Abstract
Referential communication occurs when a sender elaborates its gestures to direct the attention of a recipient to its role in pursuit of the desired goal, e.g. by pointing or showing an object, thereby informing the recipient what it wants. If the gesture is successful, the sender and the recipient focus their attention simultaneously on a third entity, the target. Here we investigated the ability of domestic horses (Equus caballus) to communicate referentially with a human observer about the location of a desired target, a bucket of food out of reach. In order to test six operational criteria of referential communication, we manipulated the recipient’s (experimenter) attentional state in four experimental conditions: frontally oriented, backward oriented, walking away from the arena and frontally oriented with other helpers present in the arena. The rate of gaze alternation was higher in the frontally oriented condition than in all the others. The horses appeared to use both indicative (pointing) and non-indicative (nods and shakes) head gestures in the relevant test conditions. Horses also elaborated their communication by switching from a visual to a tactile signal and demonstrated perseverance in their communication. The results of the tests revealed that horses used referential gestures to manipulate the attention of a human recipient so to obtain an unreachable resource. These are the first such findings in an ungulate species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deictic (or referential) gestures are mechanically ineffective body movements made to elicit specific behaviours on a recipient, repeated until the effect is obtained or failure is clearly indicated (Bates et al. 1975; Hobaiter and Byrne 2011). In referential communication, the sender elaborates its gestures to direct the attention of the recipient to its role in pursuit of the desired goal, e.g. by pointing or showing an object, thereby informing the recipient what it wants (Warneken et al. 2006). Because it reflects the construction of a mental action plan from the sender, this type of communicative gesture is considered a prerequisite for mind reading (Tomasello et al. 2005). If the gesture is successful, the sender and the recipient focus their attention simultaneously to a third entity, the target. Such shared attention should not be confused with simultaneous attention, where the attention of two individuals is drawn to the same stimulus by the stimulus itself, such as an unexpected sound (Tomasello 1995).
In contrast to gestures tuned to the mere presence or absence of an audience, referential gestures are displayed in accordance with the audience’s attentiveness. For example, when choosing between an acoustic signal and a ‘visual’ gesture, the sender will choose the latter only if the audience is already visually attending to the signaller. If the audience is not visually attentive, the sender needs to perform some attention-getting behaviours, such as vocalizations or non-indicative gestures. The meaning of these behaviours is not codified in the gesture itself, but rather derives from some accompanying behaviour (Call and Tomasello 2007). Examples of such ‘indicative behaviours’ are alternate gazing and/or pointing, indicating the location of the desired target (Leavens et al. 2005).
At around the age of 9–12 months, human infants start to use gestures to coordinate attention towards a social partner and a distal object by using indicative behaviours such as pointing, showing or offering (Bates et al. 1979). In comparison, examples of referential gestures in non-human species have been relatively scarce until recently, with evidence from different taxa accumulating in the last decade (reviewed in Pika 2012). Species for which signals have been described that may possess some if not all of the attributes of a referential gesture include non-human primates (chimpanzees, Pika and Mitani 2006; Roberts et al. 2014; orangutans, Cartmill and Byrne 2007; baboons, Bourjade et al. 2014; rhesus macaques, Canteloup et al. 2015); dogs (Miklósi et al. 2000; Gaunet and Deputte 2011; Savalli et al. 2014); dolphins (Xitco et al. 2004); ravens (Pika and Bugnyar 2011) and fish (groupers and coral trout, Vail et al. 2013). For example, Roberts et al. (2014) found that chimpanzees use both indicative (pointing with the hand) and non-indicative (bobbing with the head) gestures to direct an experimenter in a search for hidden food. Dolphins convey the attention of a recipient to a food target by maintaining the alignment of their bodies with it while alternating gaze between the target and the recipient (Xitco et al. 2004).
So far, among domesticated species, only dogs have been used as a model of referential communication (Hare et al. 1998; Miklósi et al. 2000; Gaunet 2010; Gaunet and Deputte 2011; Savalli et al. 2014). In the experimental set-ups so far used, the ability of dogs to communicate with a human observer about the location of an unreachable reward (toy or food) was tested. Hare et al. (1998) were the first to adapt experimental settings used to test primate-human referential communication to dog–human dyads. They found that dogs used gazes and vocalizations socially, i.e. less frequently in the absence of a human recipient. Subsequent experiments showed that dogs use alternate gazing when communicating the location of a hidden target (Miklósi et al. 2000; Gaunet and Deputte 2011), and even adjust their own position in space to gaze at their owner according to the direction of the owner’s gaze (Gaunet and Deputte 2011). When dogs obtained a different target to the requested one, they showed persistence, i.e. repeated gazes and vocalizations until they obtained the correct target, but did not elaborate their requests; e.g. no new behavioural technique emerged (Gaunet 2010). Recently, Savalli et al. (2014) found evidence that dogs communicate referentially with humans according to all operational criteria described by Leavens (2004): they use gaze alternation between a subject and a target, accompanied by attention-getting behaviours, such as vocalizations, that are modulated according to the attentional state and presence of the recipient. Dogs remained persistent in their communicative attempts and elaborated them if unsuccessful, i.e. if dogs did not obtain the full amount of food requested.
