Abstract
The tendency of fish to perceive the Ebbinghaus illusion was investigated. Redtail splitfins (Xenotoca eiseni, family Goodeidae) were trained to discriminate between two disks of different sizes. Then, fish were presented with two disks of the same size surrounded by disks of large or small size (inducers) arranged to produce the impression (to a human observer) of two disks of different sizes (in the Ebbinghaus illusion, a central disk surrounded by small inducers appears bigger than an identical one surrounded by large inducers). Fish chose the stimulus that, on the basis of a perception of the Ebbinghaus illusion, appeared deceptively larger or smaller, consistent with the condition of training. These results demonstrate that redtail splitfins tend to perceive this particular illusion. The results are discussed with reference to other related illusions that have been recently observed to be experienced by fish (such as the Navon effect), and with regard to their possible evolutionary implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Visual illusions are instances of systematic discrepancy between the physical properties of the external world and their representation in the visual system. Thus, visual illusions offer insight into the brain mechanisms that integrate the visual stimulation in a coherent percept, through its representation in the visual system (Mascalzoni and Regolin 2011; Vallortigara 2004, 2006, 2009, 2012; Vallortigara et al. 2010; Wade 2005, 2010). The comparative study of visual illusions provides us with information on the evolution of visual systems and the principles of perceptual organization. In geometric size illusions, the appearance of the properties of a target stimulus (e.g., length, width or diameter) is distorted by the surrounding context, providing an important tool for the study of perceptual integration of local elements into the global context created by the surrounding visual elements. Geometrical illusions such as the Ponzo, the Müller-Lyer and the horizontal–vertical illusion, seem to be perceived by species as distant as apes (chimpanzees, Fujita 1997), monkeys (e.g., Barbet and Fagot 2002; Bayne and Davis 1983; Suganuma et al. 2007; Tudusciuc and Nieder 2010; see also Fujita 1996), ungulates (e.g., horses, Timney and Keil 1996) and birds (domestic chickens, Winslow 1933; Rosa Salva et al. 2013; ring doves, Warden and Baar 1929; pigeons, Fujita et al. 1991, 1993; Nakamura et al. 2006, 2009; and gray parrots, Pepperberg et al. 2008).
Despite the paucity of research on geometric illusions in fish, they are a promising model for the comparative investigation of such phenomena (see Agrillo et al. 2014; Rosa Salva et al. 2014 for reviews). In fact, redtail splitfin, goldfish and two species of reef fish have been found to be susceptible to perceptual phenomena such as amodal completion and perception of illusory contours (Darmaillacq et al. 2011; Sovrano and Bisazza 2008, 2009; Wyzisk and Neumeyer 2007; see Fuss et al. 2014 for evidence in elasmobranchs; see Regolin and Vallortigara 1995; Regolin et al. 2004; Forkman and Vallortigara 1999 for similar evidence in other taxonomic groups). This is particularly remarkable in light of the phylogenetic distance between these three fish species (Steinke et al. 2006) and from other vertebrates (Kumar and Hedges 1998).
Studies on visual illusions in fish species are very informative about the phylogenesis of these traits. The susceptibility of this taxonomic group to amodal completion and illusory contours suggests a conserved trait rather than a case of convergent evolution in fish, birds and mammals. Within fish, illusory contours are perceived by teleosts as distant as Ostariophysi (redtail splitfin fish, Xenotoca eiseni) and Acanthopterygii (goldfish, Carassius auratus) (Sovrano and Bisazza 2009; Wyzisk and Neumeyer 2007). Similarly, amodal completion is observed in two species of Acanthopterygii (Variola louti and Scarus niger), in addition to the redtail splitfin fish (Darmaillacq et al. 2011; Sovrano and Bisazza 2008). Recently, it has been found that even cartilaginous fish (bamboo sharks, Chiloscyllium griseum) are susceptible to amodal completion and illusory contours (Fuss et al. 2014). Surprisingly, redtail splitfins also recognized illusory shapes created by phase shifts or by interruption of diagonal lines (Sovrano and Bisazza 2009), whereas in another study, goldfish did not recognize phase-shifted illusory shapes (Wyzisk and Neumeyer 2007). However, this may be due to a methodological problem in the stimuli of Wyzisk and Neumeyer (2007), which consisted of very thin lines, reducing the strength of the illusory perception.
