANOVA: N E = N F = N H = 24, F = 8.45, P < 0.001), with H lower than E (Tukey’s post hoc: Q = 5.81, P < 0.001). Furthermore, there was a trend toward a lower duration of owner directed behaviors in E compared to F (Tukey’s post hoc: Q = 3.09, P = 0.081).
Temporal unfolding of the duration of the recorded behaviors during the 2 min of trial. The temporal pattern was represented by a smoothing spline approach where the smoothing parameter was automatically selected minimizing the residuals. In Y axes, the durations in seconds; in X axes the sample points
Stranger directed behaviors
The frequency of the stranger directed behaviors showed an initial increase across all conditions. E and the F conditions showed a similar pattern, with a marked decrease after 45 s, whereas the H condition maintained higher values during the entire trial (Fig. 2b). The statistical analysis revealed no significant differences among the sample points representing the conditions (Kruskal–Wallis test: N E = N F = N H = 24, H = 4.56, P = 0.097).
The duration of the stranger directed behaviors showed a pattern similar to the frequency one (Fig. 3b). However, the sample points were significantly different for duration (ANOVA: N E = N F = N H = 24, F = 6.59; P = 0.002), with the H condition higher than both F (Tukey’s post hoc: Q = 4.42, P = 0.007) and E (Tukey’s post hoc: Q = 4.47, P = 0.007).
Apparatus directed behaviors
The trend of the frequency of the apparatus directed behaviors was very similar across all three conditions, decreasing during the first minute and remaining constant during the second minute (Fig. 2c). No statistical differences were recorded among the sample points of the conditions (Kruskal–Wallis test: N E = N F = N H = 24, H = 4.55, P = 0.097).
The pattern described for the frequency can be applied unchanged for the duration (Fig. 3c), including no statistical differences (Kruskal–Wallis test: N E = N F = N H = 24, H = 4.34, P = 0.109).
Stressful behaviors
The frequency of the stressful behaviors showed a similar trend in E and H conditions, showing a growing trend at the beginning, peaking at the 35th (H) and 50th (E) seconds, then decreasing progressively. The F condition differed from the other two conditions revealing constantly a higher level, with a peak around 55th second that held until the end of the trial (Fig. 2d). A significant difference was recorded for the sample points of stressful behaviors (ANOVA: N E = N F = N H = 24, F = 31.51, P < 0.001), with F giving a higher value than both the H (Tukey’s post hoc: Q = 9.02, P < 0.001) and E (Tukey’s post hoc: Q = 10.30, P < 0.001) conditions.
The duration of the stressful behaviors showed a similar pattern to the frequency variable (Fig. 3d), with statistical differences between the sample points of the conditions (ANOVA: N E = N F = N H = 24, F = 17.14, P < 0.001). Post hoc test showed that F was higher than both H (Tukey’s post hoc: Q = 7.77, P < 0.001) and E (Tukey’s post hoc: Q = 6.36, P = 0.001).
Heart rate monitoring
In the E condition, the heart rate (HR) was initially high, starting with a value comparable to the F condition. It then decreased as the trial progressed. In the F condition, the HR remained constantly higher than the other conditions. In the H condition, the values were initially lower than the F and E conditions, then increasing in the first 40 s and decreasing until the end of the trial. During the phase when it was decreasing, the HR values remained above condition E and below condition F (Fig. 4). The dog’s HR sample points differed (ANOVA: N E = N F = N H = 24, F = 15.91, P < 0.001). Data from E were significantly lower than F (Tukey’s post hoc: Q = 7.86, P < 0.001). Moreover, the HR in the H condition was also significantly lower than in the F condition (Tukey’s post hoc: Q = 4.64, P = 0.005). A tendency toward a higher H with respect E was also detected (Tukey’s post hoc: Q = 3.22, P = 0.066).
