Abstract
In an ever-changing environment, the ability to adapt choices to new conditions is essential for daily living and ultimately, for survival. Behavioural flexibility allows animals to maximise survival and reproduction in novel settings by adjusting their behaviour based on specific information and feedback acquired in their current environments. However, a growing body of evidence indicates that an individual’s personality type can limit the extent to which the individual might behave flexibly, by influencing the way an individual pays attention to novelty and how much information it collects and stores, which in turn affects the individual’s decision-making and learning process. In this study, the behavioural flexibility of a generalist predator, the Chimango Caracara, Milvago chimango, was analysed using the reversal learning paradigm, focusing on the comparison between age classes, and the relation of learning flexibility with a personality trait, the level of neophobia. Due to the low number of male individuals captured, this study was carried out only with female birds. The results showed that age had no significant effect either on the acquisition of a stimulus-reward association, or on the capacity of reversing this previously learned association. Reversal of the response was a harder task for these birds in comparison with the initial acquisition process. The individual’s performances in the learning tasks seemed to be uncorrelated with each other, suggesting that they involve different neural mechanisms. Contrary to the general pattern observed in the majority of previous work on personality and cognition in non-human animals, the level of neophobia did not correlate with the initial associative learning performance in both adults and juveniles, yet it showed a significant negative relationship with reversal learning ability, mainly in the regressive phase of this task, for the two age classes. Our results suggest that the predatory and generalist lifestyle of female individuals of M. chimango along with the selective pressures of the environment of the individuals studied might play a critical role in the degree and direction of the linkage between novelty response and learning flexibility observed in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In an ever-changing environment, the ability to adapt new choices or behavioural responses to new conditions is essential for daily living and ultimately, for survival. The capacity to respond appropriately will depend on the detection of cues that indicate these changes, the cognitive processes involved, and the flexibility required for making the proper response in each situation (Nussey et al. 2007). Learning is a form of plasticity, described as the capacity to modify behaviour based on experience, and involves forming internal representations of new information obtained from the current external and internal environments (Shettleworth 2010). Behavioural flexibility allows animals to exploit new environmental features that are unique to certain times and places more efficiently, to rapidly respond to a large variety of characteristics, and to increase their behavioural repertoire (Brown 2012; Dukas 2013). Therefore, through this process, animals can explore ways to maximise survival and reproduction in novel settings (Dukas 2013).
The experimental approach that has been used most frequently to study the process underlying behavioural flexibility is the reversal learning paradigm (Capaldi and Stevenson 1957; Menzel 1969; Pubols 1956; Sutherland and Mackintosh 1971). Briefly, this paradigm is an operant procedure that involves training an animal on a particular discrimination task and then, when a criterion level of accuracy has been reached, abruptly reversing the stimulus–reward contingency. To continue to be rewarded, the animal must inhibit or suppress the acquired response habit from the initial acquisition phase, while learning a new, competing, response to the previously unreinforced alternative (Dias et al. 1997). Thus, at the beginning of reversal learning, it is generally considered that the initial response habit remains dominant, at least temporarily, because of the initial training history. The inability to suppress this initial response habit is characterised by an increase in the perseveration response (i.e. perseverative errors), whereas the inability to learn the new, competing, response habit is characterised by an increase in regressive errors. These regressive or “non-perseverative” errors are seen later in the reversal session after the initial perseveration state has ceased (Palencia and Ragozzino 2004). In this sense, it has been suggested that the ability to inhibit previously successful responses (i.e. low perseveration) is one factor that could potentially enhance flexibility under changing environments (Day et al. 1999; Bond et al. 2007; Liedtke and Schneider 2014).
In the last few years, there has been an increase in studies in non-human animals, analysing the influence of personality traits on attention, learning, and memory regarding environmental features, especially regarding with respect to changes in the value-signalling cues that reveal relevant ecological and social information (i.e. Carere and Locurto 2011; Sih and Del Giudice 2012; Griffin et al. 2015). Personality refers to stable long-term behavioural, emotional, and physiological differences between individuals of the same species or population in suites of correlated traits (Groothuis and Carere 2005; Réale et al. 2010). Animal personality has been most commonly characterised along five behavioural axes: shyness–boldness, fast–slow during exploration, neophilia–neophobia, activity, aggressiveness, and sociability (Réale et al. 2007). These different but correlated behaviours evolve as an integrated pattern that can generate trade-offs and boundaries to otherwise unlimited behavioural flexibility (Sih et al. 2004). In this regard, Sih and Del Giudice (2012) postulated that individual differences in personality could be related to differences in cognitive styles, that is, the individual’s specific strategy for acquiring, processing, storing, and acting on information, independently of its cognitive ability per se (Gruszka et al. 2009). This hypothesis was based on the connection between the fast–slow behavioural continuum and the cognitive speed–accuracy trade-off (Chittka et al. 2009). According to this, for example, fast-explorers individuals would acquire a specific stimulus–reward association quicker than slow-explorers which, on the other side, are quicker than the former to react adequately if this clue–reward association changes. It has been proposed that this pattern arises as the result of a higher environmental sensitivity (i.e. attention to environmental cues) shown by shy and slow-explorers compared to bold and fast-explorers individuals (Benus et al. 1987; Groothuis and Carere 2005). Also, from the perspective of associative learning mechanisms, Pavlov (1906) was the first to propose that personality types differ in their ability to form excitatory and inhibitory connections. In agreement, Verbeek and collaborators (1994) argued that fast-explorers great tits were quicker to acquirer a foraging habit (i.e. quicker to form excitatory connections), but slower at changing it (i.e. low inhibitory control), whereas the opposite was observed in slow-explorers individuals.