Fewer attempts have been made to test fishes’ tendency to perceive geometrical optical illusion. Two existing studies have failed to demonstrate susceptibility to the Müller-Lyer illusion in either bamboo sharks (Fuss et al. 2014) or goldfish (Wyzisk 2005). Contradictory results have been obtained in animals with regard to the perception of the Ebbinghaus illusion (or Titchener circles): In this display, a central circle surrounded by large circular inducers is perceived as smaller than an identical circle surrounded by small inducers (Aglioti et al. 1995; Choplin and Medin 1999; Coren and Enns 1993; Ebbinghaus 1902; de Grave et al. 2005; Girgus et al. 1972; Massaro and Anderson 1971; Weintraub 1979). It is one of the strongest and most robust geometric size illusions spontaneously reported by human observers (de Fockert et al. 2007), apparent already in pre-verbal infants (Yamazaki et al. 2010; but see Happé 1996; Kaldy and Kovacs 2003; Phillips et al. 2004). The Ebbinghaus illusion has initially been studied in baboons (Parron and Fagot 2007) and pigeons (Nakamura et al. 2008), revealing a different perception of the Titchener circles than that in the human species (see also Murayama et al. 2012 for a preliminary study on a single bottlenose dolphin). While baboons were not affected by the illusory display, estimating the size of the central target accurately, pigeons responded to the Ebbinghaus illusion in an opposite way with respect to humans, overestimating the size of a target surrounded by large inducers and underestimating that of a target encircled by small inducers. This suggested an assimilation effect in the study of Nakamura and colleagues, with the size of the central circle assimilated to that of the inducers. These results led to theories that the perceptual mechanisms at the basis of our perception of the Ebbinghaus illusion were evolved in primates (Parron and Fagot 2007). In particular, the neural substrate determining the perception of the Ebbinghaus illusion is thought to be located in the human neocortex, where the two independent neural pathways, the dorsal and the ventral stream, are responsible for visual awareness and for action control (Goodale and Milner 1992). This could explain why the Ebbinghaus illusion is reduced when tested through motor tasks (Aglioti et al. 1995; Danckert et al. 2002). In non-mammalian species, the neural circuits composing the visual pathways are differently organized than that in mammals (e.g., birds, Shimizu 2004; Shimizu and Bowers 1999; but see Reiner et al. 2005). These differences have been claimed to be the cause of the different processing of the Ebbinghaus illusory configuration in mammalian and non-mammalian species (Nakamura et al. 2008; see Rosa Salva et al. 2013 for a discussion focused on avian species).
In humans, the Ebbinghaus illusion is believed to reflect the action of perceptual grouping mechanisms, i.e., the fact that human subjects perceive objects in relation to the surrounding context. This is supported by the negative correlation of the target-inducers’ distance with the magnitude of the illusion (Roberts et al. 2005). Moreover, erasing the distant portions of larger inducers reverses the effect of the illusion (Oyama 1960; Weintraub 1979). This indicates that when humans perceive only the immediate surroundings of the target, they also perceive the Ebbinghaus illusion as an assimilation illusion. It has thus been proposed that human’s globally oriented perceptual tendencies (Navon 1977) determine their perception of the Ebbinghaus illusion as a “contrast illusion,” in which the size of the central target is contrasted with that of the inducers. Conversely, species characterized by a more locally oriented perceptual style would be immune to the illusion or perceive it in the opposite direction, assimilating the size of the central circle to that of the inducers, since they would focus only on the target and the parts of the inducers that are located in its close proximity (Nakamura et al. 2008, 2014). Previous studies indicate that some non-human species, including pigeons and baboons, could have a more locally oriented perception than humans (Cavoto and Cook 2001; Cerella 1980; Chiandetti et al. 2014; Deruelle and Fagot 1998; Fagot and Deruelle 1997; Ushitani et al. 2001).
However, it would be an extreme oversimplification to draw a clear dichotomy between globally oriented humans and other locally oriented species. In both humans and non-human animals, the level of processing adopted in a given moment is flexibly influenced by context and task demands (Cook 1992; Cook et al. 1996; Fremouw et al. 1998; Kimchi 1992; Wasserman et al. 1993). Of particular relevance for the present paper, redtail splitfin fish (X. eiseni) have been recently shown to prefer processing of hierarchical stimuli at the global rather than that at the local level (Truppa et al. 2010). The organization of the visual system of redtail splitfins is certainly not more human-like than that of pigeons or baboons. Thus, we can dismiss the argumentation according to which a human-like visual system is needed for globally oriented perception. More likely, the test procedures adopted by Truppa et al. (2010) favored a globally oriented perception, contrary to most previous researches. In this study, fish learned to use hierarchical patterns as a cue for orientation in a square tank, in order to locate the exit from it and to reach a more comfortable environment.