Discussion
The effect of the emotional responses triggered by the visual and acoustic signals in dogs has been widely studied, while the involvement of the olfactory system, which in dogs is probably the more reliable sensory system, has been barely studied. The reason for the paucity of research examining the communicative potential of the olfactory sensory system in dogs is probably due to the assumption that there is considerable difference in the sensitivities of the olfactory systems of dogs and humans (Marshall and Moulton 1981). However, dogs and humans have a long co-evolutionary history and their communicative potential through visual and acoustic systems has been repeatedly demonstrated (Pongrácz et al. 2009; MacLean et al. 2017). It would have been surprising if their olfactory system, which has extraordinary potential to detect airborne odors, did not contribute to the regulation of their relations with humans. This would mean that they should display differential responses to the distinctive biochemical signatures of the body odors humans excrete under different emotional states.
To examine the interspecies communicative potential of human chemosignals of fear and happiness, we analyzed the behavioral changes and the physiological heart activation in dogs as a consequence of the exposition to these odors. Our data showed that the human chemosignals affect the physiological status of dogs and induce systematically different behaviors. Indeed, the discriminant analysis showed that the responses displayed by the dogs allow the identification of the body odor conditions the dogs were in, with a near perfect accuracy.
The time monitoring analysis of the data showed how the behavioral patterns unfolded over the duration of trials and revealed that the patterns were different, except for the apparatus interest, which followed a similar trend across all 3 conditions. The statistical analyses revealed that the dog’s heart activation was significantly higher in the fear condition compared to both the happiness and the empty condition, thus confirming the previous results reporting a cardiac effect on dogs exposed the human emotional fear chemosignals (Siniscalchi et al. 2016). Interestingly, the pattern observed for the stressful behaviors was very much like that of cardiac activation, with higher levels of stress displayed only in the fearful condition, which is again in line with an earlier study (Siniscalchi et al. 2016). Although a behavioral analysis alone may be regarded as insufficient to elucidate what dogs experience or even to argue that their emotional responses are like human ones (Panksepp 2004; Bekoff 2007), the heart rate indicator has the advantage of showing the emotional activity of the dogs. Indeed, it is well-established that heart rate in well controlled conditions is a suitable measure of behavioral states associated with sympathetic stimulation, as has been shown across a variety of species (rats: Ashida 1972; sheep: Baldock et al. 1988; chicken: Candland et al. 1969; pigs: Dantzer and Baldwin 1974; rabbits: Eisermann 1992; wolves: Fox and Andrews 1973; monkeys: Weisbard and Graham 1971). Thus, our finding of an enhanced heart rate in our dogs reflects a higher arousal when exposed to fear chemosignals.
As proposed by Siniscalchi et al. (2016), human fear chemosignals could have activated a predatory instinct of dogs, which could also explain anecdotal observations of a dog attacking people when they are afraid of dogs. This type of hypothetical situation would be the result of dogs using their left nostril when monitoring the human chemosignals of fear, which would be expected to lead to the activation of the left hemisphere. This in turn is involved in the control of predatory behavior (Siniscalchi et al. 2013). However, while an increase in the heartbeat rate is a sensible response in these circumstances, the physical expression of stressful signals is not necessarily a precursor of a predatory act, but could be regarded as an indicator of an emotional state of fear (together with the increased heartbeat). Thus, an alternative argument would suggest that stress signals are evidences of emotional contagion rather predatory behavior. Indeed, evidence of interspecies emotional contagion from dogs to humans has been shown on numerous occasions (Zahn-Waxler et al. 1984; Custance and Mayer 2012; Sümegi et al. 2014; Yong and Ruffman 2014; Huber et al. 2017). These studies demonstrate the role of visual and/or acoustic stimuli, whereas here for the first time, we show the involvement of the olfactory system in emotional transfer. Notably, the chemosignals used in our research came from a composite sweat stimulus pooled over 4 individuals unknown to dogs. It is worthwhile considering the possible effect of the emotional chemosignals from the owners themselves.
This is the first study revealing the effects of distinctive body odors on social behavioral responses. In line with our prevision, dogs adjusted their social interests after being exposed to different chemosignals, increasing the interest toward the stranger, as revealed by the increased duration of stranger directed behaviors in the happiness condition. On the other hand, our hypothesis was also supported for fearful chemosignals, which increased the owner interest above the other conditions as secure base effect, as showed by the higher frequency and duration of the owner directed behaviors in the fear. It should be pointed out that dogs have different attachment styles (see Udell and Brubaker 2016), which may have influenced trends in the fear condition, especially with respect to the time-based measures, as insecure attachment styles would likely drive greater differences between conditions than secure ones.