Primates, canines, dolphins and ravens live in complex social societies. It has been suggested that referential gesturing is an extremely rare form of communication (Tomasello et al. 2005), one that most likely evolved in species with highly complex social systems such as fission–fusion societies, where the negotiation of interactions and the use of visual social signals plays a fundamental role (Emery 2000; Pika 2012). Here we investigated the ability of referential communication in a highly social species that has so far not been studied for this skill: the domestic horse (Equus caballus). Horses live in fission–fusion societies (Rubenstein and Wrangham 1986) and are prey animals, for which concerting group actions and the associated communicative skills are of primary importance for survival. These animals communicate primarily visually, through fine-tuned body postures and ear, eye and head movements (Waring 2003; Wathan and McComb 2014). Given their social and cognitive skills, horses may be able to use referential communication at the intraspecific level. Nevertheless, unlike most species mentioned above, the horse is a domesticated species and it provides, in addition to dogs, a good model for studying interspecies communication, in particular animal–human communication. Therefore, we tested the ability of horses to communicate referentially with humans at the interspecific level. Research has already shown that horses understand human attentional cues (such as body and head orientation, eyes opened/closed, Proops and McComb 2010), permanent pointing (Maros et al. 2008) and, to some extent, gazing (Krueger et al. 2011), but research on how horses may use indicative behaviours to communicate with humans is lacking.
The aim of this study was to test the ability of domestic horses to communicate referentially with a human about the location of a desired target, a bucket of food, visible but out of reach. By varying the attentional states of the experimenter, we tested whether the horses would understand the difference among them and act accordingly. Like in many previous studies (Hare et al. 1998; Miklósi et al. 2000; Xitco et al. 2004; Gaunet and Deputte 2011; Savalli et al. 2014) a within-subject design was employed. We aimed to empirically answer the following questions according to the six operational criteria for intentional communication first described by Bates et al. (1975) in humans, and then adapted by Leavens (2004) to non-human animals: (1) Are the horses using gaze alternations, i.e. successive visual orientation between the communicative partner and the target? (2) Do the horses deploy attention-getting behaviours, head jerks, nods and shakes? (3) Are the signals used socially, i.e. is a recipient required to exhibit the behaviour? (4) Does the attentional status of the communicative partner (the human) influence the propensity of the sender (the horse) to exhibit the communicative gestures? (5) Is the communication persistent, i.e. does the signaller (the horse) repeat its signals in relation to different attentional and/or comprehension states of the recipient (the human), until the goal is obtained? (6) Is there elaboration of communicative gestures and related behaviours following persistent and ineffective signalling, such as changing the communicative channel?
Our main prediction was that horses use alternated gazes between the target and a human to convey their attention towards the desired object, thus activating attention-sharing mechanisms. This would be done by attracting the attention of the human with gazes or head gestures or by pointing at the target, modulating signalling according to her attentional state or the presence. We thus expect the highest rate of gaze alternation and attention-getting behaviours when the human (the experimenter or other humans present) is attentive towards the horse, and the lowest rate when her attention is elsewhere or she is absent. We predicted that horses were persistent in their communicative efforts, thus repeating their request after a period of absence of the human. Finally, we expected elaboration from horses in terms of switching from a visual to a tactile communicative channel if visual signalling turns out to be ineffective, i.e. walking back to the human and prompting action via physical contact with her.
If horses were found able to communicate referentially with a human observer, we propose that they acquired this skill individually during repeated interactions with conspecifics, being gradually reinforced with more and more subtle signals, and then adapted their signalling to interspecific communication with humans, where instead they needed to signal with more evident cues. This is similar to what is hypothesized in non-human primates, where referential gestures seem to arise from a ritualization of actions during interactions at the individual level (Tomasello et al. 1993; Tomasello 2006).
Materials and methods
Subjects
In total, 14 horses participated (mean age 10.9 ± 4.6; 8 geldings, 6 females; mixed breeds), hosted at EquiLuna A.S.D (Italy), Study Center for Ethical Equitation. All horses lived in the same social group in a paddock of 6000 m2, allowing natural social interactions, and were regularly involved in riding activities with humans. They were trained under the principles of the so-called natural horsemanship training (NHT), which are believed to provide a better experience compared to traditional training for horses and also to build better relationships between the horse and the trainer (for a review, see Birke 2007). They were provided with grass, hay and water ad libitum.
Experimental setting
We tested the horses in a familiar environment (refer to Schwab and Huber 2006) to avoid unnatural behavioural responses or stress induced by testing in novel settings. During tests, horses were able to see their conspecifics, confined at a distance of about 30 m. Occasionally, and due to the schedule of equestrian activities, conspecifics were at a closer distance and able to see the experimental area and the procedure, but physical contact was still not possible. Both the experimenter (RM) and the helpers involved in the tests were familiar to the horses.
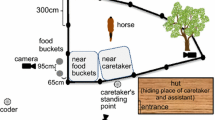
The experiment was conducted in a fenced arena (size 64 sqm). Two food buckets without lids were placed behind gates in equal distances from a release point, 16 m from each other (Fig. 1). Carrots, apples or oat were randomly used to fill both buckets, each with a different bait. We used two buckets instead of one because we did not know the individual food preferences of the horses and wanted to maintain their attention by providing in each session at least one preferred reward. During training and tests, only the experimenter (RM) and an assistant with a video camera were present, with the exception of one experimental condition (‘Many’) in which two helpers were included. The assistant with the video camera was shielded behind a panel and was visible to the horse only at the beginning of the test session during hiding, but not during the tests.
