Abstract
Understanding animals’ spatial perception is a critical step toward discerning their cognitive processes. The spatial sense is multimodal and based on both the external world and mental representations of that world. Navigation in each species depends upon its evolutionary history, physiology, and ecological niche. We carried out foraging experiments on wild vervet monkeys (Chlorocebus pygerythrus) at Lake Nabugabo, Uganda, to determine the types of cues used to detect food and whether associative cues could be used to find hidden food. Our first and second set of experiments differentiated between vervets’ use of global spatial cues (including the arrangement of feeding platforms within the surrounding vegetation) and/or local layout cues (the position of platforms relative to one another), relative to the use of goal-object cues on each platform. Our third experiment provided an associative cue to the presence of food with global spatial, local layout, and goal-object cues disguised. Vervets located food above chance levels when goal-object cues and associative cues were present, and visual signals were the predominant goal-object cues that they attended to. With similar sample sizes and methods as previous studies on New World monkeys, vervets were not able to locate food using only global spatial cues and local layout cues, unlike all five species of platyrrhines thus far tested. Relative to these platyrrhines, the spatial location of food may need to stay the same for a longer time period before vervets encode this information, and goal-object cues may be more salient for them in small-scale space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our understanding of the ways that animals use environmental and sensory information to navigate has increased markedly over the last two decades. Foraging experiments have been instrumental in revealing important aspects of animals’ spatial cognition, especially for wild primates (Garber and Dolins 1996; Garber and Lavallee 1999; Garber and Brown 2006; Janson 1996, 2007, 2011; Bicca-Marques and Garber 2004; Bicca-Marques 2005; Janmaat et al. 2006a, b). Though experiments conducted in the wild cannot be as tightly controlled as those in captivity, they have the advantage of testing questions in a similar environment to which the abilities in question evolved (Janson and Byrne 2007). However, foraging experiments addressing questions of spatial cognition have overwhelmingly been investigated in New World monkeys (platyrrhines, e.g., Saguinus mystax mystax, Garber and Dolins 1996; Cebus capucinus, Garber and Paciulli 1997; Garber and Lavallee 1999; Garber and Brown 2006; C. apella nigritus, Janson 1996, 2007, 2011; Aotus nigiceps, S. imperator imperator, S. fuscicollis weddelli, Callicebus cupreus, Bicca-Marques and Garber 2004; Bicca-Marques 2005). For Old World monkeys (catarrhines), spatial perception and the cues used while foraging are much less understood (but see: Lophocebus albigena johnstoni, Janmaat et al. 2006a, b; Papio ursinus, Noser and Byrne 2007, 2010; Byrne et al. 2009), though some work has been done on lemurs, apes, and humans (e.g., Propithecus edwardsi, Eulemur fulvus rufus, Erhart and Overdorff 2008; Hylobates lar, Asensio et al. 2011; Pongo pygmaeus, MacKinnon 1974; Pan troglodytes, Normand and Boesch 2009; Homo sapiens, Foo et al. 2005). It is essential that a wide range of species are examined because important differences in spatial cognition within the primate order may be revealed (MacLean et al. 2012), especially given variation in sensory systems. This paper aids in redressing the taxonomic bias in foraging experiments on wild primates by replicating previous experiments on platyrrhines (Garber and Dolins 1996; Garber and Lavallee 1999; Bicca-Marques and Garber 2004) on an African monkey (vervet monkeys, Chlorocebus pygerythrus). Our goal was to examine the hierarchy of sensory information vervets used in food detection and determine whether they could find hidden food with the use of associative cues. Little is known about spatial perception in vervets (or other cercopithecine monkeys, for that matter), but a tantalizing study by Cramer and Gallistel (1997) on captive vervets showed that they were able to find the shortest route among six sites and that they considered at least the next three site visits before choosing an efficient route (three-step look ahead). This contrasts with some other studies on primates, where individuals looked only one to two steps ahead while making foraging decisions (Cebus apella, Janson 2007; Cercopithecus ascanius whitesidei; yellow-nosed monkeys, MacDonald and Wilkie 1990; Pan troglodytes, Menzel 1973). These results make further investigation into vervet spatial cognition intriguing.
When foraging, animals may use many sensory modalities (i.e., vision, olfaction, audition, tactual perception, electrical perception, echolocation, and proprioception) to perceive the presence of food. For primates, food detection is primarily done through sight, smell, memory of spatial position or some combination of these cues. The relative importance of vision and olfaction for a species depends on their perceptive abilities and the properties of their resources. For instance, early in primate evolution, the acuity and color perception of the visual system was enhanced. Among other changes, the tapetum lucidum was lost in haplorhines, and some primate radiations developed an increase in the ratio of cones to rods in the retina and evolved trichromatic color vision from a dichromatic ancestor (Martin 1990; Jacobs 2009; Isbell 2009). Species with such enhanced visual systems may predominantly rely on vision to locate food. In other species, scent may be most salient in locating food resources, depending on olfactory capacity (Smith and Rossie 2006; Barton 2006), the time of the day spent foraging (Charles-Dominique 1977; Pariente 1979), and the strength and reliability of an odor signal (Vickers 2000). Strepsirrhine primates retain the primitive primate rhinarium and the entire vomeronasal complex (reviewed in: Colquhoun 2011), so their detection of odorants may be greatest within the primate order (e.g., Rushmore et al. 2012). Nonetheless, sensory adaptations may be specific to certain social or ecological signals and may not be transferrable to different situations. For example, functional vomeronasal organs are specific to the processing of pheromonal signals produced by the anogenital glands and do not aid in food detection (Døving and Trotier 1998). In addition to these sensory modalities, memory of the spatial location and positioning of previously used food sources have been shown to play an important role for primates when making foraging decisions (e.g., Garber 1989, 2000; Garber and Lavallee 1999; Bicca-Marques and Garber 2004; Janmaat et al. 2006a, b; Asensio et al. 2011).