In closing, we should note that the majority of domesticated dogs in our samples were Labrador retrievers, which are ranked very high in hereditable behavioral traits related to sociability and curiosity/fearlessness (Svartberg 2006). Brain changes take place rapidly given highly responsive evolutionary plasticity resulting in considerable differences between closely related species (Pinelli et al. 2014; D’Aniello et al. 2016b). These types of differences can be seen in the course of the domestication of dog breeds that came to evolve with considerable differences in their behavioral traits (Svartberg 2006). For this reason, it is possible that other breeds with a different selective history could show different behavioral reactivity when exposed to human emotional smells. Hence, further studies on the matter in different breeds are likely to shed more light to the issues we examined here. The important message that the current study provides is the remarkable symmetry that human chemosignals of fear and happiness induces in the pet dogs we have examined. The fact that the oldest sensory system is tuned across these two species may suggest that the specific biochemical signature of chemosignals has remained a relatively invariable carrier of information that although susceptible to contextual variations remains a major medium of interspecies communication.
References
Ainsworth MDS, Bell SM (1970) Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev 41:49–67
Albuquerque N, Guo K, Wilkinson A, Savalli C, Otta E, Mills D (2016) Dogs recognize dog and human emotions. Biol Lett 12:20150883
Ashida S (1972) Development changes in the basal and evoked heart rate in neonatal rats. J Comp Physiol Psych 78:368
Baldock NM, Sibly RM, Penning PD (1988) Behavior and seasonal variation in heart rate in domestic sheep, Ovis aries. Anim Behav 36:35–43
Bekoff M (2007) The emotional lives of animals: a leading scientist explores animal joy, sorrow, and empathy-and why they matter. New World Library, Novato
Berns GS, Brooks AM, Spivak M (2012) Functional MRI in awake unrestrained dogs. PLoS ONE 7:e38027
Berns GS, Brooks AM, Spivak M (2013) Replicability and heterogeneity of awake unrestrained canine fMRI responses. PLoS ONE 8:e81698
Berns GS, Brooks AM, Spivak M (2015) Scent of the familiar: an fMRI study of canine brain responses to familiar and unfamiliar human and dog odors. Behav Process 110:37–46
Candland DK, Taylor DB, Dresdale L, Leiphart JM, Solow SP (1969) Heart rate, aggression, and dominance in the domestic chicken. J Comp Physiol Psychol 67:70
Custance D, Mayer J (2012) Empathic-like responding by domestic dogs (Canis familiaris) to distress in humans: an exploratory study. Anim Cogn 15:851–859
D’Aniello B, Scandurra A (2016) Ontogenetic effects on gazing behavior: a case study of kennel dogs (Labrador retrievers) in the impossible task paradigm. Anim Cogn 19:565–570
D’Aniello B, Scandurra A, Prato-Previde E, Valsecchi P (2015) Gazing toward humans: a study on water rescue dogs using the impossible task paradigm. Behav Process 110:68–73
D’Aniello B, Scandurra A, Alterisio A, Valsecchi P, Prato-Previde E (2016a) The importance of gestural communication: a study of human–dog communication using incongruent information. Anim Cogn 19:1231–1235
D’Aniello B, Polese G, Luongo L, Scandurra A, Magliozzi L, Aria M, Pinelli C (2016b) Neuroanatomical relationships between FMRF-amide-immunoreactive components of the nervus terminalis and the topology of olfactory bulbs in teleost fish. Cell Tissue Res 364:43–57
D’Aniello B, Alterisio A, Scandurra A, Petremolo E, Iommelli MR, Aria M (2017) What’s the point? Golden and Labrador retrievers living in kennels do not understand human pointing gestures. Anim Cogn 20:777–787
Dantzer R, Baldwin BA (1974) Changes in heart rate during suppression of operant responding in pigs. Physiol Behav 12:385–391