Sketch of the experimental procedure from above. A horse is depicted at the release point. Human pictures resemble the position of the experimenter and the helpers, if present. The Bucket and the Experimenter Area had a radius of one horse body length. The dotted straight line outside the test area in the bottom of the figure indicates the direction taken by the experimenter when walking out and coming back to the test area during the condition ‘Walk-away’. Exp experimenter
Experimental conditions
We designed four experimental conditions (Online Resource 1), consisting of 3 trials each, totalling 12 trials for each horse. The sequence of trials was determined in a pseudo-random manner, i.e. avoiding that the same condition was administered in two consecutive trials. Each horse participated in only one trial per day. Each trial lasted for about 1 min (57 ± 7 s). The duration of trials varied because the number of seconds that the experimenter took to walk 100 m away from the arena was not always the same (condition ‘Walk-away’), or because on some occasions the horse would walk back to the experimenter.
During each trial, the experimenter guided the horse on the leash into the arena by entering randomly from the left or the right side (Fig. 1). As soon as they reached the gate on the opposite side of the arena, the experimenter crossed the gate and showed the filled bucket to the horse, then she led it to the other bucket and did the same. She handled the horse to the release point (see Fig. 1), made it turn towards the centre of the arena and placed herself at its left or right side at random. She then released the horse, and it would walk to the gate in front of the chosen reward. The conditions were set as follows:
-
Forward: The experimenter was frontally oriented towards the centre of the arena, i.e. looking at a point equally distanced from the two gates.
-
Backward: The experimenter faced away from the gates, i.e. her back was oriented to the centre of the arena. Being turned is identified as a state of inattention by many species, the horse included (Krueger et al. 2011). The ‘Forward’ and ‘Backward’ conditions were similar to those used in Hare et al. (1998) and Savalli et al. (2014), but we prolonged the ‘Backward’ for up to 3 additional min (Backward_prolonged) to investigate elaborated communication. For the analyses, Backward_prolonged was considered separately from the original Backward condition.
In these two conditions, after releasing the horse, the experimenter kept her eyes open and looked at it as soon as it caught her attention, e.g. when the horse made noises, pulled the tape on the gate or looked back at her. As soon as the horse stopped these behaviours for 2 s, the experimenter looked again straight to the centre of the arena. She opened the gate to the bucket after the set time, regardless of the horse’s attention, or sooner if the horse walked back into the experimenter’s area (Fig. 1). The experimenter did not open the gate after the communicative attempts of the horses because we did not want to decide a priori which was the ‘right’ communication to have the gate opened, but instead wanted the horses to choose what to do. We therefore fixed a certain time (about 57 s) after which the experimenter opened the gate.
The other two experimental conditions were:
-
Many: Behind each of the two buckets, a helper was facing towards the arena. During the test, as soon as the horse approached the gate while led by the experimenter, the helpers showed the rewarded bucket to the horse. The helpers were asked to alternate looks between the horse and the bucket. If the horse approached a bucket and waited in front of the gate, the helper behind that gate opened it after 50 s. If the horse gazed back at the experimenter, she would open the gate instead of the helper. This was done to assure the horse that in the presence of the helpers, communication with the experimenter was still effective. This is a completely novel condition, not administered in any previous experiments.
-
Walk-away: The experimenter left the arena at the rear side and walked away as indicated in Fig. 1. This condition is similar to that used by Miklósi et al. (2000), Xitco et al. (2004), Gaunet and Deputte (2011) and Savalli et al. (2014), but in order to test whether horses were persistent in their request, we modified this condition by allowing the horse to observe the human walking away from the test area (about 100 m along a straight line) and returning after 60 s. For the analyses, we considered the walking away and the returning phases as two separate conditions: Walk-away and Returning.
Training phase
The aim of the training was to familiarize the horse to the procedure, namely to approach the bucket as soon as it was baited and to wait for the experimenter to open the gate, and to habituate to the experimental area. During the training, the experimenter guided the subject on the leash into the arena as she did during the test phase. The experimenter remained still and oriented to the centre of the arena until the horse reached the gate. Then she followed and opened the gate to provide access to the baited bucket. The waiting time from when the horse stopped in front of the gate and the experimenter opened the gate was increased stepwise from 5 to 10, 20 and finally 30 s. This was done because we did not want to inadvertently reinforce communicative behaviours of the horse. For example, the horse might have turned back as soon as it arrived at the gate (as often happened): if we just opened the gate, we would have reinforced the behaviour. Instead, fixed durations allowed for a random combination with communicative attempts.
The training occurred for each horse before testing, until it reached a bucket 5 consecutive times (once per day in consecutive days) after being released. If the horse did not approach a bucket after being released, it was prompted to move (by swinging a rope close to the horse, without touching it). If it did not move or if it showed no interest in the rewards 5 consecutive times, it was excluded from the sample.