Animals are not only confronted with the problem of detecting food when they are near it, they must also find their way, to and from, variable food sources and other important areas of their home range such as sleep sites (Shettleworth 2010). Primates generally use a two-system model for navigation where spatial information can be represented in an egocentric (internal, relative to the body position and direction of motion) and/or an allocentric (external, relative to environmental cues) framework (Wehner and Srinivasan 1981; Etienne et al. 1988, 1998; Gallistel 1990; Wehner et al. 1996; Dolins and Mitchell 2010; but see Wang and Spelke 2002). Use of egocentric representations alone may lead to the accumulation of errors in navigation (Séguinot et al. 1993), and egocentric and allocentric localization appear to work in parallel in most situations to keep an animal oriented (Burgess 2006). The predominant use of one representation over the other may depend upon how much the animal is moving, the size and structure of the environment it is moving through, and prior experience within that environment (Burgess 2006). Egocentric localization can be accomplished by orienting relative to the distance, angle, and direction from an object (or beacon) in space. Allocentric localization can be done by making relational associations between objects (or landmarks) in the environment, independent of an individual’s spatial perspective (Dolins and Mitchell 2010). Allocentric representations may be more permanent than egocentric ones because the body position changes as an animal moves, and knowledge of directions in the environment is not gained (Burgess 2006; Waller and Lippa 2007). Associative cues such as beacons and landmarks are used in both egocentric and allocentric navigation by a wide variety of animals including insects (Wehner and Raeber 1979; Cartwright and Collett 1983), fish (Warburton 1990; Cain and Malwal 2002), birds (Balda and Turek 1984; Cheng 1989; Vallortigara et al. 1990; Cheng and Sherry 1992), and mammals (Suzuki et al. 1980; Collett et al. 1986; Cheng 1986; Etienne 1987; Hermer and Spelke 1994; Garber and Dolins 1996; Dolins 2009; Hribar and Call 2011).
In this study, we used foraging experiments to examine food detection in wild vervet monkeys (Chlorocebus pygerythrus) in small-scale space, or the area that can be seen from a single vantage point (following Byrne’s (2000) definition of “large-scale space”). Specifically, we determined whether global spatial cues, local layout cues, or goal-object cues (Brodbeck 1994) were used by vervet monkeys and whether they could use an associative cue to find hidden food. For our experiments, global spatial cues were more distal and included the relational information provided by the arrangement of feeding platforms and all of the objects, such as trees and bushes, which surrounded them. Local layout cues were more proximal and were provided by the position of certain feeding platforms (e.g., those containing food versus those without food) relative to others. Goal-object cues were those that were provided by the food rewards and other objects placed on the platforms themselves, such as color, texture, or odor (Brodbeck 1994).
Widespread in savannah, woodland, and forest edge throughout sub-Saharan Africa, vervets are among the most adaptable of the cercopithecine monkeys (Cheney and Seyfarth 1992). Now, considered to be several separate species based on their morphology and biogeography (Groves 2001), vervets are both terrestrial and arboreal and are known to be flexible in their diets, eating primarily fruit, insects, and gums in some areas, but supplementing with a range of vegetation and opportunistic feeding on animals (Wrangham and Waterman 1981; Skinner and Smithers 1990). Vervets quite easily adapt to human-modified landscapes, and they are considered pests in many areas for their crop-raiding behaviors (Estes 1992; Saj et al. 2001); however, these habits allow wild vervets to quickly adapt to an experimental study regime.
Our first set of experiments (1A and 1B) sought to determine whether vervet monkeys used goal-object cues more than global spatial cues and/or local layout cues (Brodbeck 1994) when detecting food resources (Table 1; following Garber and Lavallee 1999). We hypothesized that when presented with all of these cues, goal-object cues would be most important for vervets because these allow food to be directly seen or smelled and approached, so navigation does not need to be accomplished with spatial cues provided by objects other than the food in the environment. Of the goal-object cues available, we hypothesized that visual cues would be most salient for vervets because they are catarrhines with an enhanced visual system (Jacobs 2009).
Our second set of experiments (2A and 2B) examined whether vervets could use global spatial cues and/or local layout cues to locate food resources, without the availability of goal-object cues (Table 1). In these experiments, vervet monkeys could also have successfully found food by applying a win-stay foraging rule, though this required that they used either global spatial cues or local layout cues to remember the location of food resources. Application of a win-stay rule means that animals return to a particular food source if they have successfully foraged there previously (Garber 1989; Bicca-Marques 2005). If the global spatial position or local layout of food remains consistent, animals can apply previously learned spatial information to predict the location of real rewards in the same spot at a later time. This type of rule may be used whenever animals return to a feeding tree after a certain amount of time to monitor the renewal of resources (Garber and Lavallee 1999) and returning to a platform that previously contained real food indicates that a win-stay rule is being used. We predicted that though goal-object cues would be the dominant means to locate food for vervets, in the absence of these cues, global spatial and/or local layout cues would be used. This implied that vervets would be able to apply a win-stay foraging rule, which has been found to be used successfully by other primates (e.g., Leontopithecus rosalia, Callithrix kuhli, Platt et al. 1996; Saguinus mystax mystax, Garber and Dolins 1996; S. imperator, S. fuscicollis weddelli, Callicebus cupreus cupreus, Bicca-Marques 2005; Saimiri sciureus, Andrews 1988; Cebus capucinus, Garber and Paciulli 1997; Cercopithecus ascanius whitesidei, MacDonald and Wilkie 1990; Pongo pygmaeus abelii, MacDonald and Agnes 1999; Gorilla gorilla gorilla, MacDonald 1994).
Our last experiment (3) assessed whether vervets could use a nearby, reliable associative cue (a single beacon) to find hidden food resources (Table 1). Use of an associative cue would show egocentric localization by the vervets, who would be defining their axis of orientation relative to the beacon (Burgess 2006). Given that use of associative cues is common throughout the animal kingdom (reviewed in: Spetch and Kelly 2006) and by other primate species (e.g., Saguinus mystax mystax, Garber and Dolins 1996; Cebus capucinus, Garber and Lavallee 1999), we hypothesized vervets would be able to use these cues for spatial orientation.