The view that human and non-human animals may or may not show globally oriented perception depending on contextual variables (e.g., Fremouw et al. 2002; Kinchla and Wolf 1979; Kinchla et al. 1983; Pomerantz 1983; Robertson et al. 1993) is strongly supported by two recent studies on domestic chickens that used two radically different procedures, obtaining opposite results within the same species (Rosa Salva et al. 2013; Nakamura et al. 2014). In the study of Rosa Salva et al. (2013), few-day-old chicks of the hybro strain learnt during rearing, according to an observational-learning paradigm, to find food in proximity to either a big or a small circle. When tested with illusory displays, subjects that were used to finding food near the small circle approached the configuration with big inducers, whereas subjects habituated to food near the big circle approached the configuration with small inducers, indicating a contrast illusion analogous to that reported by humans. On the other hand, Nakamura et al. (2014) trained three adult bantam chickens to classify screen-presented circles as “small” or “large” by pecking appropriate keys in an operant conditioning chamber. This is the same procedure previously used by Nakamura et al. (2008) with pigeons, and in this case too, it resulted in the illusion being apparently perceived in the opposite way than in humans. These discordant results could theoretically be due to the different age and breed of the subjects in the two studies, or to the limited sample size tested by Nakamura and colleagues. However, a much more likely explanation involves the use of training and testing paradigms based on operant conditioning of pecking responses, causing an inclination to pay attention to local details of the configuration. Given that pecking is the main manipulative response of birds, it is tempting to draw an analogy with the human literature showing reduction or absence of the illusion when associated with grasping (Aglioti et al. 1995; Danckert et al. 2002). More importantly, the procedure adopted by Nakamura et al. (2008, 2014) required the subjects to peck at the target central circle before emitting the choice response. This is likely to promote focalized attention toward the target and its immediate surroundings (see also Chiandetti et al. 2014, for evidence of a locally oriented perceptual style in chicks trained on touch screen-based pecking tasks).
Thus, the overall evidence suggests that the perceptual processes leading to the perception of the Titchener circles as a contrast illusions might be present in both mammals and birds and that the previously apparently discordant results are just a by-product of the testing procedure employed. This could be due to convergent evolution in the two classes of tetrapoda, or it could reflect the presence of conserved mechanisms inherited by a common ancestor in different taxonomic groups of vertebrates, including fish species (whose divergence may date back to approximately 450 million years ago, Kumar and Hedges 1998). In order to test this hypothesis on the evolutionary origins of the neural substrate of the Ebbinghaus illusion, we thus proceeded to investigate its perception in redtail splitfin fish, according to a procedure analogous to that of Truppa et al. (2010).
Methods
Subjects
Subjects were eight male mature fish (ranging 3–5 cm in length) of the species X. eiseni from a stock maintained in our laboratory within vegetation rich (Ceratophyllum sp.) large tanks (55–120 l) provided with artificial illumination, 14 h per day. Water temperature was maintained at 25 ± 2 °C. Fish were daily fed dry fish food (Sera GVG-Mix®).
Apparatus
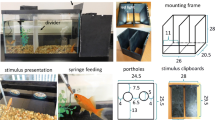
The apparatus was located in a darkened room and was identical to the apparatus used in Sovrano and Bisazza (2008, 2009; see Fig. 1). It consisted of a square tank (15 cm long, 15 cm wide and 15 cm high), with uniform white walls and lit centrally with a 75-W light bulb. The testing tank was inserted in a larger tank (60 cm × 36 cm × 25 cm) so as to create an external surrounding region with vegetation and food, where the test fish was located together with other two conspecifics (females were not tested) that provided motivation for social reinstatement.
Schematic representation and measures of the apparatus used in the experiment: a bird’s-eye view of the inner and outer tanks, with an experimental subject portrayed in the center of the inner tank; the dotted lines indicate the tunnels connecting the inner and the outer tank (one of which was blocked preventing fish passage), b front view of one of the tunnel’s doors as seen from the subjects’ perspective within the inner tank; one of the test stimuli is visible below the door
In two diagonally opposite corners of the square apparatus, a small tunnel (2.5 cm in length, 2 cm in size, 3 cm in height, located 4.5 cm from the floor of the tank) of white plastic material was inserted, allowing the fish to pass through it to rejoin conspecifics in the surrounding region in the outer tank (see Fig. 1). At the end of each tunnel was a door (2.5 × 3.5 cm) made of a sheet of an opaque plastic material on top and of a transparent very flexible plastic material on the bottom. The two doors were visually identical, but only one door could be opened, the other being blocked with an external plastic transparent panel. Fish could thus open the correct door to rejoin conspecifics by pressing on it with the snout; choices for each door were clearly visible from video recordings, because of characteristic movements of the tail and the most caudal part of the body of the fish that remained visible outside the tunnel.
The stimuli used for visual discrimination learning and for the illusion test were located below each door (see Fig. 2). The stimuli were made of a plastic material designed to resist the aquatic environment. Transparent screens (9 × 4 cm) were located 2.3 cm in front of the stimuli, so that the fish saw the stimuli at a certain distance; this was done because visual exploration of the stimuli in close proximity could prevent Gestalt-like perception of the overall pattern. During training, the stimuli to be discriminated consisted of a large (9 mm of diameter), a medium (6.5 mm) and a small (4.5 mm in diameter) orange disk, situated below a line of linearly or circularly arranged gray inducer disks of alternating sizes (the bigger disks were 4 mm in the circular condition and 4.5 mm in the linear condition; the smaller disks were 1 mm in diameter in both conditions). During the testing of the Ebbinghaus illusion, the stimuli consisted of orange disks (4.5 mm in diameter) that were bordered by large or small gray disks (9 and 3 mm in diameter, respectively). Across conditions, the gray disks (inducers) varied in number and in the distance to the target disk (see Fig. 3).