The primary source of evidence supporting these previous predictions about the link between cognition and fast-slow behavioural types comes from studies using exploratory speed as a target personality trait to relate to learning flexibility (i.e. Benus et al. 1987, 1990; Verbeek et al. 1994; Bolhuis et al. 2004; Range et al. 2006; Guillette et al. 2009, 2011). Shyness and neophobia are strongly associated with the slow–fast exploratory styles (shy and neophobic individuals being usually on the slow part of this continuum). Also, exploratory style is known to strongly affect individual responses to cognitive challenges (Sih and Del Giudice 2012; Sol et al. 2013). However, despite the consistency observed in the results from these studies, there are cases in which such correlations were found not to be significant or even took the opposite direction. For example, Titulaer and collaborators (2012) concluded that, though slow-explorers females of great tits outperformed fast-explorers females in reversal learning tasks, fast-exploring males showed more flexible learning than slow-exploring males. Tebbich and collaborators (2012) observed a negative relationship between neophobia and reversal learning speed in woodpecker finches, though there was a lack of correlation between these variables in a closely related and sympatric species, the small tree finch. The inconsistency between these results is a reflexion of the complex link between cognition and personality, which probably depends on several factors like age, sex, experience, and lifestyles, as well as the ecological and social context in which an individual is immersed (Thomson et al. 2012; Sih et al. 2015). Thus, much still needs to be explored and investigated more deeply about personality-dependent learning and cognitive styles in animals.
In the present study, we investigated the behavioural flexibility of a generalist predator, the Chimango Caracara, Milvago chimango (Falconiformes) (Biondi et al. 2005), using the reversal learning paradigm. In particular, we focused on the relationship between neophobia level and performance during the acquisition of a cue–reward association and also, during the reversal learning phase, taking into account the individual’s age class. Based on the previously mentioned link between personality and cognition, and taking into account that in M. chimango neophobia correlates negatively with exploration speed (Biondi et al. 2010), we predict that there would be a negative relationship between neophobia and the speed to acquire an initial stimulus–reward association and a positive relationship between neophobia and learning flexibility in M. chimango. Moreover, if more neophobic birds have a higher environmental sensitivity (i.e. are more attentive to changes in their surroundings) than less neophobic ones, we expect that neophobia level will mainly correlate with the quantity of regressive errors performed during reversal. Alternatively, if more neophobic individuals are quicker to suppress or inhibit their response habit on the basis of negative feedback after the stimulus–reward contingency has changed, we predict a negative correlation mainly between this personality trait and the number of perseverative errors made during reversal. Also, we expect to find a higher performance in juveniles than adults individuals of M. chimango during both the acquisition and reversal tasks, since this difference has been noted especially in the reversal phase (Bond et al. 2007). This prediction is based on previous studies in mammals regarding the higher learning flexibility found in younger individuals compared to adults, mainly due to the greater preservation response of the latter (i.e. Moore et al. 2003, 2005, 2006; Bontè et al. 2011).
Methods
Subjects and housing
Eleven adult and ten juveniles (less than 2 years old) individuals of M. chimango were captured with baited walk-in traps (Bloom 1987) in a suburban area around Mar del Plata city, Argentina (7950 ha, and half a million inhabitants) between June and August (non-breeding period). We used plumage colour (mainly tail feathers), tarsus colour and moult stage to determine age (White et al. 1994; Ferguson-Lees and Christie 2001, Sarasola et al. 2011). Birds were identified with leg bands and weighed, and a blood sample was taken from the brachial vein to perform molecular sexing (Fridolfsson and Ellegren 1999). Immediately after capture, birds were housed in individual outdoor aviaries (1.5 × 1.5 × 1.2 m) following housing and care conditions described by Bloom (1987) and Aprile and Bertonatti (1996). Aviaries were visually isolated from one another by black synthetic fabric, ensuring that individuals performed on their own, without social motivation (Biondi et al. 2008, 2010, 2015). Birds were given at least a 5-day period to become habituated to captivity, during which they were fed once a day from a dish containing beef meat, and water was provided ad libitum (Biondi et al. 2008). Birds were considered to have habituated to captivity when they were comfortable enough to feed shortly after food presentation (Biondi et al. 2008). During all tests, the subjects were video-recorded for later analysis of behavioural variables with a Sony Sx-85 camcorder placed at 20 m from the aviaries. All individuals were identified with a plastic ring in their tarsus and then released at their capture sites at the end of the experimental tests. In this way, we prevented the use of the same individuals in subsequent experimental settings. The capture of raptors adhered to guidelines for the use of animals in research and to the legal requirements of Argentina: Disposition N°45, Exp. N 22500-24126/13, Dirección Contralor y Uso de Recursos Naturales y pesqueros, Ministerio de Asuntos Agrarios de la Provincia de Buenos Aires. The birds were cared for in accordance with the Guidelines for the Use of Animals in Behavioural Research and Teaching (ASAB/ABS 2003).