Methods
Study site and subjects
This study was conducted at Lake Nabugabo, Masaka District, central Uganda (0°22′–12°S and 31°54′E). Lake Nabugabo (8.2 × 5 km) is a satellite lake to Lake Victoria lying at an elevation 1,136 m. The landscape around the lake is modified by humans and is a matrix that includes wetlands, grasslands, patches of forest, areas with natural regenerating vegetation, farmers’ fields, and a few buildings. One habituated group of vervet monkeys (Chlorocebus pygerythrus) at Nabugabo called M group was followed for 2 months (June–July 2012) from dawn to dusk, 5 days per week (41 days). Vervets are ideal subjects for foraging experiments because they are partially terrestrial, eat a varied diet, and easily take food from human sources (Isbell et al. 1998; Saj et al. 2001). This allows experiments to be conducted on the ground and the use of locally grown bananas to be offered as food rewards. The group contained 24 individuals (2 adult males, 5 adult females, 3 subadult males, 3 subadult females, 11 juveniles and infants), and dye-marking was employed at the beginning of the study, after which all individuals could be individually recognized by features of the face and body.
Study design and detailed hypotheses
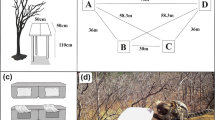
Experiments used methods and a number of trials similar to Garber and Lavallee (1999) (12 trials for Experiments 1A, 1B, 2A, and 2B; 30 trials for Experiment 3). Individuals were not trained prior to experimentation because their naturalistic foraging behavior and the method with which they found food in each context was of interest. However, depending on the question, the data were analyzed after a certain number of predetermined trials (“learning trials”, see below) to examine the effect of experience on the monkeys’ abilities to find food. M group had a predictable daily path, which was partially due to them only using two sleep sites, along the edge of the lake at the NE and SW ends of their home range boundary. Seven feeding platforms, arranged in a circle with each platform 3 m apart, were set up between their sleep sites. The placement of feeding platforms was not changed between trials or experiments, so the global spatial cues (platforms themselves, trees, bushes, etc.) surrounding each platform remained consistent. Platforms were wooden tables approximately 0.75 m high, with a square flat top 0.75 × 0.75 m in size (Fig. 1). The group visited the platforms relatively predictably (usually twice per day) on their way to and from their sleep site. JAT completed 78 trials of five different experimental conditions during the 2 months of observation. After an initial six-day period of habituation to the platforms (Garber and Brown 2006), trials were carried out on most days, whenever the monkeys passed by the platform array (mean number of trials per day: 1.77; range 0–3). It was important for the experimental protocol that the monkeys did not observe the platforms being baited, so all items were placed on the platforms prior to the group’s arrival. This usually meant that two trials could not be done in close succession, so trials on the same day were separated by a mean of 7 h and 5 min (range 0:08–12:58). Analyses were done on the number and order of visits to each platform during trials. A visit was scored any time an individual jumped onto one of the feeding platforms. All repeat visits were also scored and the order of visitation was noted. Not all platforms were visited during every trial, depending in part on participants’ knowledge of where food was located.
Though vervets are often terrestrial, at our study site they appear more at ease when off the ground or when within a meter or two of the safety of the trees (MMJ White, unpubl. data). We noted that the monkeys tended to run up to the platforms and leap onto the first one in their line of travel, regardless of what was contained on that platform. Indeed, if the platform contents were covered with a leaf, the vervets often did not even lift the leaf to see what was underneath. This suggests that the first platform leaped upon was not always selected as a foraging site, but as a “safe” site. We therefore provide data on the first, second, and third platforms chosen by individual(s) that reached the site relative to the probability that this was a platform with a real banana (Table 2). This approach was also deemed appropriate in the experiments conducted by Garber and Lavallee (1999) and Bicca-Marques and Garber (2004).
Experiments 1A and 1B sought to determine what sensory information vervet monkeys use to detect food resources (Table 1). Global spatial cues, local layout cues, and goal-object cues were all available to the animals. One banana was placed on three of the seven feeding platforms (platform selection done at random), while a sham that resembled a real banana but was made of weighted styrofoam was placed on the other four. The spatial layout of real versus sham bananas was left the same (i.e., spatial location predictable) for 6 days (12 trials). Bananas were left uncovered and provided visual, olfactory, and spatial cues to the monkeys. We hypothesized that the vervets would initially be deceived by the sham bananas, but over time they would be able to distinguish between real and sham bananas, since all sensory information was available, and would go to platforms containing real bananas, avoiding those with sham bananas more often than by chance (Garber and Lavallee 1999).
In Experiment 1B, all sensory cues were again available to the monkeys. Bananas were again left uncovered (Table 1), with three real and four sham bananas. However, on Day 1 the spatial layout of real and sham bananas was rotated from the set up in 1A and remained the same (i.e., spatial location predictable) for the duration of the experiment (6 days, 12 trials). If global spatial cues and/or local layout cues were foremost in the information used to find food, we hypothesized that the monkeys would initially return to the platforms that had previously contained real bananas in Experiment 1A, showing their transfer of knowledge from the previous condition. Then, over additional trials with the new spatial locations, they would improve in their abilities to locate real bananas, avoiding platforms with sham bananas, at levels greater than chance. If goal-object cues were the most salient in navigating to real food sources, the change in spatial layout was predicted to have no significant effect, initially or in the long-term, on the vervets’ ability to locate real food rewards.
Experiments 2A and 2B sought to determine if vervets could use global spatial cues and/or local layout cues to locate food resources without any available goal-object cues (Table 1). On Day 1 of Experiment 2A, the spatial position of three real and four sham bananas was rotated from what it was in Experiment 1B and remained the same (i.e., spatial location predictable) until 12 trials were completed (6 days). However, bananas on all platforms were covered with large leaves to remove visual cues and banana skins were placed with sham bananas so that all platforms smelled like bananas (again following Garber and Lavallee 1999). It was hypothesized that if the spatial layout of food, in either a global or local context, was used to find real rewards, after a single experiential trial with the new spatial position, the vervets would begin to learn the predictable placement of real bananas, and using a win-stay foraging rule, they would locate real rewards at levels greater than chance.