Fish were trained to discriminate between two orange disks of different size. After the end of training, fish were presented with two stimuli in which the size of the two orange disks were identical, but the context in which they were inserted (size, number and spacing of the gray inducers) was changed in such a way to produce the impression (to a human observer) of two disks of different dimensions.
Procedure
Before testing, for at least one week, fish underwent a “shaping procedure” in their home tank (30 × 40 × 20 cm). We used a partition that divided their home tank in two halves, one (‘enriched’) with food and vegetation and the other (‘unenriched’) without any food and vegetation. Two tunnels, with moveable doors identical to those subsequently used during testing, were positioned on the partition, allowing the fish to move between the two compartments. This made the fish accustomed to the use of the moveable doors before testing. Below the two tunnels, we placed the stimuli (disks of different dimensions, large for one group of fish and small for another group) that were then used during the subsequent training. This accustomed the fish to the stimuli and facilitated training. The stimuli used were a large or a small orange disk, surrounded by a row, linear or curved, of alternating large and small gray disks (see Fig. 2). Note that during this phase, both moveable doors were associated with the figures later reinforced during training and both allowed the fish to move from one compartment to the other. Selective reinforcement by blocking one door was done only during the subsequent training phase, in which the two stimuli of different size were presented together. Fish previously exposed to the large orange disk were then rewarded for approaching the large orange disk, while fish exposed to the small orange disk were rewarded on the small one.
The experiment comprised two parts: training and test. During training, the fish were required to discriminate between the two orange disks of different dimension. The animals were first trained on an easy discrimination (large vs. small disk), followed by a difficult discrimination (medium vs. small disk) (Fig. 2). Training on the difficult discrimination started only after the completion of training on the easy one. Two pairs of stimuli were employed for training on the easy discrimination (pair 1, Fig. 2a vs. 2c; pair 2, Fig. 2d vs. 2f) and two other pairs for training on the difficult discrimination (pair 3, Fig. 2b vs. 2c; pair 4 Fig. 2e vs. 2f).
Four animals were trained with the large disk as positive (reinforced); four animals with the small disk as positive. Fish were given daily sessions of ten trials. During each session, the two pairs of stimuli employed for the currently trained discrimination were alternated in blocks of two consecutive trials, except that for the last two trials, in which the two different pairs were alternated. For example, for the easy discrimination, the training schedule could be as follows: pair 1, pair 1, pair 2, pair 2, pair 1, pair 1, pair 2, pair 2, pair 1, pair 2. The precise ordering was randomized between sections.
Training on the discrimination continued until fish reached the learning criterion (70 % of correct choices in two consecutive sessions).
In each trial, the fish was placed in the inner tank by gently inserting it into a transparent plastic cylinder placed in the center. After 20 s, the cylinder was removed. In each trial, the number of choices of the two doors was video-recorded, until the fish was able to exit and rejoin conspecifics (the maximum time was 15 min). During training, a correction method (Sutherland and Mackintosh 1971) was used: if the fish made a wrong choice, it was allowed to change it until it was able to exit or until the overall time allowed for the trial elapsed. This standard correction method has been used in previous works (e.g., Sovrano and Bisazza 2008, 2009; Truppa et al. 2010), since initial observations revealed that it is optimal for learning in this task. The inter-trial interval was of 7 min after a correct choice and 3 min if fish choices showed mixed responses (incorrect and correct). During the inter-trial interval, the fish was allowed to remain in the external surrounding region (reinforcement time). Occasionally, food (the same as that used in the home tank) was given in the outer tank after a correct choice but not more often than twice in each daily session. After every two trials, the test tank was rotated 90° clockwise, in order to avoid any possible reference to the use of extra-tank cues.
After reaching the learning criterion, the fish performed test trials in which they faced two stimuli: two orange disks of identical diameter, differing only in their context, in terms of size, number and spacing of the gray inducers: one stimulus consisted of a orange disk (4.5 mm, diameter), surrounded by six large gray disks (9 mm, diameter), that could be compared with four types of stimuli: all composed of the same central orange disk (4.5 mm, diameter), but varying in the size (3 mm, diameter) and the number (either six or eight) of gray small circles of the context and the spacing from the central circle (exactly the same of the big context condition—3.5 mm—and adjacent to the central circle—0.5 mm) (see Fig. 3).
The test consisted of four single sessions of ten trials (each trial lasting 2 min), one session for each of the four test conditions (comparison between the large context condition with each of the small context conditions). During the test, both doors were closed. If animals did not make a choice in the 2 min allotted for each trial, they were left in the test tank up to a maximum of 15 min, or until they produced at least one choice. This is the standard procedure employed for this task in previous studies (Sovrano and Bisazza 2008, 2009; Truppa et al. 2010), which keep the animal motivated to make a choice in order to avoid staying in the central tank for long periods. A minimum of two trials with the same stimuli as those used during training were inter-mixed every 2–3 test trials, in order to reinstate the motivation of the animals (these trials were, of course, discarded in the test data analyses). In order to proceed to the next test trials, fish had to perform correctly in both the trials with familiar stimuli. If this was not the case, training trials were repeated until the fish performed correctly two times consecutively.