Experimental protocol
Apparatus
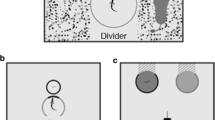
Two boxes of equal dimensions but of different colour were used to evaluate the acquisition and reversal capacity of M. chimango individuals. These boxes had two swinging doors that could be opened by pushing inwards (Fig. 1). The boxes were built from an opaque plastic material (one green and one yellow, 10.5 × 13.5 × 15 cm each) attached to wooden bases of 15 × 1 × 19 cm. This wooden base helped to hide the interior remote door locking mechanism (a modification of a car locking system) used as punishment after an incorrect choice was made (see experimental procedure). The boxes were placed at the front of the aviary facing the perches. The apparatus doors were located on the side that faced the individuals and the opposite side of the box was oriented to the aviary side with easy human access (Fig. 1). On that side, the boxes had a 3-cm groove by which food could be introduced during each trial, without being detected by the individuals being tested. To ensure that the individuals were not influenced by the researcher’s hand during the food placement, the same movements were performed around the rewarded and the unrewarded boxes.
Training
All individuals were exposed to a training phase during which they had to habituate to the experimental apparatus and to the continual approaching of the researcher during the baiting of the boxes. Simultaneously, they were shaped to extract food from the boxes by opening their doors. To accomplish this, the birds were exposed to the boxes baited with 1 g of meat each, during one daily session of five consecutive trials separated by 1-min inter-trials. After the birds consumed the first pieces of food and the 1-min inter-trial period was finished, the researcher approached to the aviary to bait the boxes once again. The right–left location of each box was randomly altered in each trial to avoid colour–side associations. This procedure was repeated during all subsequent trials. All birds were given a maximum of ten sessions to habituate to the apparatus and to learn to retrieve the food from it, by opening the doors in each trial. Once a bird could open the two boxes and extract the food from it in all consecutive trials, they were ready to proceed to the first task.
Acquisition task
Birds were trained to discriminate between two colours (yellow and green), with only one of them associated with a reward. The green and yellow boxes were placed alongside each other at the front of the aviary, separated by 50 cm (Fig. 1), with the doors closed and only one of them containing a food reward inside (2 g of red meat). The birds were randomly assigned to have reward associated with a yellow or a green box, ensuring that there was the same proportion of adults and juveniles in each group. To avoid association between reward and location (right or left), the boxes were changed randomly from one to the other between trials (as in habituation), trying not to present the box on the same side of the aviary more than three trials in a row. Individuals were given a maximum of ten sessions of nine trials each, to reach a learning criterion of five consecutive correct trials (i.e. to first open the rewarded box). On each trial, individuals were given a 4-min period to approach the boxes and to open one of them. If this did not occur within this period, the trial was considered null, and an additional trial was added to the session. If the individual made a correct choice, the next trial began 40 s later. On the other hand, if an error was made, the doors of the rewarded box were remotely locked, and the individual had to wait a 2-min period until the next trial. On the next day after the learning criterion was reached, one additional acquisition trial was given to each individual to control memory differences and to make sure birds had learned the task. If a mistake was made in these additional trials, the corresponding individual stayed in the acquisition phase until the learning criterion was achieved again.
Reversal task
The reversal phase test started immediately after the successful completion of the additional acquisition trial. In this case, the colour–reward contingency was reversed, that is, using the same coloured boxes but placing the reward in the opposite coloured box than in the acquisition trial. The test was conducted in the same way as the colour discrimination test, that is, individuals were given nine trials per session separated by 40-s or 4-min inter-trial periods depending on the outcome of the previous trial (success or error, respectively), with a maximum of ten sessions for each bird. The variables measured in both acquisition and reversal tasks were the total number of sessions and trials needed to reach the learning criterion, as well as the number of errors made by each individual. Additionally, response perseveration during the reversal phase was also analysed. Perseveration involved continuing to choose the colour that was designated positive in the acquisition phase and was operationally defined as opening the incorrect box for three or more trials in consecutive blocks of four trials each (Ragozzino et al. 1999, 2002; Kim and Ragozzino 2005). Once a bird had made fewer than three errors in a block for the first time, all subsequent errors were counted as regressive errors. In this way, we were able to measure the ability to learn and maintain a new choice after initially shifting away from the previously correct choice.
Object neophobia test
Twenty-four hours after the last reversal learning trial, a standard experimental design to assess reaction to novelty (Greenberg 1983) was presented to each bird. This task was performed using the same object and experimental protocol published in Biondi and collaborators (2010). Briefly, each test subject was presented with a dish containing pieces of meat (40 g in total). After the bird approached and consumed the first piece of meat (approximately 5 g), a researcher interrupted the feeding, approached the bird, and placed a novel object next to the remaining food. Each individual was then given 900 s to return to the dish. If a bird did not eat within this time, we recorded a 900-s maximum latency. The difference between the latency to consume in the presence of the novel object and the consumption latency in its absence was used as a measure of neophobia level.
Data analysis
Sex was not considered as explanatory variable in the analyses due to the low number of males captured in this study (two adults and one juvenile). These individuals were removed from the sample, and consequently, only females were investigated in this study.