Experiment 2B provided the same set up as 2A, with global spatial and local layout cues provided and goal-object cues disguised. Three real and four sham bananas were placed on platforms, covered with leaves, with banana skins next to sham rewards; but on Day 1, the spatial layout of the real and sham rewards were rotated from the set up used in Experiment 2A, remaining the same (i.e., spatial location predictable) until 12 trials were completed (6 days; Table 1). Again, if global spatial or local layout cues were used to navigate we predicted that, on their first trial with the new reward locations, the monkeys would initially return to platforms that previously contained real food, showing transfer of spatial knowledge from the previous condition. Then, after a few trials and using the win-stay foraging rule, they would get better at locating food and would go to platforms with real rewards at levels significantly greater than chance (Garber and Lavallee 1999).
Experiment 3 assessed whether the vervets could use only associative cues to locate hidden food resources (Table 1). Three platforms were again baited with real bananas and four with sham bananas, all platforms were covered with leaves, and banana skins were placed with sham bananas to remove useable goal-object cues (visual and scent cues). The spatial layout of real and sham rewards was randomized on every trial but a reliable beacon (green plastic boxes) was placed on platforms that contained real rewards (Fig. 2). We hypothesized that if the monkeys used an associative cue to find hidden food, after several training trials, they would learn to associate the green boxes with food and visit platforms with food more often than non-food platforms at levels greater than chance.
Data analyses
During trials, visits to the platforms were scored using all-occurrences sampling. After a certain number of trials in which the monkeys gained experience with the protocol (one or six “learning trials”, see “Results”), the numbers of visits to real and sham sites for each trial and in each experiment were analyzed. Pooled data from multiple individuals were used but since visits were not independent, trials were analyzed separately and combined using repeated G tests of goodness-of-fit. Expected values were calculated based on chance levels (i.e., proportion of sites that contained real bananas (3/7) and the proportion that contained shams (4/7) multiplied by the number of visits for each experiment). In repeated G tests, the results of groups of G tests can be summed and the overall difference from expected values can be determined for groups of trials within a single experiment (Sokal and Rohlf 1981). This allows the testing of multiple null hypotheses, and can provide information on: (1) whether results from each trial differ from expected (individual G-values), (2) whether the relative proportions of data in each category for each trial differ from expected (total G-values), (3) whether the pooled data differs from expected (pooled G-value), and (4) whether each individual trial is significantly different from one another (heterogeneity G-value), which is especially useful for examining the differences between individuals (McDonald 2009). For our purposes, the total G-values and pooled G-values were of interest and were reported. Percent success for each experiment was calculated as the number of platforms with real rewards visited, divided by the number of total visits, times 100 (Fig. 3).
For Experiments 2A and 2B, results did not differ from chance and it was considered advantageous to determine the strength of the null hypothesis relative to the alternative hypothesis using a Bayesian analysis (Gallistel 2009; Kruschke 2011). The distribution of individual success rates (visitations to real vs. sham sites) during these two experiments were examined with one-sample t tests, first with a theoretical mean of chance (3/7 = 0.43) and second, as the alternative hypothesis, with a theoretical mean consisting of the success rate in Experiment 1A (0.60). The Bayes factor (K) was calculated from the ratio of the instantaneous probability of each t-value under the t-distribution for that particular mean. G tests were run using The Handbook for Biological Statistics (http://udel.edu/~mcdonald/statrepgtestgof.html) and t tests were calculated using the Vassar Stats: Website for Statistical Computation (http://vassarstats.net/). Significance was set at P = 0.05.
Results
Food detection
When provided with global spatial cues, local layout cues and goal-object cues (Experiment 1A), after only a single trial with which to gain experience (a “learning trial”), vervet monkeys were successful 60 % of the time and visited platforms with real bananas more often than those with sham bananas (11 trials, N = 98 visits, total G = 19.60, df = 11, P = 0.051, pooled G = 11.02, df = 1, P = 0.001; Table 2; Fig. 3). When the spatial layout of real versus sham rewards was rotated (Experiment 1B), the monkeys again went to platforms containing real bananas more often than those with sham bananas after a single learning trial (11 trials, N = 67 visits, total G = 30.35, df = 11, P = 0.001, pooled G = 22.48, df = 1, P < 0.0001; Fig. 3) with a performance of 72 % success (Table 2).
Goal-object cues appeared to be more salient than global spatial cues or local layout cues for vervets. This was indicated by the first two trials of Experiment 1B, when initially, after the spatial layout of real versus sham bananas had been altered, 60 % (3/5) of arriving individuals did not first go to a platform that had previously contained a real banana; rather these new arrivals studied the platforms before going to one that had previously contained a sham banana but now had a real reward. The remaining 40 % (2/5) of arriving individuals went to platforms that had previously contained a real reward. Only one of these individuals acted as though he expected that a real reward would be at that site; this subadult male picked up the sham banana, sniffed it, and banged it against the platform before moving on.
Of the goal-object cues that were available to the monkeys, visual cues appeared to be the dominant means with which vervets located real bananas. An indication that visual cues were used more than olfactory cues were the initial reactions of individuals to the styrofoam bananas. During the first three trials of Experiment 1A, when the monkeys were first exposed to the sham bananas, they visited sham sites as often as real sites (3 trials, N = 39 visits, total G = 5.78, df = 3, P = 0.123, pooled G = 1.85, df = 1, P = 0.174) and often behaved as though they thought the sham bananas were real.