The order of presentation of the test stimuli was randomized between animals.
The first choice made in each trial and the number of choices given to each of the two stimuli during the 2 min of test were recorded for each animal. From these figures, the percentage of first choices and of overall choices for the configuration with large inducers was computed. We expected that the choice for the figure in which the central orange circle appears smaller would be above chance level in the fish rewarded on the smaller circle, and below chance level in the fish rewarded on the bigger circle.
The data were submitted to an analysis of variance with type of stimuli used during the test (comparison between large subjective condition with each of four small subjective conditions) as a within-subjects factor and the reinforced stimulus (large disk vs. small disk) used during training as a between-subjects factor. Significant departures from random choice (50 %) were estimated by one-sample two-tailed t tests.
Results
The results of training are shown in Fig. 4. There was no difference in the number of trials needed to reach the learning criterion between the two groups, rewarded with the large or the small orange disk (small disk: mean ± SEM 20 ± 8.6; large disk: mean ± SEM 20.5 ± 7.37; t(6) = 0.091 P = 0.93).
The results of the tests are shown in Fig. 5a–d, separately for each reinforcement condition and for the four type of test stimuli. Data were analyzed as percentage of choices for the figure with large inducers.
Percentage of choices for the configuration represented on the X axis below each group of bars. Thus, the two leftward columns represent the percentage of choice for the configuration with bigger inducers, and the two rightward columns represent choices for the configuration with smaller inducers. Blue bars represent the performance of subjects trained on the smaller target, whereas red bars represent subjects trained for the bigger target (group means with SEM are shown). In each figure, the top graph represents the percentage of first choices, the bottom graph represents the percentage of total choices. Each graph summarizes performance in one of the four testing conditions, in which number and spacing of the smaller inducers were varied: a condition with six inducers distant from the central circle, b condition with six inducers near to the central circle, c condition with eight inducers distant from the central circle, d condition with eight inducers near to the central circle
The analysis of variance with type of test (comparison between large subjective condition with each of four small subjective conditions) as a within-subjects factor and type of stimulus used during training (big or small orange circle) as between-subjects factor revealed a significant main effect of type of training, considering both only first choices (F(1,6) = 174.545 P ≤ 0.0001) and total choices (F(1,6) = 52.808 P ≤ 0.0001). There were no other statistically significant effects [first choices: type of test: (F(3,18) = 1.533 P = 0.24), type of test × type of training: (F(3,18) = 1.577 P = 0.23); total choices: type of test: (F(3,18) = 0.168 P = 0.917), type of test × type of training: (F(3,18) = 0.993 P = 0.418)].
Thus, in further analyses, we collapsed the data of all the four types of test stimuli, running separate statistical tests for the two reinforcement conditions. One-sample t tests, comparing both the percentage of first choices and that of overall choices (2 min of observation) to chance level, revealed that fish significantly chose the orange disk that appeared larger or smaller (to a human observer), consistent with the training phase. Results were as follows: fish rewarded on the bigger orange circle chose to approach the perceptually bigger stimulus significantly more than expected by chance (first choice, t(3) = 10.247 P = 0.002, overall choices t(3) = 4.607 P = 0.019); whereas fish rewarded on the smaller orange circle chose the perceptually bigger stimulus significantly less than expected by chance level (first choice t(3) = −9.000 P = 0.003; overall choices t(3) = −5.690 P = 0.011).
Discussion
In the present experiment, redtail splitfin fish that had been trained to exit from the test arena through the exit marked by a bigger orange circle preferred to approach the circle that appeared perceptually bigger in the Ebbinghaus display (i.e., the orange circle surrounded by small gray inducers). Similarly, fish rewarded on the smaller orange circle preferred to approach the illusory display in which the central circle appeared perceptually smaller (being surrounded by big inducers). This behavior indicates that our subjects perceive the Titchener circles as a contrast illusion. To the best of our knowledge, this is the first demonstration of the perception of geometric contrast illusions in teleost fish.