The number of trials that elapsed until the learning criterion was reached and the number of errors made, were compared between age classes and tasks using generalised linear mixed models (GLMM), with Poisson error distribution family and log as link function (Pinheiro and Bates 2000). The same model was used to compare the number of perseverative and regressive errors made during the reversal phase trials between adults and juveniles. The latency to approach the first box of choice during the first trial during acquisition and reversal tasks was extracted from the videos and compared with LMM between age and tasks, including individual identity as random factor. The database modelling was adjusted by using NLME- and LME4-specific packages from R statistic software, version 3.0.3 (R Development Core Team 2014). One-way ANOVA was used to compare between age classes the level of object neophobia and latency to approach the boxes during the first habituation trial. This last value was considered as an additional measurement of neophobia (i.e. initial response to the boxes). All latency variables, as well as the level of object neophobia, were normalised by taking logarithm (to base 10). Finally, the neophobia level was correlated with the number of trials needed to reach the learning criterion, as well as with the number of errors made by each individual before learning, in both acquisition and reversal tasks. Furthermore, neophobia was correlated with the quantity of perseverative and regressive errors committed during reversal phase. For this, all variables were log-transformed (in base 10) and Pearson’s product moment correlation was performed (Zar 1999).
Results
Age and learning performance
The latency to approach to the first box of choice during the first acquisition trial (11.56 ± 2.74 s) was not significantly different to that observed during the first reversal trial (9.94 ± 2.48 s) (GLMM: t = −0.81, P = 0.415). The contrast analysis between tasks within each age class yielded no significant differences (GLMM, juveniles: t = −0.30, P = 0.765; adults: t = −0.81, P = 0.411). All birds reached the learning criterion of five consecutive correct choices (i.e. to first open the rewarded box) during both the acquisition and reversal tasks. During the acquisition task, they needed, on average, 13.47 ± 1.47 trials and made 4.05 ± 0.82 errors before meeting this criterion. In reversal task, individuals needed on average 27.83 ± 2.66 trials and made 14.06 ± 1.75 errors before reaching the learning criterion. These variables (trials to criterion and errors made) did not differ between adults and juveniles in either of the two tasks (Fig. 2a, b, Table 1). However, it was apparent that the birds made more mistakes and needed a higher quantity of trials to meet the learning criterion during reversal than in the acquisition of the initial colour–reward association. This pattern occurred when considering all the individuals together as well as when adult and juvenile raptors were considered separately (Fig. 2a, b, Table 1). Moreover, the number of trials needed until reach the learning criterion during acquisition was not correlated with this same variable during reversal phase (Pearson’s correlation: r = 0.1, N = 18, P = 0.618); the same tendency was observed regarding the quantity of errors made during acquisition and reversal tasks (Pearson’s correlation: r = 0.3, N = 18, P = 0.185). The analysis of the error types during the reversal phase showed that there were no significant differences between age classes (GLM, z = −0.8, P = 0.403) either in perseverative (adult: 10.2 ± 2.8; juv: 9.0 ± 1.6) or in regressive errors (adult: 4.1 ± 1.1; juv: 3.9 ± 0.8), though the former outnumbered the latter in both adult and juvenile individuals (GLM: z = −4.9, P < 0.001).
Mean ± SE values of the number of trials (a) and errors (b), made by M. chimango individuals on discrimination (Dis) and reversal (Rev) tasks. Values are given for adults (Ad) and juveniles (Juv) separately. Asterisk indicates significant differences in the response variable values between tasks, within each age class (see Table 1)
Neophobia and learning performance
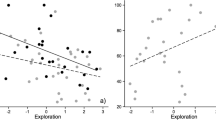
The level of neophobia towards the novel object was higher in adults (369.3 ± 116.5 s) than in juveniles (64.2 ± 23.3 s), this difference being statistically significant (F = 4.7, df = 16, P < 0.05). A similar pattern was observed regarding the latency to feed from the boxes for the first time (habituation period), with juveniles approaching and feeding faster (44.8 ± 17.9 s) compared to adults (122.9 ± 33.1 s) (F = 4.6, df = 16, P < 0.05). Because of the dissimilarities between ages in both neophobia measurements, the analysis of the relationship between learning performance and neophobia was carried out separately for adults and juveniles, despite the lack of age differences in both learning tasks. There was a significant correlation between the object neophobia level and the latency to approach the experimental apparatus for the first time, during the training phase (Fig. 3, Pearson’s correlation: adults, r = 0.86, N = 9, P = 0.003; juveniles, r = 0.92, N = 9, P < 0.0001). Thus, both adult and juvenile individuals who took longer to approach and explore the boxes for the first time expressed a higher level of neophobia when they had to feed close to a novel object. Due to this tight relationship, only the scores of neophobia towards the boxes were used in the correlational analysis with learning performance. The level of neophobia was not related to the initial acquisition of the colour–reward association, which was reflected by the low value of correlation coefficient between the numbers of trials needed to reach the criterion during the acquisition task and the latency to feed from the boxes for the first time (Pearson’s correlation: adults, r = 0.36, N = 9, P = 0.101; juveniles, r = 0.46, N = 9, P = 0.211). The same occurred with the errors made during acquisition (Pearson’s correlation: adults, r = 0.41, N = 9, P = 0.207; juveniles, r = 0.38, N = 9, P = 0.302). Nevertheless, in both adults and juveniles neophobia level was positively correlated with the number of trials needed to meet the learning criterion during the reversal task (Fig. 4; Pearson’s correlation: adults, r = 0.82, N = 9, P = 0.007; juveniles, r = 0.78, N = 9, P = 0.013). A similar pattern was observed regarding errors made before reaching the criterion, for which there was a positive correlation between this variable and neophobia level in juveniles (Fig. 5; Pearson’s correlation: r = 0.71, N = 9, P = 0.034), though only a marginally significant positive correlation in adults (r = 0.54, N = 9, P = 0.085).