In Experiment 2A, when goal-object cues were removed and only global spatial and local layout cues provided information for the locations of real rewards (Table 1), the vervets seemed unable to quickly navigate to food. After a single learning trial, performance was 48 % over the next 11 trials and they visited sites with sham bananas as often as they visited platforms with real bananas (N = 83 visits, total G = 4.45, df = 11, P = 0.954, pooled G = 0.91, df = 1, P = 0.341; Table 2; Fig. 3). Performance did not really improve after two (48 %) or three learning trials (48.5 %). Even after six learning trials, over which global spatial and local layout cues remained the same, the vervets performance was 45.8 % and they still often went to every platform and checked under the leaves (6 trials, N = 60 visits, total G = 2.03, df = 6, P = 0.917, pooled G = 3.48, df = 1, P = 0.062). After the single rotation in spatial layout (Experiment 2B), when global spatial and local layout cues changed from Experiment 2A and goal-object cues were still unavailable, the monkeys performance was 46 % and they again visited real and sham sites at the same frequency after a single learning trial (11 trials, N = 101 visits, total G = 4.91, df = 11, P = 0.936, pooled G = 0.27, df = 1, P = 0.606; Table 2). Performance did not improve after two (46.1 %) or three (46.1 %) learning trials. After six learning trials, performance was 49 % and monkeys were still checking under every leaf (6 trials, N = 51 visits, total G = 3.76, df = 6, P = 0.709, pooled G = 0.75, df = 1, P = 0.387). Bayesian analysis of individual performance (N = 6 individuals) during both Experiments 2A and 2B (after two training trials for each individual in each experiment) found positive support for the null hypothesis of chance performance over an alternative hypothesis of 60 % success (Pr (0.43) = 0.570, Pr (0.60) = 0.068, K = 8.429) (Kass and Raftery 1995). In addition, during the first two trials of Experiment 2B when the spatial layout of real and sham rewards was initially changed, only 40 % (2/5) of newly arriving individuals went to platforms that previously contained real food. While 60 % (3/5) of new arrivals went to sites that had previously contained sham bananas.
Use of associative cues
In the absence of global spatial, local layout, and goal-object cues, but the presence of reliable associative cues to indicate the presence of food (Experiment 3), vervets were found to visit sites with real food 54 % of the time (Fig. 3). After a single learning trial, though all data from each trial did not differ from expected (11 trials, N = 106 visits, total G = 11.72, df = 11, P = 0.385), the pooled data showed that real food sites were visited significantly more than sham food sites (pooled G = 4.96, df = 1, P = 0.026). Vervets’ abilities to use associative cues to find real food improved with a greater number of trials, but again only for pooled data and not when results from each trial were compared with expected frequencies (29 trials, N = 279 visits, total G = 27.48, df = 29, P = 0.546, pooled G = 13.9, df = 1, P = 0.0002; Table 2).
Discussion
When examining the sensory cues that vervet monkeys used to locate food items, our experiments showed that goal-object cues were used more readily than global spatial and/or local layout cues. When all cues were available to the monkeys, they had no problem locating real rewards. However, in Experiment 1B, when a switch in the location of real versus sham rewards was done, most arriving monkeys went to platforms where goal-object cues showed that a real banana was present, rather than going to those that had previously contained real rewards. Of the goal-object cues that were available, the sight of real rewards seemed to be the most salient information for vervets. They did not appear to pay as much attention to olfactory cues. Indeed, when first exposed to the sham bananas (which had previously been kept separate from real bananas and did not smell authentic), the monkeys reacted to them as though they should be real; repeatedly smelling them, biting them, and sometimes taking them away from the platforms and up trees. Biting of the sham bananas led to the total destruction of two of them. The dominance of sight over smell when foraging was not unexpected for vervets given their enhanced visual system and limited olfactory sense (Barton 2006; Jacobs 2009).
Reliance on goal-object cues over the cues to spatial location provided by the global spatial relationship of the platforms in the environment and the local layout of resources on each platform, however, differs for vervets compared with the results of similar studies on New World monkeys (Garber and Dolins 1996; Garber and Lavallee 1999; Bicca-Marques and Garber 2004). For white-faced capuchins (Cebus capucinus), Garber and Lavallee (1999) found that global spatial and local layout cues were largely relied upon when locating food resources, though this study did not differentiate between these distant and more near-to-site types of information. Similarly, Bicca-Marques and Garber (2004) found that night monkeys (Aotus nigriceps), emperor tamarins (S. imperator imperator), saddle-back tamarins (S. fuscicollis weddelli), and titi monkeys (Callicebus cupreus) were able to find food when only global spatial and local layout cues were available to them. For moustached tamarins (Saguinus mystax mystax) that also found food using spatial cues, the study design used by Garber and Dolins (1996) allowed differentiation of the use of global spatial and local layout cues. The results of these experiments indicated that moustached tamarins primarily used more distal global spatial cues to locate food over the use of local layout cues. In our study with a similar sample size, the poorer performance of vervet monkeys on Experiments 2A and 2B suggested that, in small-scale space, they do not readily use global spatial and/or local layout cues to find food sources. The monkeys’ visits to platforms containing real food rewards in Experiment 2A did not reach significance and upon rotation of the location of rewards, most individuals did not return initially to sites that had previously held real food. In Experiment 2B, the vervets again failed to visit platforms with real rewards more than those with sham bananas.
An obvious difference between catarrhine vervet monkeys and the platyrrhines that have been previously tested by Garber and colleagues (Garber and Dolins 1996; Garber and Lavallee 1999; Bicca-Marques and Garber 2004) is the specialization of their visual system. Relative to platyrrhines, catarrhines have an expanded parvocellular pathway, which aids in visual acuity in the central visual field and in color vision (Kaas and Huerta 1988; Isbell 2009). Indeed, trichromatic color vision is ubiquitous in Old World monkeys, but among the New World monkeys examined by Garber and colleagues (Garber and Dolins 1996; Garber and Lavallee 1999; Bicca-Marques and Garber 2004), night (or owl) monkeys are uniformly monochromatic (Jacobs et al. 1996) and capuchins, tamarins, and titi monkeys are polymorphic for this trait, with males being dichromatic and females showing a mix of trichromatic and dichromatic phenotypes (Jacobs 2009). Nevertheless, differences in color vision and visual acuity between catarrhines and platyrrhines seem unlikely to explain why vervets failed to easily locate food resources using global spatial and local layout cues, since spatial tasks are inherently visual.