The results obtained here in fish parallel those shown in humans (e.g., Choplin and Medin 1999; Coren and Enns 1993; Ebbinghaus 1902; de Grave et al. 2005; Girgus et al. 1972; Massaro and Anderson 1971; Weintraub 1979) and domestic chicks, when tested with appropriate procedures (Rosa Salva et al. 2013). Similar perceptual and neural mechanisms as those observed in our species seem thus to be available across vertebrates, including so-called lower vertebrates, suggesting the presence of homologous traits inherited from a common ancestor (however, for evidence of perception of illusory contours or even geometrical illusions in insects, see Geiger and Poggio 1975; Horridge et al. 1992). Avian species have also revealed similarities with humans in the way testing modalities affect the perception of the illusion. When chicks and pigeons are required to peck at the stimuli, they seem to perceive the Titchener circles as an assimilation illusion (Nakamura et al. 2008, 2014), whereas the same display is perceived by chicks as a contrast illusion in a task not involving direct actions on the stimuli or their close observation from a short distance (Rosa Salva et al. 2013). Likewise, in humans, grasping responses are less affected by the illusory display than explicit conscious judgements (Aglioti et al. 1995; Danckert et al. 2002). Also, Ebbinghaus displays in which the distant portion of the larger inducers has been erased (simulating the effect of close visual inspection) cause the perception of assimilation illusions in human observers (Oyama 1960; Weintraub 1979). In this regard is noteworthy that we employed training and testing procedures that did not force the subjects to manipulate or inspect stimuli from a very close distance. In the present study, fish learnt to use the size of a target disk as a visual cue to find the exit from a square tank. Two identical doors were present in the test tank, only one of which could be opened by the fish to exit from the tank. Animals could identify the correct door by the size of the target disk placed above it. This procedure allowed fish to move freely in the environment before emitting their response, choosing the viewing distance that better suit their spontaneous processing style. A very similar task was employed in previous research, demonstrating globally oriented perception and amodal completion in X. eiseni (Sovrano and Bisazza 2008; Truppa et al. 2010). This is in contrast with the procedures used by studies that have failed to demonstrate a human-like perception of the Ebbinghaus illusion in other species. For example, Nakamura et al. (2009, 2014) trained birds to respond to small and large circles by pecking at different keys in an operant conditioning chamber. We believe that the approach that we employed with fish in this study could be more informative with regard to the perceptual mechanisms available to different species in ecologically relevant settings.
References
Aglioti S, DeSouza JF, Goodale MA (1995) Size-contrast illusions deceive the eye but not the hand. Curr Biol 5:679–685
Agrillo C, Miletto Petrazzini ME, Dadda M (2014) Illusory patterns are fishy for fish, too. Front Neural Circuits 7:137
Barbet I, Fagot J (2002) Perception of the corridor illusion by baboons (Papio papio). Behav Brain Res 132:111–115
Bayne K, Davis R (1983) Susceptibility of rhesus monkeys (Macaca mulatta) to the Ponzo illusion. Bull Psychon Soc 21:476–478
Cavoto KK, Cook RG (2001) Cognitive precedence for local information in hierarchical stimulus processing by pigeons. J Exp Psychol Anim Behav Proc 27(1):3–16
Cerella J (1980) The pigeon’s analysis of pictures. Pattern Recognit 12(1):1–6
Chiandetti C, Pecchia T, Patt F, Vallortigara G (2014) Visual hierarchical processing and lateralization of cognitive functions through domestic chicks’ eyes. PLoS One 9(1):e84435
Choplin JM, Medin DL (1999) Similarity of the perimeters in the Ebbinghaus illusion. Percept Psychophys 61:3–12
Cook RG (1992) Dimensional organization and texture discrimination in pigeons. J Exp Psychol Anim Behav Proc 18:354–363
Cook RG, Cavoto KK, Cavoto BR (1996) Mechanisms of multidimensional grouping, fusion, and search. Anim Learn Behav 24:150–167
Coren S, Enns JT (1993) Size contrast as a function of conceptual similarity between test and inducers. Percept Psychophys 54:579–588
Danckert JA, Sharif N, Haffenden AM, Schiff KC, Goodale MA (2002) A temporal analysis of grasping in the Ebbinghaus illusion: planning versus online control. Exp Brain Res 144:275–280
Darmaillacq AS, Dickel L, Rahmani N, Shashar N (2011) Do reef fish, Variola louti and Scarus niger, perform amodal completion? Evidence from a field study. J Comp Psychol 125:273
De Fockert J, Davidoff J, Fagot J, Parron C, Goldstein J (2007) More accurate size contrast judgments in the Ebbinghaus illusion by a remote culture. J Exp Psychol Hum Percept Perform 3:738–742