Moreover, the separate analysis of error types showed that only regressive errors correlated positively with neophobia level (Fig. 6; Pearson’s correlation: adults, r = 0.75, N = 9, P = 0.019; juveniles, r = 0.86, N = 9, P = 0.003). In the case of perseverative errors, this correlation was non-significant (Pearson’s correlation: adults, r = 0.09, N = 9, P = 0.810; juveniles, r = 0.39, P = 0.281).
Discussion
In this study, the behavioural flexibility of female individual of the bird of prey, M. chimango, was analysed, focusing on the relationship between a personality trait, the neophobia level, and the performance during a reversal learning task, also taking in account the individuals’ age class. The results showed that age did not have a significant effect either on the acquisition of a stimulus-reward association or on the capacity to reverse this previously learnt association. As indicated by the higher number of trials required and errors committed, the reversal of a previously learnt colour–reward association, once the contingency changed, seems to be a harder task for these birds in comparison with the initial acquisition. The individuals’ performances in these two learning tasks were not correlated, probably reflecting distinct underlying neural mechanisms. The main finding of this study was that, while the level of neophobia in adults and juveniles was not related to the birds’ initial associative learning performance, it had a significant negative relationship with the reversal learning performance, particularly with the number of regressive errors committed before learning for the two age classes. This result contradicts previous findings about personality and behavioural flexibility, in which the fast exploratory and bold individuals (i.e. less neophobic) were less sensitive to changes in their surroundings and/or less willing to inhibit previously learned cue–reward associations (i.e. more prone to form routines) (Griffin et al. 2015).
As has been observed in several studies (e.g. Pagani et al. 2005; Chadman et al. 2006; Bond et al. 2007), reversal was a harder task than the initial acquisition of the novel cue–reward association for most of the birds. Indeed, only one adult and two juveniles were faster during reversal than in acquisition, and one juvenile showed similar performance (i.e. number of trials until success) in both tasks. A deeper analysis of the process occurring during the reversal phase revealed that in females of M. chimango perseveration is predominant and significantly longer in duration than the learning phase of reversal (LaClair and Lacreuse 2016). In other words, the perseverative response observed in the present study seems due to an incapacity to stop a previously acquired response, rather than to an inability to produce a new alternative behaviour. Moreover, the fact that there was no significant relationship in the number of trials and errors to reach the learning criterion between initial acquisition and reversal tasks, suggests that in females of M. chimango the two learning processes, which are well known to be underpinned by different neural centres (Watanabe 2006) and modulated by independent cognitive mechanisms, cannot be taken as a part of a “general” learning capacity (e.g. Matzel et al. 2003).
An enhanced learning ability has both advantages and costs associated with it (Dukas 1999, 2009; Brown 2012). One of the costs of shaping behaviour through learning can be the effects of being naïve (Brown 2012). Even innate behavioural responses may incur a cost because it is unlikely that the present environmental conditions are exactly the same as those during which the innate behaviour was shaped. Young individuals are a special case because they are born with no, or incomplete, knowledge about their surrounding environment, so they need a relatively fast and flexible learning capacity in their earlier and more sensitive life stages to enhance their survival chances. In fact, there is evidence that, at least in mammals, younger animals are remarkably faster in reversing a reward contingency than older individuals (Johnson and Wilbrecht 2011; Mongillo et al. 2013). However, in the present study, adult and juvenile birds had similar performances in the initial acquisition of a colour–reward association and also during the reversal learning task. It is important to note that the lack of differences in the learning performance could be due to the age of the juveniles that participated in the study. It is possible that an age difference in reversal ability might be found in much younger birds (i.e. under 1 year old). Indeed, several studies in primates have pointed out a nonlinear relationship between age classes and learning flexibility, being the oldest and youngest individuals more perseverative and less flexible than young adults (e.g. Weed et al. 2008, Manrique and Call 2015). Further research about ontogenetic changes in learning could be of great value for understanding the role of cognition in coping with the ecological and social demands experienced during each life stage of M. chimango.