We suggest that our results do not show with certainty that vervets cannot use spatial information to find food, only that spatial cues may be relatively less important when compared with platyrrhines, and that the smaller effect might require a larger sample size to be revealed. It is notable that in large-scale space (i.e., the area that cannot be seen entirely from a single vantage point, sensu Byrne 2000), the vervets’ knowledge of where in their home range the feeding platforms were located, indicated memory and use of spatial information on a daily basis. The proximal spatial location of food in small-scale space changes more often and is less stable than distal spatial layout, like the location of food trees in a home range (Poucet 1993); therefore, it may be less cognitively costly for vervets to rely heavily on goal-object cues in proximal situations, not committing proximal spatial cues to memory, and to use memory only for stable, distal cues in their environment. The ability of primates to navigate directly to out-of-sight resources in large-scale space has been shown for several species and suggests that spatial memory is important for efficient travel around large home ranges (Saguinus mystax, S. fuscicollis, Garber 1989; Cebus apella, Janson and Di Bitetti 1997; Pithecia pithecia, Cunningham and Janson 2007; Ateles geoffroyi, Valero and Byrne 2007; Lophocebus albigena johnstoni, Janmaat et al. 2006a, b; Papio ursinus, Noser and Byrne 2007; Hylobates lar, Asensio et al. 2011; Pan troglodytes, Normand and Boesch 2009).
The vervet niche may also help explain their strong reliance on goal-object cues, especially vision. Unlike many primates, vervets usually occupy savannah–woodland ecosystems and are found in open areas and along forest edges (Enstam Jaffe and Isbell 2009); thus, they often forage through areas where long-range visual information can be readily used to detect food and predators (Sumner and Mollon 2000). For species residing in closed forests, vision may not be as heavily relied upon because individuals often have their sight blocked by barriers. In more open habitats, animals may become reliant on vision and color vision may aid this adaptation. For instance, in polymorphic marmosets (Callithrix geoffroyi), Caine and Mundy (2000) found that trichromats were better than dichromats at detecting food at distances up to 6 m. To our knowledge, food detection by wild primates at ranges greater than 6 m has not been examined relative to their visual system, but species in open areas with color vision may be at a visual advantage.
Though our study did not set out to explicitly test rule-based foraging for vervet monkeys, their failure to find food using only global spatial and/or local layout cues implies that they do not use a win-stay foraging rule. This result is curious considering that this foraging strategy has been found in several primate species and that individuals would have improved foraging success if they were able to predict the location of productive feeding sites (Terborgh 1983; Garber 1989; 2000; Garber and Dolins 1996; Janson 1996). More research is needed before it can be concluded that vervets cannot use nearby spatial cues to find food and that they do not use a win-stay foraging rule. We make this statement because the results of Experiment 2A (P = 0.062) suggest that with a greater sample size, vervets would use a win-stay rule. Certainly, during their natural foraging, vervets often returned to small fruit trees that they previously fed from, indicating that win-stay is used by them in some circumstances. Though our goal here was to replicate earlier studies on platyrrhines, future research should focus on experiments specifically designed to test rule-based foraging and on increasing trial number, perhaps by having multiple feeding stations with the same platform set up, in different areas of the home range. The spatial position of food may also need to stay the same over longer time periods than those tested here (6 days) before vervet monkeys encode this information. Differences between species in the capacity to easily remember food site location may be related to their natural history. For example, Platt et al. (1996) found that the abilities of golden lion tamarins (Leontopithecus rosalia) and Wied’s marmosets (Callithrix kuhli) to use a win-stay rule and remember food location over variable time periods depended upon the time it takes for their resources to renew in natural situations. The tamarins resources were more slowly renewing than those used by the marmosets, which may have been associated with the tamarins’ better long-term memory of resource location.
Like many other animals (reviewed in: Spetch and Kelly 2006), vervet monkeys were able to use associative cues to orient themselves and find hidden food resources. The use of an associative cue shows egocentric localization (Burgess 2006) but does not inform us about the navigation strategies or the types of allocentric representations that vervets may be capable of. Future research will expand upon these data and investigate if vervets can also use the relationship between an array of landmarks to accurately find food rewards, a task that would allow it to be determined if they can use an allocentric strategy to navigate (Sutton et al. 2000; MacDonald et al. 1994; Potì et al. 2005; 2010; Marsh et al. 2011).
This study demonstrates that the use of comparable experimental methods with distantly related species can reveal important similarities and differences in their use of spatial information and different sensory modalities. The finding that documented differences often appear to map onto phylogeny and niche indicates that variation is a result of the evolutionary path followed by each species. It also raises intriguing questions concerning the selective pressures that lead to variation in spatial abilities and the reliance on certain sensory modalities over others. The fields of sensory ecology and spatial cognition appear ripe for future research taking a comparative and evolutionary perspective.