Data collection and analyses
All trials were recorded with a video camera (Sony HDR-CX220). A selection of behaviours (see ethogram in Table 1; for video examples of the coded behaviours see Online Resource 2) was continuously registered from the video using Solomon Coder beta 12.09.04 (http://solomoncoder.com). As for the behaviour ‘Gaze to the experimenter’, we considered a head rotation of 90 degrees to maintain a conservative criterion of gazing at the recipient (see Table 1 and Fig. 2a) (although Xitco et al. 2004 adopted 45 degree criterion with dolphins). Because the orientation of ears is considered to indicate the horse’s focus of attention for conspecifics (Wathan and McComb 2014), we did not consider the mere orientation of the head towards the bucket as a gaze. Instead, we scored a gaze to the bucket only when it was accompanied by active pointing, i.e. when head direction combined with the orientation of at least one ear towards the bucket (see Table 1; Fig. 2b).
Pictures of two coded behaviours: a gaze to the experimenter and b point at the bucket. Descriptions of the behaviours are available in Table 1
The experimenter did all video analysis. A person unfamiliar to the experiment analysed 10 % of the videos to ensure validity of coding. There was good correlation between the experimenter and naïve coder for all three important variables (pointing to the bucket, head gestures and gazing at the experimenter: r > 0.75, p < 0.001).
We verified each operational criteria according to the methods explained in Table 2. Because the duration of the trials varied, absolute frequencies and durations were divided by each trial’s total duration. The data were analysed nonparametrically because it did not fulfil the criteria for parametric analysis. We compared the nonparametric conditions with Friedman tests, and if the tests turned out significant, pairwise comparisons of significant variables were performed using Wilcoxon (criteria #1 to #5). Differences in proportions were tested by means of the McNemar’s test (criterion #6). These tests were performed with GNU PSPP version 0.8.4 software (Free Software Foundation, http://www.gnu.org/software/pspp). All tests were two-tailed with an alpha level of 0.05.
To evaluate whether there was a learning effect across conditions, the first and the last trial of all the conditions, and the first and the last trial regardless of condition, were compared for 4 relevant behaviours (gaze alternation, gaze at the experimenter, point at the buck and head gestures), using a two-sample Wilcoxon signed-rank test.
Results
From 18 horses, only 4 failed and were excluded. Failure was considered to have occurred when the horse remained close to the experimenter even after release (3 subjects) or did not move into the bucket’s area (1 subject). This last horse was not interested in the food and spent all the training time close to the fence of the arena and oriented towards its conspecifics.
No learning effect was found for the variables tested between the first and the last trial of each condition (additional data are given in Online Resource 3). There was a significant difference across conditions in the relative frequency of gaze alternations (Χ 2 = 18.54, p < 0.001), gazes at the experimenter (Χ 2 = 13.49, p < 0.01), head gestures (Χ 2 = 13.37, p < 0.01) and pointing at the bucket (Χ 2 = 11.29, p < 0.01), and in the relative duration of gazes at the experimenter (Χ 2 = 14.71, p < 0.001). Horses did not walk to the gate where they came in (z = −0.14, p = 0.89), rather walked to gate where oats were provided (z = −3.00, p < 0.01), thus showing a preference for this reward.
Post hoc analysis revealed that horses alternated gazes between the target and the experimenter significantly more often in the Forward than in the Walk-away (z = −2.48, p < 0.01), the Backward (z = −2.92, p < 0.01) and the Many condition (z = −3.08, p < 0.01) (Fig. 3). In the Returning condition horses showed gaze alternations at a rate similar to the Forward condition (z = −0.41, p = 0.68), but significantly more often than in the Walk-away condition (z = −2.47, p < 0.01).
Medians of the absolute numbers of gaze alternation between the experimenter and the target. Labels on the x-axis indicate the different experimental conditions. The box of the Forward condition is grey because it represents the baseline condition. Whiskers extend to the 25 and 75 % quartile and exclude outliers; with *P ≤ 0.05, **P ≤ 0.01
Gazes at the experimenter were significantly more frequent in the Forward than the Backward (z = −2.45, p < 0.01) and the Many (z = −3.23, p < 0.001) but not the Walk-away condition (z = −1.35, p = 0.18). The average duration of gazes at the experimenter during the Walk-away condition was significantly longer than the Forward (z = −2.57, p < 0.01) and the Many condition (z = −2.79, p < 0.01), but similar to the Backward condition (z = −1.71, p = 0.09).
Horses pointed more often at the bucket or produced more head gestures in the Forward condition compared to either the Backward (point at the bucket: z = −3.30, p < 0.001; head gesture: z = −2.62, p < 0.01) or the Walk-away condition (point at the bucket: z = −2.20, p < 0.02; head gesture: z = −3.30, p < 0.001). Instead, the rate of pointing or head gesturing was similar between the Forward and the Many condition (point at the bucket: z = −1.64, p = 0.11; head gesture: z = −0.50, p = 0.59) (Fig. 4).