Several studies exploring the link between behavioural syndromes and cognitive styles have shown that individuals that are fast proactive explorers behave less flexibly in response to changes than slow reactive explorers (i.e. Verbeek et al. 1994; Guillette et al. 2011). The reason for this is that fast individuals are less sensitive to modifications in their surroundings and also exhibit a higher proclivity for forming behavioural routines (resulting in less capacity for inhibiting previously learned associations) than slow explorers. Based on this, and considering that neophobia level is generally taken as part of fast–slow behavioural syndrome with a negative relationship with exploratory speed (Sih et al. 2004), we predicted that in females of M. chimango there would be a positive relationship between the neophobia level and the reversal learning performance. Results from the present study, however, provided a different perspective: we found a negative correlation between neophobia level and learning flexibility. Moreover, taking a closer look at the type of errors made by individuals during the reversal phase, we found that neophobia correlates positively with the number of regressive errors but not with the perseverative errors. Consequently, neophobia was not associated with the capacity to inhibit a previously learned response, but with the ability of M. chimango to learn that a previously non-rewarded stimulus is later rewarded. This could suggest that in this raptor species there is positive feedback between risk-sensitivity-related behaviours and responsiveness to changes in the value of the environmental clues that indicate food (Sih et al. 2015). Individuals of M. chimango inhabiting urban settings frequently make use of anthropogenic resources, like garbage, whose spatial and temporal predictability is subject to human activity (Brown 2012). Thus, not only would be advantageous to show a decreased neophobia, but also to be able to react rapidly to changes in the resources location and availability. Further studies including other potential personality traits—such as aggressiveness, exploration, or sociability—are needed to reach a full understanding of the nature of the linkage between personality and learning flexibility in M. chimango.
Current evidence about the relationship between cognitive styles and personality is still inconclusive. Other studies have also found a similar relationship between novelty responses and behavioural flexibility (e.g. Frost et al. 2007; Thomson et al. 2012). However, there is a general consensus within behavioural ecologists regarding neophobia as a crucial factor underlying behavioural flexibility in animals (e.g. Reader 2003; Greenberg 2003; Mettke-Hofmann 2014). Neophobia helps protect animals from unknown dangers, but it can also constrain the access and exploitation of novel resources, which is of vital importance especially for species with generalist lifestyles, like M. chimango, and for those colonising or dispersing into new areas (Greggor et al. 2015; Mettke-Hofmann 2014). Broadly speaking, generalist animals are known to have particularly appropriate cognitive abilities for responding more flexibly to environmental changes and the appearance of novel feeding opportunities (Reader and Laland 2002; Ratcliffe et al. 2006). For instance, it has been argued that generalists need a proficient cognitive machinery for processing and filtering a wide spectrum of environmental information (Reader and MacDonald 2003). This will depend not only on the complexity of the habitat they inhabit, but also on the inherent complexity of the generalist feeding strategy (see Mettke-Hofmann 2014). Hence, these cognitive requirements associated with the generalist lifestyle of M. chimango, along with the relatively low predation risk that a predatory bird experiences in urbanized habitats (Møller 2009; Sol et al. 2013; Rebolo-Ifrán et al. 2015), may represent key intervening factors in the observed linkage between neophobia level and learning flexibility.
In this sense, a growing body of evidence supports the idea that the degree in which different behavioural characteristics are correlated depends in part on the magnitude of the environment’s selective pressures (Coleman and Wilson 1998; Bell 2005; Bell and Sih 2007; Dingemanse et al. 2007). Thus, the existence of individual variation in personality traits that correlate with one another may have an adaptive function in nature, allowing the species to cope with fluctuations in their ecological and social settings (Koolhaas et al. 2010). This can also be translated into the relationship between cognition and personality traits, in the sense that the way these characteristics relate between each other might be determined by species ecological and lifestyle-specific trade-offs, which may vary across different environments (Bell and Sih 2007; Koolhaas et al. 2010; Sih et al. 2015). Consequently, it should be of paramount importance, not only to analyse other personality traits and its relationship with different measures of behavioural flexibility in M. chimango, but also to study populations of this species currently experimenting distinct selection pressures in their habitats—i.e. comparing individuals across a rural–urban gradient—to gather more evidence of the consistency (or variation) in the linkage between learning ability and the novelty responses observed in this study.
References
Aprile G, Bertonatti C (1996) Manual sobre rehabilitación de fauna. Boletín Técnico FVSA (Fundación Vida Silvestre Argentina), Buenos Aires
Bell AM (2005) Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus): behavioural syndromes. J Evol Biol 18:464–473. doi:10.1111/j.1420-9101.2004.00817.x
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834. doi:10.1111/j.1461-0248.2007.01081.x
Benus RF, Koolhaas JM, Van Oortmerssen GA (1987) Individual differences in behavioural reaction to a changing environment in mice and rats. Behaviour 100:105–122