de Groot JH, Smeets MA, Kaldewaij A, Duijndam MJ, Semin GR (2012) Chemosignals communicate human emotions. Psychol Sci 23:1417–1424
de Groot JH, Smeets MA, Rowson MJ, Bulsing PJ, Blonk CG, Wilkinson JE, Semin GR (2015) A sniff of happiness. Psychol Sci 26:684–700
de Groot JH, Semin GR, Smeets MA (2017) On the communicative function of body odors: a theoretical integration and review. Perspect Psychol Sci 12:306–324
Deputte BL, Doll A (2011) Do dogs understand human facial expressions? J Vet Behav 6:78–79
Eisermann K (1992) Long-term heart rate responses to social stress in wild European rabbits: predominant effect of rank position. Physiol Behav 52:33–36
Essner A, Sjöström R, Ahlgren E, Lindmark B (2013) Validity and reliability of Polar® RS800CX heart rate monitor, measuring heart rate in dogs during standing position and at trot on a treadmill. Physiol Behav 114:1–5
Fox MW, Andrews RV (1973) Physiological and biochemical correlates of individual differences in behavior of wolf cubs. Behavior 46:129–139
Fukuzawa M, Mills DS, Cooper JJ (2005) More than just a word: non-semantic command variables affect obedience in the domestic dog (Canis familiaris). Appl Anim Behav Sci 91:129–141
Gácsi M, Gyoöri B, Virányi Z, Kubinyi E, Range F, Belényi B, Miklósi Á (2009) Explaining dog wolf differences in utilizing human pointing gestures: selection for synergistic shifts in the development of some social skills. PLoS ONE 4:e6584
Hamilton J, Vonk J (2015) Do dogs (Canis lupus familiaris) prefer family? Behav Process 119:123–134
Hare B, Tomasello M (2005) Human-like social skills in dogs? Trends Cogn Sci 9:439–444
Hare B, Brown M, Williamson C, Tomasello M (2002) The domestication of social cognition in dogs. Science 298:1634–1636
Hepper PG (1988) The discrimination of human odor by the dog. Perception 17:549–554
Huber A, Barber AL, Faragó T, Müller CA, Huber L (2017) Investigating emotional contagion in dogs (Canis familiaris) to emotional sounds of humans and conspecifics. Anim Cogn 20:703–715
Izuma K, Saito DN, Sadato N (2008) Processing of social and monetary rewards in the human striatum. Neuron 58:284–294
Jacob S, McClintock MK, Zelano B, Ober C (2002) Paternally inherited HLA alleles are associated with women’s choice of male odor. Nat Genet 30:175–179
Jonckheer-Sheehy VS, Vinke CM, Ortolani A (2012) Validation of a Polar® human heart rate monitor for measuring heart rate and heart rate variability in adult dogs under stationary conditions. J Vet Behav 7:205–212
Knutson B, Adams CM, Fong GW, Hommer D (2001) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159
MacLean EL, Herrmann E, Suchindran S, Hare B (2017) Individual differences in cooperative communicative skills are more similar between dogs and humans than chimpanzees. Anim Behav 126:41–51
Marshall DA, Moulton DG (1981) Olfactory sensitivity to α-ionone in humans and dogs. Chem Senses 6:53–61
McClintock MK (2000) Human pheromones: primers, releasers, signalers, or modulators? In: Wallen K, Schneider JE (eds) Reproduction in context. MIT Press, Cambridge, pp 355–420
Merola I, Prato-Previde E, Marshall-Pescini S (2012) Social referencing in dog–owner dyads? Anim Cogn 15:175–185
Merola I, Marshall-Pescini S, D’Aniello B, Prato-Previde E (2013) Social referencing: water rescue trained dogs are less affected than pet dogs by the stranger’s message. Appl Anim Behav Sci 147:132–138
Miklósi Á (2007) Human–animal interactions and social cognition in dogs. In: Jensen P (ed) The behavioral biology of dogs. CAB International, Wallingford, pp 205–222
Miklósi Á, Topál J (2013) What does it take to become ‘best friends’? Evolutionary changes in canine social competence. Trends Cogn Sci 17:287–294
Mitro S, Gordon AR, Olsson MJ, Lundström JN (2012) The smell of age: perception and discrimination of body odors of different ages. PLoS ONE 7:e38110