De Grave DDJ, Biegstraaten M, Smeets JBJ, Brenner E (2005) Effects of the Ebbinghaus figure on grasping are not only due to misjudged size. Exp Brain Res 163:58–64
Deruelle C, Fagot J (1998) Visual search for global/local stimulus features in humans and baboons. Psychon Bull Rev 5:476–481
Ebbinghaus H (1902) Grundzüge der psychologie. Veit, Leipzig
Fagot J, Deruelle C (1997) Processing of global and local visual information and hemispheric specialization in humans (Homo sapiens) and baboons (Papio papio). J Exp Psychol Hum Percept Perform 23:429–442
Forkman B, Vallortigara G (1999) Minimization of modal contours: an essential cross species strategy in disambiguating relative depth. Anim Cogn 4:181–185
Fremouw T, Herbranson WT, Shimp CP (1998) Priming of attention to local and global levels of visual analysis. J Exp Psychol Anim Behav Proc 24:278–290
Fremouw T, Herbranson WT, Shimp CP (2002) Dynamic shifts of pigeon local/global attention. Anim Cogn 5:233–243
Fujita K (1996) Linear perspective and the Ponzo illusion: a comparison between rhesus monkeys and humans. Jpn Psychol Res 38:136–145
Fujita K (1997) Perception of the Ponzo illusion by rhesus monkeys, chimpanzees, and humans: similarity and difference in the three primate species. Percept Psychophys 59:284–292
Fujita K, Blough DS, Blough PM (1991) Pigeons see the Ponzo illusion. Anim Learn Behav 19:283–293
Fujita K, Blough DS, Blough PM (1993) Effects of the inclination of context lines on perception of the Ponzo illusion by pigeons. Anim Learn Behav 21:29–34
Fuss T, Bleckmann H, Schluessel V (2014) The brain creates illusions not just for us: sharks (Chiloscyllium griseum) can “see the magic” as well. Front Neural Circuits 20:8–24
Geiger G, Poggio T (1975) The Müller-Lyer figure and the fly. Science 190:479–480
Girgus JS, Coren S, Agdern M (1972) The interrelationship between the Ebbinghaus and Delboeuf illusions. J Exp Psychol 95:453–455
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Happé F (1996) Studying weak central coherence at low levels: children with autism do not succumb to visual illusions. A research note. J Child Psychol Psychiatry 37:873–877
Horridge GA, Zang S-W, O’Carrol D (1992) Insect perception of illusory contours. Philos Trans R Soc Lond B 337:59–64
Kaldy Z, Kovacs I (2003) Visual context integration is not fully developed in 4-year-old children. Perception 32:657–666
Kimchi R (1992) Primacy of wholistic processing and global/local paradigm: a critical review. Psychol Bull 112:24–38
Kinchla RA, Wolf JM (1979) The order of visual processing: top-down, bottom-up, or middle-out. Percept Psychophys 25:225–231
Kinchla RA, Solis-Macias V, Hoffman J (1983) Attending to different levels of structure in a visual image. Percept Psychophys 33:1–10
Kumar S, Hedges SB (1998) A molecular timescale for vertebrate evolution. Nature 392:917–920
Mascalzoni E, Regolin L (2011) Animal visual perception. Wiley Interdiscip Rev Cogn Sci 2:106–116
Massaro DW, Anderson NH (1971) Judgemental model of the Ebbinghaus illusion. J Exp Psychol 89:147–151
Murayama T, Usui A, Takeda E, Kato K, Maejima K (2012) Relative size discrimination and perception of the Ebbinghaus illusion in a bottlenose dolphin (Tursiops truncatus). Aquat Mamm 38:333–342
Nakamura N, Fujita K, Ushitani T, Miyata H (2006) Perception of the standard and the reversed Müller-Lyer figures in pigeons (Columba livia) and humans (Homo sapiens). J Comp Psychol 120:252–261
Nakamura N, Watanabe S, Fujita K (2008) Pigeons perceive the Ebbinghaus–Titchener circles as an assimilation illusion. J Exp Psychol Anim Behav Proc 34(3):375–387
Nakamura N, Watanabe S, Fujita K (2009) Further analysis of perception of reversed Müller-Lyer figures for pigeons (Columba livia). Percept Mot Skills 108:239–250
Nakamura N, Watanabe S, Fujita K (2014) A reversed Ebbinghaus–Titchener illusion in bantams (Gallus gallus domesticus). Anim Cogn 17:471–481
Navon D (1977) Forest before trees: precedence of global features in visual perception. Cogn Psychol 9:353–383
Oyama T (1960) Japanese studies on the so-called geometrical-optical illusions. Psychologia 3:7–20
Parron C, Fagot J (2007) Comparison of grouping abilities in humans (Homo sapiens) and baboons (Papio papio) with Ebbinghaus illusion. J Comp Psychol 121:405–411
Pepperberg IM, Vicinay J, Cavanagh P (2008) Processing of the Müller-Lyer illusion by a grey parrot (Psittacus erithacus). Perception 37:765–781
Phillips WA, Chapman KL, Berry PD (2004) Size perception is less context sensitive in males. Perception 33:79–86
Pomerantz JR (1983) Global and local precedence: selective attention in form and motion perception. J Exp Psychol Gen 112:516–540
Regolin L, Vallortigara G (1995) Perception of partly occluded objects by young chicks. Percept Psychophys 57:971–976
Regolin L, Marconato F, Vallortigara G (2004) Hemispheric differences in the recognition of partly occluded objects by newly-hatched domestic chicks (Gallus gallus). Anim Cogn 7:162–170