Benus RF, Den Daas S, Koolhaas JM, Van Oortmerssen GA (1990) Routine formation and flexibility in social and non-social behaviour of aggressive and non-aggressive male mice. Behaviour 112:176–193
Biondi LM, Bó MS, Favero M (2005) Dieta del chimango (Milvago chimango) durante el periodo reproductivo en el sudeste de la provincia de Buenos Aires, Argentina. Ornitol Neotrop 16:31–42
Biondi LM, Bo MS, Vassallo AI (2008) Experimental assessment of problem solving by Milvago chimango (Aves: Falconiformes). J Ethol 26:113–118
Biondi LM, Bó MS, Vassallo AI (2010) Inter-individual and age differences in exploration, neophobia and problem-solving ability in a Neotropical raptor (Milvago chimango). Anim Cogn 13:701–710
Biondi LM, Guido JM, Bó MS et al (2015) The role of stimulus complexity, age and experience in the expression of exploratory behaviour in the Chimango Caracara, Milvago chimango. Anim Cogn 18:139–150
Bloom PH (1987) Capturing and handling raptors. In: Giron Pendelton BA, Millsap BA, Cline KW, Bird DM (eds) Raptor management techniques manual. National Wildlife Federation, Washington, pp 99–123
Bolhuis JE, Schouten WG, de Leeuw JA et al (2004) Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav Brain Res 152:351–360
Bond AB, Kamil AC, Balda RP (2007) Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica). J Comp Psychol 121:372–379
Bontè E, Flemming T, Fagot J (2011) Executive control of perceptual features and abstract relations by baboons (Papio papio). Behav Brain Res 222:176–182
Brown C (2012) Experience and learning in changing environments. In: Candolin U, Wong BBM (eds) Behavioral responses to a changing world, 1st edn. Oxford University Press, Oxford, pp 46–60
Capaldi EJ, Stevenson HW (1957) Reversal following different amounts of training. J Comp Physiol Psychol 50:195–198
Carere C, Locurto C (2011) Interaction between animal personality and animal cognition. Curr Zool 57:491–498
Chadman KK, Watson DJ, Stanton ME (2006) NMDA receptor antagonism impairs reversal learning in developing rats. Behav Neurosci 120:1071–1083
Chittka L, Skorupski P, Raine NE (2009) Speed–accuracy tradeoffs in animal decision making. Trends Ecol Evol 24:400–407. doi:10.1016/j.tree.200902.010
Coleman K, Wilson DS (1998) Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim Behav 56:927–936
Day LB, Crews D, Wilczynski W (1999) Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim Behav 57:393–407
Dias R, Robbins TW, Roberts AC (1997) Dissociable forms of inhibitory control within prefrontal cortex with an analogue of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci 17:9285–9297
Dingemanse NJ, Wright J, Kazem AJN et al (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138
Dukas R (1999) Costs of memory: ideas and predictions. J Theor Biol 197:41–50
Dukas R (2009) Learning: mechanisms, ecology, and evolution. In: Dukas R, Ratcliffe JM (eds) Cognitive ecology II. University of Chicago Press, Chicago, pp 7–26
Dukas R (2013) Effects of learning on evolution: robustness, innovation and speciation. Anim Behav 85:1023–1030
Ferguson-Lees J, Christie DA (2001) Raptors of the world. Houghton Mifflin Harcourt, Boston
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing non-ratite birds. J Avian Biol 30:116–121
Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU (2007) Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc R Soc Lond B Biol Sci 274:333–339
Greenberg R (1983) The role of neophobia in determining the degree of foraging specialization in some migrant warblers. Am Nat 122:444–453
Greenberg R (2003) The role of neophobia and neophilia in the development of innovative behaviour of birds. In: Reader SM, Laland KN (eds) Animal innovation. Oxford University Press, Oxford, pp 175–196
Greggor AL, Thornton A, Clayton NS (2015) Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Curr Opin Behav Sci 6:82–89. doi:10.1016/j.cobeha.2015.10.007
Griffin AS, Guillette LM, Healy SD (2015) Cognition and personality: an analysis of an emerging field. Trends Ecol Evol 30:207–214. doi:10.1016/j.tree.2015.01.012
Groothuis TG, Carere C (2005) Avian personalities: characterization and epigenesis. Neurosci Biobehav Rev 29:137–150
Gruszka A, Matthews G, Szymura B (2009) Handbook of individual differences in cognition: attention, memory, and executive control. Springer, New York
Guillette LM, Reddon AR, Hurd PL, Sturdy CB (2009) Exploration of a novel space is associated with individual differences in learning speed in black-capped chickadees, Poecile atricapillus. Behav Process 82:265–270
Guillette LM, Reddon AR, Hoeschele M, Sturdy CB (2011) Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc R Soc Lond B Biol Sci 278:767–773. doi:10.1098/rspb.2010.1669
Johnson C, Wilbrecht L (2011) Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev Cogn Neurosci 1:540–551. doi:10.1016/j.dcn.2011.05.008
Kim J, Ragozzino ME (2005) The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem 83:125–133
Koolhaas JM, De Boer SF, Coppens CM, Buwalda B (2010) Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31:307–321
LaClair M, Lacreuse A (2016) Reversal learning in gonadectomized marmosets with and without hormone replacement: are males more sensitive to punishment? Anim Cogn 19:619–630
Liedtke J, Schneider JM (2014) Association and reversal learning abilities in a jumping spider. Behav Process 103:192–198. doi:10.1016/j.beproc.2013.12.015
Manrique HM, Call J (2015) Age-dependent cognitive inflexibility in great apes. Anim Behav 102:1–6
Matzel LD, Han YR, Grossman H et al (2003) Individual differences in the expression of a “general” learning ability in mice. J Neurosci 23:6423–6433
Menzel R (1969) On honey bees memory of spectral colours. 2. Reversal learning and learning of several colours. Z Vgl Physiol 63:290–309
Mettke-Hofmann C (2014) Cognitive ecology: ecological factors, life-styles, and cognition. Wiley Interdiscip Rev Cogn Sci 5:345–360. doi:10.1002/wcs.1289