Medians of the absolute numbers of attention-getting behaviours (gazes at the experimenter, head gestures, point at the bucket). The legend indicates the patterns associated with each experimental condition. Whiskers extend to the 25 and 75 % quartile and exclude outliers; with *P ≤ 0.05, **P ≤ 0.01
Only during the Forward and the Backward conditions did some horses walk away from the bucket’s area in the direction of the experimenter. In the Forward condition, this was done by four out of 14 horses (28.6 %), after a latency of 35 ± 16 s. In the Backward condition, seven out of 14 horses (50 %) walked back to the experimenter, after a latency of 40 ± 10 s. During the Backward condition, five out of seven horses established physical contact with her. Four horses walked back to the experimenter in both the Forward and Backward condition, but only three during the Backward condition alone (Χ 2 = 1.33, df = 1, p = 0.25, McNemar’s test). During the Backward_prolonged condition, ten out of 14 (71.4 %) horses walked back to the experimenter, after a latency of 82 ± 31 s. Nine of these 10 horses established physical contact with her. With respect to the Forward condition, seven more horses walked back to the experimenter’s area during the Backward_prolonged condition (Χ 2 = 5.14, df = 1, p < 0.05, McNemar’s test).
Discussion
The aim of this study was to test the ability of domestic horses to communicate referentially with humans about a desired, but inaccessible food. The question was whether horses would try to achieve the goal by manipulating the attention of a human experimenter, which requires an understanding of the human’s different attentional states. By applying well-established criteria for referential communication, we found evidence in support of referential communication. Horses used gaze alternation to coordinate attention with a human recipient in the direction of the desired target (operational criterion #1): the frequency of this behaviour was higher when the human was frontally oriented than when it was backward oriented, absent or when more appropriate helpers were present. Therefore, horses alternated their gaze depending on the presence (criterion #3) and attentional state of the human (criterion #4).
One may argue that gaze alternation is the outcome of associative learning and is not related to intentional communication. The horses involved in this study had lived their whole lives up to this point with humans and may have incidentally learned that turning the head back towards a human increases the chances of obtaining what they desire, without understanding the meaning of the gesture. This did not happen during our study, as evidenced by the results exploring possible learning effects. Even if the horses had learned this behaviour prior to the study, our data confirm that they were able to use this behaviour in a flexible way suggesting they comprehend its meaning. Horses behaved differently depending on the attentional state of the human experimenter: when her attention was elsewhere (‘Backward’ and ‘Walk-away’ conditions), the rate of gaze alternation decreased, suggesting that horses understood that turning the head back was a visual signal and thus needed the visual attention of the human experimenter. In a recent study, Bourjade et al. (2015) found that a similar rate of gaze alternation in trained and untrained baboons, which demonstrates that this behaviour was spontaneous and not incidentally learned from humans.
Still, one may argue that horses learned that their head needs to be turned in combination with a frontal position of the human, without really understanding the attentional state of the human. The use of attention-getting behaviours by horses may rule out this explanation (criterion #2). We took into account the direction of the horses’ ears together with head orientation to infer the attention towards the target, or the use of head gestures like head nods and shakes as attention getters. According to our results, when the experimenter was backward oriented and when she was walking away from the arena, not only the rate of gaze alternation, but also that of head gestures and pointing dropped to chance level. That horses may have incidentally learned that both gaze alternation and attention-getting behaviours had to be used when the human was frontally oriented seems unlikely. During the condition ‘Many’, one helper was behind each bucket. These helpers, located closer to the bucket and with their attention focused alternatively at it and at the horse, would be perceived as more appropriate recipients than the more distant and not focused experimenter. In this condition, horses pointed at the bucket and gestured with the head, but did not use gaze alternation. This suggests that horses understood that the outcome of gaze alternation with the experimenter was for her to open the gate, whereas in this case the helper was closer to a chain of actions that might bring them to the baited bucket. This result furthermore suggests that horses comprehended the use of attention getters, which were needed because the helpers were inactive.
Horses pointed at the bucket and executed head gestures with the same frequency in both the ‘Forward’ and the ‘Many’ conditions, where helpers and the experimenter were frontally oriented. This supports the hypothesis that these two behaviours are used in conjunction with eye contact to prompt action of the recipient and/or to indicate the position of the desired target. This interpretation of head gestures in terms of communicative signals stands in contrast to the widespread view in the equestrian community who consider head gestures as mere signs of arousal or distress.
Unexpectedly, the frequency of gazes (not of gaze alternation) at the experimenter walking away was similar to the baseline condition (‘Forward’). This is the opposite of our prediction, but a careful consideration of the features of these gazes led us consider them to have a checking rather than a communicative function. In fact, horses did not look—as they did in the ‘Forward’ condition—at the bucket after gazing at the experimenter. Whereas gaze alternation, if performed together with attention-seeking behaviours and in the presence of a recipient, can be considered a referential gesture, gazes alone do not necessarily have communicative means. These gazes not only were performed without alternation towards the bucket, but also were not accompanied by other attention-getting behaviours, such as head gestures. The results on the rate of gazing are similar to those obtained in dolphins (Xitco et al. 2004) and in dogs (Savalli et al. 2014). In particular, if food was taken away from the testing room, dogs gazed at the exit door at the same rate as they did at the food when it was present. However, in the condition when the food was absent, dogs did not alternate their gaze between the exit door and the owner as frequently as they did between the food and the owner. Savalli and co-authors (2014) interpreted the rate of gazes at the exit door as a ‘waiting’ reaction, whereas gazes used during alternation were given a communicative meaning. We agree with them that some behaviours that are used as attention getters can change their function with conditions (see also Gaunet and Deputte 2011), so that when the possible helper is leaving, gazing could serve as checking/monitoring behaviour. Further, in our experiment gazes at the experimenter walking away were on average longer than during the two conditions in which the experimenter was visually attentive (‘Many’ and ‘Forward’). Longer gazes were interpreted as a violation of expectation in horses in both Proops et al. (2009) and Sankey et al. (2011), where horses gazed longer in the direction of a mismatched cue. The long gazes given by our horses may have been triggered likewise by violation of expectation if the horse was expecting the experimenter to remain in the arena as in the other conditions.