Montague PR, Berns GS (2002) Neural economics and the biological substrates of valuation. Neuron 36:265–284
Morisaki A, Takaoka A, Fujita K (2009) Are dogs sensitive to the emotional state of humans? J Vet Behav 4:49
Mujica-Parodi LR, Strey HH, Frederick B, Savoy R, Cox D, Botanov Y, Tolkunov D, Rubin D, Weber J (2009) Chemosensory cues to conspecific emotional stress activate amygdala in humans. PLoS ONE 4:e6415
Mutic S, Parma V, Brünner YF, Freiherr J (2015) You smell dangerous: communicating fight responses through human chemosignals of aggression. Chem Senses 41:35–43
Nagasawa M, Murai K, Mogi K, Kikusui T (2011) Dogs can discriminate human smiling faces from blank expressions. Anim Cogn 14:525–533
Nelson NL, Russell JA (2013) Universality revisited. Emot Rev 5:8–15
Palestrini C, Prato-Previde E, Spiezio C, Verga M (2005) Heart rate and behavioral responses of dogs in the Ainsworth’s strange situation: a pilot study. Appl Anim Behav Sci 94:75–88
Palmer R, Custance D (2008) A counterbalanced version of Ainsworth’s strange situation procedure reveals secure-base effects in dog–human relationships. Appl Anim Behav Sci 109:306–319
Panksepp J (2004) Affective neuroscience: the foundations of human and animal emotions. Oxford University Press, New York
Pause BM (2012) Processing of body odor signals by the human brain. Chemosens Percept 5:55–63
Penn DJ, Oberzauche E, Grammer K, Fischer G, Soini HA, Wiesler D, Novotny MV, Novotny MV, Dixon SJ, Xu Y, Brereton RG (2007) Individual and gender fingerprints in human body odor. J R Soc Interface 4:331–340
Pinelli C, Rastogi RK, Scandurra A, Jadhao AG, Aria M, D’Aniello B (2014) A comparative cluster analysis of nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase histochemistry in the brains of amphibians. J Comp Neurol 522:2980–3003
Pongrácz P, Molnár C, Miklósi Á (2009) Dog barking: a specific way of dog–human communication. J Vet Behav 4:54
Prato-Previde E, Custance DM, Spiezio C, Sabatini F (2003) Is the dog–human relationship an attachment bond? An observational study using Ainsworth’s strange situation. Behavior 140:225–254
Prehn A, Ohrt A, Sojka B, Ferstl R, Pause BM (2006) Chemosensory anxiety signals augment the startle reflex in humans. Neurosci Lett 394:127–130
Racca A, Guo K, Meints K, Mills DS (2012) Reading faces: differential lateral gaze bias in processing canine and human facial expressions in dogs and 4-year-old children. PLoS ONE 7:e36076
Rehn T, McGowan RT, Keeling LJ (2013) Evaluating the strange situation procedure (SSP) to assess the bond between dogs and humans. PLoS ONE 8:e56938
Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD (2002) A neural basis for social cooperation. Neuron 35:395–405
Scandurra A, Prato-Previde E, Valsecchi P, Aria M, D’Aniello B (2015) Guide dogs as a model for investigating the effect of life experience and training on gazing behavior. Anim Cogn 18:937–944
Scandurra A, Alterisio A, D’Aniello B (2016) Behavioral effects of training on water rescue dogs in the Strange Situation Test. Appl Anim Behav Sci 174:121–127
Scandurra A, Alterisio A, Marinelli L, Mongillo P, Semin GR, D’Aniello B (2017) Effectiveness of verbal and gestural signals and familiarity with signal-senders on the performance of working dogs. Appl Anim Behav Sci 191:78–83
Schoon GA, De Bruin JC (1994) The ability of dogs to recognize and cross-match human odors. Forensic Sci Int 69:111–118
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599
Semin GR (2007) Grounding communication: Synchrony. In: Kruglanski AW, Higgins ET (eds) Social psychology: handbook of basic principles. Guilford Press, New York, pp 630–649
Semin GR, de Groot JH (2013) The chemical bases of human sociality. Trends Cogn Sci 17:427–429
Siniscalchi M, Sasso R, Pepe AM, Dimatteo S, Vallortigara G, Quaranta A (2011) Sniffing with the right nostril: lateralization of response to odor stimuli by dogs. Anim Behav 82:399–404