Møller AP (2009) Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159:849–858. doi:10.1007/s00442-008-259-8
Mongillo P, Araujo JA, Pitteri E et al (2013) Spatial reversal learning is impaired by age in pet dogs. Age 35:2273–2282. doi:10.1007/s11357-013-9524-0
Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB (2003) Impairment in abstraction and set shifting in aged rhesus monkey. Neurobiol Aging 24:125–134
Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB (2005) A non-human primate test of abstraction and set shifting: an automated adaptation of the Wisconsin card sorting test. J Neurosci Methods 146:165–173
Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB (2006) Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging 27:1484–1493
Nussey DH, Wilson AJ, Brommer JE (2007) The evolutionary ecology of individual phenotypic plasticity in wild populations. J Evol Biol 20:831–844. doi:10.1111/j.1420-9101.2007.01300.x
Pagani JH, Brown KL, Stanton ME (2005) Contextual modulation of spatial discrimination reversal in developing rats. Dev Psychobiol 46:36–46
Palencia CA, Ragozzino ME (2004) The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem 82:81–89
Pavlov IP (1906) The scientific investigation of the psychical faculties or processes in the higher animals. Science 24:613–619
Pinheiro J, Bates D (2000) Mixed-effects models in S and S-PLUS. Springer Science & Business Media, Berlin
Pubols BHJr (1956) The facilitation of visual and spatial discrimination reversal by overtraining. J Comp Physiol Psychol 49:243–248
Ragozzino ME, Detrick S, Kesner RP (1999) Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19:4585–4594
Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP (2002) Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci 116:105–115
Range F, Bugnyar T, Schlögl C, Kotrschal K (2006) Individual and sex differences in learning abilities of ravens. Behav Process 73:100–106. doi:10.1016/j.beproc.2006.04.002
Ratcliffe JM, Fenton MB, Shettleworth SJ (2006) Behavioral flexibility positively correlated with relative brain volume in predatory bats. Brain Behav Evol 67:165–176
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2013. ISBN 3-900051-07-0
Reader SM (2003) Innovation and social learning: individual variation and brain evolution. Anim Biol 53:147–158
Reader SM, Laland KN (2002) Social intelligence, innovation, and enhanced brain size in primates. Proc Natl Acad Sci 99:4436–4441
Reader SM, MacDonald K (2003) Environmental variability and primate behavioural flexibility. In: Reader SM, Laland KN (eds) Animal innovation. Oxford University Press, Oxford, pp 83–116
Réale D, Reader SM, Sol D et al (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Réale D, Dingemanse NJ, Kazem AJ, Wright J (2010) Evolutionary and ecological approaches to the study of personality. Philos Trans R Soc Lond B Biol Sci 365:3937–3946
Rebolo-Ifrán N, Carrete M, Sanz-Aguilar A et al (2015) Links between fear of humans, stress and survival support a non-random distribution of birds among urban and rural habitats. Sci Rep. doi:10.1038/srep13723
Sarasola JH, Negro JJ, Bechard MJ, Lanusse A (2011) Not as similar as thought: sexual dichromatism in Chimango Caracaras is expressed in the exposed skin but not in the plumage. J Ornithol 152:473–479
Shettleworth SJ (2010) Cognition, evolution, and behavior. Oxford University Press, New York
Sih A, Del Giudice M (2012) Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc Lond B Biol Sci 367:2762–2772. doi:10.1098/rstb.20120216
Sih A, Bell AM, Johnson JC, Ziemba RE (2004) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277
Sih A, Mathot KJ, Moirón M et al (2015) Animal personality and state–behaviour feedbacks: a review and guide for empiricists. Trends Ecol Evol 30:50–60
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112
Sutherland NS, Mackintosh NJ (1971) Mechanisms of animal discrimination learning. Academic Press, New York
Tebbich S, Stankewitz S, Teschke I (2012) The relationship between foraging, learning abilities and neophobia in two species of Darwin’s finches. Ethology 118:135–146
Thomson JS, Watts PC, Pottinger TG, Sneddon LU (2012) Plasticity of boldness in rainbow trout, Oncorhynchus mykiss: do hunger and predation influence risk-taking behaviour? Horm Behav 61:750–757
Titulaer M, van Oers K, Naguib M (2012) Personality affects learning performance in difficult tasks in a sex-dependent way. Anim Behav 83:723–730
Verbeek ME, Drent PJ, Wiepkema PR (1994) Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav 48:1113–1121
Watanabe S (2006) The neural basis of cognitive flexibility in birds. In: Wasserman EA, Zentall TR (eds) Comparative cognition: experimental explorations of animal intelligence. Oxford University Press, Oxford, pp 619–639
Weed MR, Bryant R, Perry S (2008) Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience 157:22–28
White CM, Olsen PD, Cliff LF (1994) New world vultures to Guineafowl. In: Del Hoyo J, Sargalat EA (eds) Handbook of the birds of the world 2. Lynx Editions, Barcelona, pp 216–247
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall Inc., Upper Saddle River
Acknowledgements
We thank Maria Susana Bó and Enrique Madrid for her assistance during the capture and management of raptors and Susana Rosso and Jorge Sanchez for allowing access to their properties to capture birds. We appreciate the improvements in English usage made by Elena Okada. This work was conducted with funds provided by the Grant EXA622/12 from the National University of Mar del Plata, Grant PIP 0893 from the CONICET and by Grant PICT 2243 from FONCYT, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guido, J.M., Biondi, L.M., Vasallo, A.I. et al. Neophobia is negatively related to reversal learning ability in females of a generalist bird of prey, the Chimango Caracara, Milvago chimango . Anim Cogn 20, 591–602 (2017). https://doi.org/10.1007/s10071-017-1083-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-017-1083-9