The last two criteria for intentional communication are persistence (#5) and elaboration (#6). Horses were persistent in their communicative effort: they diminished the frequency of gaze alternation while observing the experimenter walking away from the test area, but resumed alternating gazes between the experimenter and the target when the experimenter returned (‘Returning’ condition). The horses also elaborated their communicative strategy when first attempts failed, by entering the recipient’s visual field or getting closer to her during ‘Forward’ and ‘Backward’ conditions, and switching their communicative channel from visual to tactile. Walking back to the experimenter and touching her is a behaviour known from earlier reports about horses in a human-given cue experiment (Proops and McComb 2010) and dogs during tests for referential communication (Savalli et al. 2014). Whereas we did not find significant differences between the number of horses that approached the experimenter in the two conditions, we found that most of the horses elaborated their communication during the additional time given when we compared the ‘Forward’ and the ‘Backward_prolonged’ condition (characterized by longer trials). It is not clear whether this difference arose because horses understood the different attentional state of the recipient or simply because trials were longer. Horses understand the asymmetry of the human’s front and back side and often prefer facing a human when monitoring him (Sankey et al. 2011), so it is possible that they walked back to the experimenter more in the Backward_prolonged condition for this reason.
One hypothesis for the origin of referential gestures in non-human primates is that they arise from a ritualization of actions during interactions at the individual level (Tomasello et al. 1993, Tomasello 2006). Similarly, horses may individually learn that a gaze directed to a conspecific is reinforced with obtaining its attention and that a sequence of gazes to a conspecific followed by gazes to the target points the conspecific’s attention to the target itself. We therefore consider intentional gaze alternation in horses to be a behaviour learned within their social environment rather than an innate behaviour. In species with protruded faces, such as horses, head orientation has always been considered a cue salient enough to indicate an interest towards a specific direction or item, without calling eye gaze into action (Emery 2000). This consideration may have led to an underestimation of horse’s communicative skills, supported by the difficulty to detect gaze alternation when performed between conspecifics: horse’s lateral vision covers about a 340° field view (Murphy et al. 2009), and a slight turn of the head may be sufficient to catch the attention of, for example, a conspecific in the rear.
So why in our experiment did horses use large-scale head movement to communicate with the human experimenter? Xitco et al. (2004) noted that dolphins, which also have lateral eyes, produced large-scale head movements in the direction of the experimenter that were not needed by these animals for their own perceptual benefit, because they could establish eye contact with an experimenter in the rear even with a slight turn of their head. In domestic settings, horses are completely dependent on humans and even accept the help of humans to complete a task (Lesimple et al. 2012). During its individual experience with humans, a horse may learn that we respond to eye contact when associated with head orientation and may adapt its communicative signals accordingly. In other words, the horse may learn that when it does not turn its head to the human recipient, its communicative efforts are not reinforced by obtaining the attention of the human. If so, the fact that horses turned their head back when alternating gaze with the human is another piece of evidence supporting the communicative meaning of this behaviour. If interspecific gaze alternation is instead a product domestication, it is an open question worthy of being investigated. In fact, a comparison between prehistoric and modern horse genome recently revealed that domestication affected some genes related to cognitive functions, such as social cognition (Schubert et al. 2014). In dogs, for example, it seems that domestication played an important role in the shaping of socio-cognitive abilities and the establishment of a special sensitivity towards human’s communicative behaviour such as ‘pointing’ or ‘showing’ (for a recent review, see Kaminski and Marshall-Pescini 2014).
Conclusion
Several studies provide evidence for the existence of advanced cognitive abilities in horses, such as categorization (Hanggi 1999), cross-modal individual recognition (Proops et al. 2009), social learning (Krueger 2007; Krueger et al. 2014) and numerical discrimination (Petrazzini 2014). Our results add to this body of evidence by suggesting that horses act like active informers and are able to recognize recipients as communicative agents. In our experiment, they tended to influence the behaviour of a human experimenter using their eyes, providing evidence they understand that intentions can be communicated between individuals using gaze. They also activated mechanisms promoting shared attention depending on that individual’s attentional state. We therefore suggest adding horses to the species capable of flexible and intentional use of communicative signals, along with several primate species, dogs, corvids, dolphins and reef fish. It remains an open question whether intentional communication and referential signalling require advanced cognitive processes like perspective taking and strategic thinking (Pika and Mitani 2006; Vail et al. 2013). After dogs, horses would be the second domesticated species for which this ability to communicate with humans has been shown.