References
Andrews MW (1988) Selection of food sites by Callicebus moloch and Saimiri sciureus under spatially and temporally varying food distribution. Learn Motiv 19:254–268
Asensio N, Brockelman WY, Malaivijitnond S, Reichard UH (2011) Gibbon travel paths are goal oriented. Anim Cogn 14:395–405
Balda RP, Turek RJ (1984) The cache-recovery system as an example of memory capabilities in Clark’s nutcrackers. In: Roiblat HL, Bever TG, Terrace HS (eds) Animal cognition. Erlbaum, Hillsdale, pp 513–532
Barton RA (2006) Olfactory evolution and behavioral ecology in primates. Am J Primatol 68:545–558
Bicca-Marques JC (2005) The win-stay rule in within-patch foraging decisions in free-ranging titi monkeys (Callicebus cupreus cupreus) and tamarins (Saguinus imperator imperator and S. fuscicollis weddelli). J Comp Psychol 119:343–351
Bicca-Marques JC, Garber PA (2004) Use of spatial, visual, and olfactory information during foraging in wild nocturnal and diurnal anthropoids: a field experiment comparing Aotus, Callicebus, and Saguinus. Am J Primatol 62:171–187
Brodbeck DR (1994) Memory for spatial and local cues: a comparison of a storing and nonstoring species. Anim Learn Behav 22:119–133
Burgess N (2006) Spatial memory: how egocentric and allocentric combine. Trends Cogn Sci 10:551–557
Byrne RW (2000) How monkeys find their way: leaderships, coordination, and cognitive maps of African baboons. In: Boinski Sue, Garber Paul A (eds) On the move: how and why animals travel in groups. Academic Press, London, pp 239–264
Byrne RW, Noser R, Bates LA, Jupp PE (2009) How did they get here from there? Detecting changes of direction in terrestrial ranging. Anim Behav 77:619–631
Cain P, Malwal S (2002) Landmark use and development of navigational behaviour in the weakly electric fish Gnathoneus petersii (Mormyridae; Teleostei). J Exper Biol 205:3915–3923
Caine NG, Mundy NI (2000) Demonstration of a foraging advantage for trichromatic marmosets (Callithrix geoffroyi) dependent on food colour. Proc R Soc Lond B Biol Sci 267:439–444
Cartwright BA, Collett TS (1983) Landmark learning in bees: experiments and models. J Comp Physiol A 151:521–543
Charles-Dominique P (1977) Ecology and behaviour of nocturnal primates. Columbia University Press, New York
Cheney DL, Seyfarth RM (1992) How monkeys see the world. University of Chicago Press, Chicago
Cheng K (1986) A purely geometric module in the rat’s spatial representation. Cognition 23:149–178
Cheng K (1989) The vector sum model of pigeon landmark use. J Exp Psychol 15:366–375
Cheng K, Sherry DF (1992) Landmark-based spatial memory in birds (Parus atricapillus and Columba livia): the use of edges and distances to represent spatial positions. J Comp Psychol 106:331–341
Collett TS, Cartwright BA, Smith BA (1986) Landmark learning and visuo-spatial memories in gerbils. J Comp Physiol A 158:835–851
Colquhoun IC (2011) A review and interspecific comparison of nocturnal and cathemeral strepsirhine primate olfactory behavioural ecology. Int J Zool 2011:1–11
Cramer AE, Gallistel CR (1997) Vervet monkeys as travelling salesmen. Nature 387:464
Cunningham EP, Janson CH (2007) Integrating information about location and value of resources by white-faced saki monkeys (Pithecia pithecia). Anim Cogn 10:293–304
Dolins FL (2009) Captive cotton-top tamarins’ (Saguinus oedipus oedipus) use of landmarks to localize hidden food items. Am J Primatol 71:316–323
Dolins FL, Mitchell RW (2010) Linking spatial cognition and spatial perception. In: Dolins FL, Mitchell RW (eds) Spatial cognition, spatial perception: mapping the self and space. Cambridge University Press, UK, pp 1–31
Døving KB, Trotier D (1998) Structure and function of the vomeronasal organ. J Exp Biol 201:2913–2925
Enstam Jaffe K, Isbell LA (2009) After the fire: benefits of reduced ground cover for vervet monkeys (Cercopithecus aethiops). Am J Primatol 71:252–260
Erhart EM, Overdorff DJ (2008) Spatial memory during foraging in Prosimian primates: Propithecus edwardsi and Eulemur fulvus rufus. Folia Primatol 79:185–196
Estes RD (1992) Behaviour guide to African mammals. University of California Press, Los Angeles
Etienne AS (1987) The control of short-distance homing in the golden hamster. In: Ellen P, Thinus-Blanc C (eds) Cognitive processes and spatial orientation in animal and man, vol 1., Experimental animal psychology and ethologyMartinus Nijohoff, Dordrectht, pp 233–251
Etienne AS, Maurer R, Saucy F (1988) Limitations in the assessment of path dependent information. Behaviour 106:81–111
Etienne AS, Berlie J, Georgakopoulos J, Maurer R (1998) Role of dead reckoning in navigation. In: Healy S (ed) Spatial representations in animals. Oxford University Press, Oxford, pp 54–68
Foo P, Warren WH, Duchon A, Tarr MJ (2005) Do humans integrate routes into a cognitive map? Map- versus landmark-based navigation of novel shortcuts. J Exp Psychol 31:195–215
Gallistel CR (1990) The organization of learning. MIT Press, Cambridge
Gallistel CR (2009) The importance of proving the null. Psychol Rev 116:439–453
Garber P (1989) Role of spatial memory in primate foraging patterns: Saguinus mystax and Sagunius fuscicollis. Am J Primatol 19:203–216
Garber PA (2000) The ecology of group movement: evidence for the use of spatial, temporal, and social information by some primate foragers. In: Boinski S, Garber PA (eds) On the move: how and why animals travel in groups. Chicago University Press, Chicago, pp 261–298
Garber PA, Brown E (2006) Use of landmark cues to locate feeding sites in wild capuchin monkeys (Cebus capucinus): an experimental field study. In: Estrada A, Garber PA, Pavelka MSM, Luecke L (eds) New perspectives in the study of Mesoamerican primates. Springer, New York, pp 311–332