Reiner A, Yamamoto K, Karten HJ (2005) Organization and evolution of the avian forebrain. Anat Rec A Discov Mol Cell Evol Biol 287A:1080–1120
Roberts B, Harris MG, Yates TA (2005) The roles of inducer size and distance in the Ebbinghaus illusion (Titchener circle). Perception 34:847–856
Robertson LC, Egly R, Lamb MR, Kerth L (1993) Spatial attention and cuing to global and local levels of hierarchical structure. J Exp Psychol Hum Percept Perform 19:471–487
Rosa Salva O, Rugani R, Cavazzana A, Regolin L, Vallortigra G (2013) Perception of the Ebbinghaus illusion in four-day-old domestic chicks (Gallus gallus). Anim Cogn 16:895–906
Rosa Salva O, Sovrano VA, Vallortigara G (2014) What can fish brains tell us about visual perception. Front Neural Circuits 8:119. doi:10.3389/fncir.2014.00119
Shimizu T (2004) Comparative cognition and neuroscience: misconceptions about brain evolution. Jpn Psychol Res 46:246–254
Shimizu T, Bowers AN (1999) Visual circuits of the avian telencephalon: evolutionary implications. Behav Brain Res 98:183–191
Sovrano VA, Bisazza A (2008) Recognition of partly occluded objects by fish. Anim Cogn 11:161–166
Sovrano VA, Bisazza A (2009) Perception of subjective contours in fish. Perception 38:579–590
Steinke D, Salzburger W, Meyer A (2006) Novel relationships among ten fish model species revealed based on a phylogenomic analysis using ESTs. J Mol Evol 62:772–784
Suganuma E, Pessoa VF, Monge-Fuentes V, Castro BM, Tavares MCH (2007) Perception of the Müller-Lyer illusion in capuchin monkeys (Cebus apella). Behav Brain Res 182:67–72
Sutherland NS, Mackintosh NJ (1971) Mechanisms of animal discrimination learning. Academic Press, London
Timney B, Keil K (1996) Horses are sensitive to pictorial depth cues. Perception 25:1121–1128
Truppa V, Sovrano VA, Spinozzi G, Bisazza A (2010) Processing of visual hierarchical stimuli by fish (Xenoteca eiseni). Behav Brain Res 207(1):51–60
Tudusciuc O, Nieder A (2010) Comparison of length judgments and the Müller-Lyer illusion in monkeys and humans. Exp Brain Res 207:221–231
Ushitani T, Fujita K, Yamanaka R (2001) Do pigeons (Columba livia) perceive object unity? Anim Cogn 4:153–161
Vallortigara G (2004) Visual cognition and representation in birds and primates. In: Rogers LJ, Kaplan G (eds) Vertebrate comparative cognition: are primates superior to non-primates?. Kluwer Academic/Plenum Publishers, New York, pp 57–94
Vallortigara G (2006) The cognitive chicken: visual and spatial cognition in a non-mammalian brain. In: Wasserman EA, Zentall TR (eds) Comparative cognition: experimental explorations of animal intelligence. Oxford University Press, Oxford, pp 41–58
Vallortigara G (2009) Original knowledge and the two cultures. In: Carafoli E, Danieli GA, Longo GO (eds) The two cultures: shared problems. Springer, Berlin, pp 125–145
Vallortigara G (2012) Core knowledge of object, number, and geometry: a comparative and neural approach. Cogn Neuropsychol 29:213–236
Vallortigara G, Chiandetti C, Rugani R, Sovrano VA, Regolin L (2010) Animal cognition. Wiley Interdiscip Rev Cogn Sci 1:882–893
Wade NJ (2005) Perception and illusions, historical perspectives. Springer, Dordrecht
Wade NJ (2010) Visual illusions. Corsini encyclopedia of psychology. Wiley, Hoboken, pp 1–2
Warden CJ, Baar J (1929) The Müller-Lyer illusion in the ring dove, Turtur risorius. J Comp Psychol 9(4):275–292
Wasserman EA, Kirkpatrick-Steger K, Van Hamme LJ, Biederman I (1993) Pigeons are sensitive to the spatial organization of complex visual stimuli. Psychol Sci 4:336–341
Weintraub DJ (1979) Ebbinghaus illusion: context, contour, and age influence the judged size of a circle admist circles. J Exp Psychol Hum Percept Perform 5:353–364
Winslow CN (1933) Visual illusions in the chick. Arch Physiol 153:1–83
Wyzisk K (2005) Experimente zur Form- und Größenwahrnehmung beim Goldfisch (Carassius auratus) unter Verwendung von Scheinkonturen und Größentäuschungen. Ph.D. thesis, Johannes-Gutenberg-Universität Mainz, Germany
Wyzisk K, Neumeyer C (2007) Perception of illusory surfaces and contours in goldfish. Vis Neurosci 24:291–298
Yamazaki Y, Otsuka Y, Kanazawa S, Yamaguchi MK (2010) Perception of the Ebbinghaus illusion in 5-to-8-month-old infants. Jpn Psychol Res 52(1):33–40
Acknowledgments
This study was supported by research grant from the Cassa di Risparmio of Trento e Rovereto. We wish to thank Matteo Kettmaier for his help with the experiments and Francesco Cerri for the maintenance of the aquaria.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The experiments reported here comply with the current Italian and European Community laws for the ethical treatment of animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sovrano, V.A., Albertazzi, L. & Rosa Salva, O. The Ebbinghaus illusion in a fish (Xenotoca eiseni). Anim Cogn 18, 533–542 (2015). https://doi.org/10.1007/s10071-014-0821-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-014-0821-5