References
Bates E, Camaioni L, Volterra V (1975) The acquisition of performatives prior to speech. Merrill-Palmer Q Behav Dev 21(3):205–226
Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V (1979) The emergence of symbols: cognition and communication in infancy. Academic Press, New York
Birke L (2007) Learning to speak horse: the culture of natural horsemanship. Soc Anim 15(3):217–239
Bourjade M, Meguerditchian A, Maille A, Gaunet F, Vauclair J (2014) Olive baboons, Papio anubis, adjust their visual and auditory intentional gestures to the visual attention of others. Anim Behav 87:121–128
Bourjade M, Canteloup C, Meguerditchian A, Vauclair J, Gaunet F (2015) Training experience in gestures affects the display of social gaze in baboons’ communication with a human. Anim Cogn 18(1):239–250
Call JE, Tomasello ME (2007) The gestural communication of apes and monkeys. Taylor & Francis Group/Lawrence Erlbaum Associates, New York
Canteloup C, Bovet D, Meunier H (2015) Intentional gestural communication and discrimination of human attentional states in rhesus macaques (Macaca mulatta). Anim cogn 18:875–883
Cartmill EA, Byrne RW (2007) Orangutans modify their gestural signaling according to their audience’s comprehension. Curr Biol 17:1345–1348
Emery NJ (2000) The eyes have it: the neuroethology, function and evolution of social gaze. Neurosc Biobehav R 24(6):581–604
Gaunet F (2010) How do guide dogs and pet dogs (Canis familiaris) ask their owners for their toy and for playing? Anim Cogn 13:311–323
Gaunet F, Deputte BL (2011) Functionally referential and intentional communication in the domestic dog: effects of spatial and social contexts. Anim Cogn 14:849–860
Hanggi EB (1999) Categorization learning in horses (Equus caballus). J Comp Psychol 1113:243–252
Hare B, Call J, Tomasello M (1998) Communication of food location between human and dog (Canis familiaris). Evolution of communication 2(1):137–159
Hobaiter C, Byrne RW (2011) The gestural repertoire of the wild chimpanzee. Anim Cogn 14(5):745–767
Kaminski J, Marshall-Pescini S (2014) The social dog: behaviour and cognition. Academic Press/Elsevier, San Diego (CA)
Krueger K (2007) Behaviour of horses in the “round pen technique”. Appl Anim Behav Sci 104(1):162–170
Krueger K, Flauger B, Farmer K, Maros K (2011) Horses (Equus caballus) use human local enhancement cues and adjust to human attention. Anim Cogn 14:187–201
Krueger K, Farmer K, Heinze J (2014) The effects of age, rank and neophobia on social learning in horses. Anim Cogn 17(3):645–655
Leavens DA (2004) Manual deixis in apes and humans. Interact Stud 5:387–408
Leavens DA, Russell JL, Hopkins WD (2005) Intentionality as measured in the persistence and elaboration of communication by chimpanzees (Pan troglodytes). Child Dev 76(1):291–306
Lesimple C, Sankey C, Richard MA, Hausberger M (2012) Do horses expect humans to solve their problems? Front Psyc 3:306
Maros K, Gácsi M, Miklósi Á (2008) Comprehension of human pointing gestures in horses (Equus caballus). Anim Cogn 11:457–466
Miklósi Á, Polgárdi R, Topál J, Csányi V (2000) Intentional behaviour in dog-human communication: an experimental analysis of showing behaviour in the dog. Anim Cogn 3:159–166
Murphy J, Hall C, Arkins S (2009) What horses and humans see: a comparative review. Intl J Zool 2009:721798. doi:10.1155/2009/721798
Petrazzini MEM (2014) Trained quantity abilities in horses (Equus caballus): a Preliminary Investigation. Behav Sci 4(3):213–225
Pika S (2012) The case of referential gestural signaling. Where next? Commun Integr Biol 5(6):578–582
Pika S, Bugnyar T (2011) The use of referential gestures in ravens (Corvus corax) in the wild. Nat Commun 2:56
Pika S, Mitani J (2006) Referential gestural communication in wild chimpanzees (Pan troglodytes). Curr Biol 16(6):R191–R192
Proops L, McComb K (2010) Attributing attention: the use of human-given cues by domestic horses (Equus caballus). Anim Cogn 13:197–205
Proops L, McComb K, Reby D (2009) Cross-modal individual recognition in domestic horses (Equus caballus). Proc Natl Acad Sci USA 106:947–951
Roberts AI, Vick SJ, Roberts SGB, Menzel CR (2014) Chimpanzees modify intentional gestures to coordinate a search for hidden food. Nat Commun 5:3088
Rubenstein DI, Wrangham RW (1986) Ecological aspects of social evolution: birds and mammals, 1st edn. Princeton University Press, Princeton (NJ)
Sankey C, Henry S, André N, Richard-Yris M, Hausberger M (2011) Do horses have a concept of person? Plos One 6:e18331
Savalli C, Ades C, Gaunet F (2014) Are dogs able to communicate with their owners about a desirable food in a referential and intentional way? Plos One 9(9):e108003
Schubert M, Jónsson H, Chang D, Der Sarkissian C, Ermini L, Ginolhac A et al (2014) Prehistoric genomes reveal the genetic foundation and cost of horse domestication. P Natl Acad Sci 111(52):E5661–E5669
Schwab C, Huber L (2006) Obey or not obey? Dogs (Canis familiaris) behave differently in response to attentional states of their owners. J Comp Psychol 120:169–175
Tomasello M (1995) Joint attention as social cognition. In: Moore C, Dunham PJ (eds) Joint attention: its origin and role in development. Erlbaum, Hillsdale (NJ), pp 103–130
Tomasello M (2006) Why don’t apes point? In: Enfield NJ, Levinson SC (eds) Roots of human sociality: culture, cognition and interaction. Berg, Oxford, pp 506–524