Siniscalchi M, Pergola G, Quaranta A (2013) Detour behavior in attack-trained dogs: left-turners perform better than right-turners. Laterality 18:282–293
Siniscalchi M, d’Ingeo S, Quaranta A (2016) The dog nose “KNOWS” fear: asymmetric nostril use during sniffing at canine and human emotional stimuli. Behav Brain Res 304:34–41
Stevenson RJ (2009) The psychology of flavour. Oxford University Press, Oxford
Sümegi Z, Oláh K, Topál J (2014) Emotional contagion in dogs as measured by change in cognitive task performance. Appl Anim Behav Sci 160:106–115
Svartberg K (2006) Breed-typical behavior in dogs—historical remnants or recent constructs? Appl Anim Behav Sci 96:293–313
Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA (2014) Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed 113:210–220
Thesen A, Steen JB, Doving KB (1993) Behavior of dogs during olfactory tracking. J Exp Biol 180:247–251
Topál J, Miklósi Á, Csányi V, Dóka A (1998) Attachment behavior in dogs (Canis familiaris): a new application of Ainsworth’s (1969) Strange Situation Test. J Comp Psychol 112:219
Topál J, Gergely G, Erdőhegyi Á, Csibra G, Miklósi Á (2009) Differential sensitivity to human communication in dogs, wolves, and human infants. Science 325:1269–1272
Udell MA, Brubaker L (2016) Are dogs social generalists? Canine social cognition, attachment, and the dog–human bond. Curr Dir Psychol Sci 25:327–333
Udell MA, Wynne CD (2008) A review of domestic dogs’ (Canis familiaris) human-like behaviors: or why behavior analysts should stop worrying and love their dogs. J Exp Anal Behav 89:247–261
Udell MA, Wynne CD (2010) Ontogeny and phylogeny: both are essential to human-sensitive behavior in the genus Canis. Anim Behav 79:e9–e14
Udell MA, Dorey NR, Wynne CD (2010) What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol Rev 85:327–345
Van der Ploeg HM (1980) Validity of the Zelf-Beoordelings-Vragenlijst (A Dutch version of the Spielberger State-Trait Anxiety Inventory). Ned Tijdschr Geneeskd 35:243–249
Weisbard C, Graham FK (1971) Heart-rate change as a component of the orienting response in monkeys. J Comp Physiol Psychol 76:74
Wyatt TD (2015) How animals communicate via pheromones. Am Sci 103:114
Wynne CD, Udell MA, Lord KA (2008) Ontogeny’s impacts on human–dog communication. Anim Behav 76:e1–e4
Yong MH, Ruffman T (2014) Emotional contagion: dogs and humans show a similar physiological response to human infant crying. Behav Process 108:155–165
Zahn-Waxler C, Hollenbeck B, Radke-Yarrow M (1984) The origins of empathy and altruism. In: Fox MW, Mickley LD (eds) Advances in animal welfare science. Kluwer Academic, Norwell, pp 21–41
Zhou W, Chen D (2009) Fear-related chemosignals modulate recognition of fear in ambiguous facial expressions. Psychol Sci 20:177–183
Acknowledgements
The authors would like to thank all the handlers who participated in the test with great enthusiasm. This research was supported by ordinary funding from the University of Naples “Federico II”. G.R. Semin gratefully acknowledges the financial support provided by the Portuguese Science Foundation (IF/00085/2013/CP1186/CT0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethical Animal Care and Use Committee of the University of Naples “Federico II” (Protocol Number 2017/0025509). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Informed consent was obtained from the owners of all dogs included in the study.
Rights and permissions
About this article
Cite this article
D’Aniello, B., Semin, G.R., Alterisio, A. et al. Interspecies transmission of emotional information via chemosignals: from humans to dogs (Canis lupus familiaris). Anim Cogn 21, 67–78 (2018). https://doi.org/10.1007/s10071-017-1139-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-017-1139-x