Tomasello M, Kruger A, Ratner H (1993) Cultural learning. Behav Brain Sci 16:495–552
Tomasello M, Carpenter M, Call J, Behne T, Moll H (2005) Understanding and sharing intentions: the origins of cultural cognition. Behav Brain Sci 28(05):675–691
Vail AL, Manica A, Bshary R (2013) Referential gestures in fish collaborative hunting. Nat Commun 4:1765
Waring G (2003) Horse behavior, 2nd edn. Noyes Publications/William Andrew Publishing, Norwich (NY)
Warneken F, Chen F, Tomasello M (2006) Cooperative activities in young children and chimpanzees. Child Dev 77:640–663
Wathan J, McComb K (2014) The eyes and ears are visual indicators of attention in domestic horses. Curr Biol 24(15):R677–R679
Xitco MJ, Gory JD, Ii SAK (2004) Dolphin pointing is linked to the attentional behavior of a receiver. Anim Cogn 7:231–238
Acknowledgments
We wish to thank EquiLuna A.S.D. for granting us the permission to use their facilities and involve in this research the horses they host. In particular, we thank Laura Ascione, Claudio Saba, Andrea Montagnani and Lesley Moore for the help provided, and all those volunteered in this research. Our warmest thanks go to Christian Postiglione, our camera operator and technical assistant. We are grateful to Christian Postiglione, Elisabetta Visalberghi, Ian Couzin and Corsin Müller for helpful discussions and statistical consultation, to Debbie Kelly and Amelia Wein for improving the English and to Alan McElligott for his invaluable help at the very beginning of this project. This research was supported exclusively by private funding.
Funding information
Authors declare not to have any source of funding.
Author’s contribution
RM conceived of the study, designed the study, collected field data, participated in data analyses and in statistical analyses and contributed to draft the manuscript; LH participated in data analyses and in statistical analyses and contributed to draft the manuscript. Both authors gave final approval for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10071_2016_987_MOESM1_ESM.jpg
Online Resource 1. Pictures of the different phases of the trials. The horse is handled by the experimenter into the arena from one of two entrances (1). It is shown a baited bucket at the opposite side of the arena (2) and taken back at the entrance (3), where it is shown the other baited bucket (4). The horse is then handled at the release point (5) and released (6). During the experimental condition ‘Forward’, the experimenter remains in the same position at the release point, whereas she is faced away from the arena in the condition ‘Backward’ (7a), and walks away from the arena in the condition ‘Walk-away’ (7b, the white circle shows the experimenter). During the condition ‘Many’, two helpers show the horse the baited buck, and remain behind the bucket until the end of the trial (7c). (JPEG 777 kb)
Online Resource 2. The video shows samples of the experimental conditions and coded behaviours. The target (bucket of food) is on the other side of the visible fence. The first two samples show gaze alternation between the horse and the experimenter during the condition ‘Forward’ (the experimenter was frontally oriented towards the center of the arena). In both samples, the experimenter stayed about in the direction of the video camera. The third sample shows a horse pointing to the bucket while at the same time performing a head gesture (very quick movement of the head along the sagittal plane). The fourth sample shows a horse elaborating its communication from visual to tactile during the condition ‘Forward’: while close to the target, she first pointed at it and used some head gestures, then walked back to the experimenter, touched her and again to the target. When she stopped close to it, she alternated her gaze between the walking experimenter and the target. The fifth sample shows another case of elaboration of communication, this time during the condition ‘Backward’ (the experimenter faced away from the gates, i.e. her back was oriented to the center of the arena). The horse walked back to the experimenter and touched her. (MP4 70,313 kb)

10071_2016_987_MOESM3_ESM.jpg
Online Resource 3. The first and the third trial of each condition, and the first and the last trial regardless of condition, were compared for each coded behaviour to test for learning during the experiment. In the figure, the medians of the absolute numbers of the coded behaviours are shown, with whiskers extending to the 25 % and 75 % quartile. The abbreviations on the x-axis refer to the different experimental conditions: M = Many, WA = Walk-away, F = Forward, B = Backward; the number next to each condition refers to the trial (1 = first, 3 = third). 1st and Last refer to the first and last trial regardless of condition. Under each tested pair, the z and p values of the two-sample Wilcoxon Signed-rank test. (JPEG 315 kb)
Rights and permissions
About this article
Cite this article
Malavasi, R., Huber, L. Evidence of heterospecific referential communication from domestic horses (Equus caballus) to humans. Anim Cogn 19, 899–909 (2016). https://doi.org/10.1007/s10071-016-0987-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-016-0987-0