Garber PA, Dolins FL (1996) Testing learning paradigms in the field: evidence for use of spatial and perceptual information and rule-based foraging in wild moustached tamarins. In: Norconk MA, Rosenberger AL, Garber PA (eds) Adaptive radiation of neotropical primates. Plenum Press, New York, pp 201–216
Garber PA, Lavallee A (1999) Experimental approaches to the study of primate cognition in natural and near-to-wild field settings. In: Garber PA, Leigh S (eds) Readings in the biological bases of human behavior. Pearson Custom Publishers, Massachusetts, pp 71–98
Garber PA, Paciulli LM (1997) Experimental field study of spatial memory and learning in wild capuchin monkeys (Cebus capucinus). Folia Primatol 68:236–253
Groves C (2001) Primate taxonomy. Smithsonian, Washington
Hermer L, Spelke ES (1994) A geometric process for spatial reorientation in young children. Nature 370:57–59
Hribar A, Call J (2011) Great apes use landmark cues over spatial relations to find hidden food. Anim Cogn 14:623–635
Isbell LA (2009) The fruit, the tree, and the serpent: why we see so well. Harvard University Press, Cambridge
Isbell LA, Pruetz JD, Young TP (1998) Movements of vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) as estimators of food resource size, density, and distribution. Behav Ecol Sociobiol 42:123–133
Jacobs GH (2009) Evolution of colour vision in mammals. Phil Trans R Soc Lond B 364:2957–2967
Jacobs GH, Neitz M, Neitz J (1996) Mutations in S-cone pigment genes and the absence of colour vision in two species of nocturnal primate. Proc R Soc Lond B Biol Sci 263:705–710
Janmaat KRL, Byrne RW, Zuberbühler K (2006a) Evidence for a spatial memory of fruiting states of rainforest trees in wild mangabeys. Anim Behav 72:797–807
Janmaat KRL, Byrne RW, Zuberbühler K (2006b) Primates take weather into account when searching for fruits. Curr Biol 16:1232–1237
Janson CH (1996) Towards an experimental socioecology of primates: examples for Argentine brown capuchin monkeys (Cebus apella nigritus). In: Norconk MA, Rosenberger AL, Garber PA (eds) Adaptive radiation of neotropical primates. Plenum Press, New York, pp 309–325
Janson CH (2007) Experimental evidence for route integration and strategic planning in wild capuchin monkeys. Anim Cogn 10:341–356
Janson CH (2011) Reconciling rigor and range: observations, experiments, and quasi- experiments in field primatology. Int J Primatol 33:520–541
Janson CH, Byrne R (2007) What wild primates know about resources: opening up the black box. Anim Cogn 10:357–367
Janson CH, Di Bitetti MS (1997) Experimental analysis of food detection in capuchin monkeys: effects of distance, travel speed, and resource size. Behav Ecol Sociobiol 41:17–24
Kaas JH, Huerta MF (1988) The subcortical visual system of primates. In: Steklis HD, Erwin J (eds) Comparative primate biology, vol 4. Alan RLiss, New York, pp 327–391
Kass RE, Raftery AE (1995) Bayes factors and model uncertainty. J Am Statist Ass 90:773–795
Kruschke JK (2011) Bayesian assessment of null values via parameter estimation and model comparison. Prospect Psychol Sci 6:299–312
MacDonald SE (1994) Gorillas’ (Gorilla gorilla gorilla) spatial memory in a foraging task. J Comp Psychol 104:382–397
MacDonald SE, Agnes MM (1999) Orangutan (Pongo pygmaeus abelii) spatial memory and behavior in a foraging task. J Comp Psychol 113:213–217
MacDonald SE, Wilkie DM (1990) Yellow-nosed monkeys (Cercopithecus ascanius whitesidei) spatial memory in a simulated foraging experiment. J Comp Psychol 104:382–387
MacDonald SE, Pang JC, Gibeault S (1994) Marmoset (Callithrix jacchus jacchus) spatial memory in a foraging task: win-stay versus win-shift strategies. J Comp Psychol 108:328–334
MacKinnon J (1974) The behaviour and ecology of wild orang-utans (Pongo pygmaeus). Anim Behav 22:3–74
MacLean EL, Matthews LJ, Hare BA, Nunn CL, Anderson RC, Aureli F, Brannon EM, Call J, Drea CM, Emery NJ, Haun DBM, Herrmann E, Jacobs LF, Platt ML, Rosati AG, Sandel AA, Schroepfer KK, Seed AM, Tan J, van Schaik CP, Wobber V (2012) How does cognition evolve? Phylogenetic comparative psychology. Anim Cogn 15:223–238
Marsh HL, Spetch ML, MacDonald SE (2011) Strategies in landmark use by orangutans and human children. Anim Cogn 14:487–502
Martin RD (1990) Primate origins and evolution. Princeton University Press, New Jersey
McDonald JH (2009) Handbook of biological statistics, 2nd edn. Sparky House Publishing, Baltimore
Menzel EW (1973) Chimpanzee spatial memory organization. Science 182:943–945
Normand E, Boesch C (2009) Sophisticated Euclidean maps in forest chimpanzees. Anim Behav 77:1195–1201
Noser RW, Byrne RW (2007) Mental maps in chacma baboons (Papio ursinus): using intergroup encounters as a natural experiment. Anim Cogn 10:331–347
Noser RW, Byrne RW (2010) How do wild baboons (Papio ursinus) plan their routes? Travel among multiple high-quality food sources with inter-group competition. Anim Cogn 13:145–155
Pariente G (1979) The role of vision in prosimian behavior. In: Doyle GA, Martin RD (eds) The study of prosimian behavior. Academic Press, New York, pp 411–459
Platt ML, Brannon EM, Briese TL, French JA (1996) Differences in feeding ecology predict differences in performance between golden lion tamaris (Leontopithecus rosalia) and Wied’s marmosets (Callithrix kuhli) on spatial and visual memory tasks. Anim Learn Behav 24:384–393
Potì P, Bartolommei P, Saporiti M (2005) Landmark use by Cebus apella. Int J Primatol 26:921–948
Potì P, Kanngiesser R, Saporiti M, Amiconi A, Bläsing B, Call J (2010) Searching the middle- capuchins’ (Cebus apella) and bonobos’ (Pan paniscus) behavior during a spatial search task. J Exp Psychol Anim Behav Process 36:92–109
Poucet B (1993) Spatial cognitive maps in animals: new hypotheses on their structure and neural mechanisms. Psychol Rev 100:163–182
Rushmore J, Leonhardt SD, Drea CM (2012) Sight or scent: lemur sensory reliance in detecting food quality varies with feeding ecology. PLoS One 7:e41558
Saj TL, Paterson JD, Sicotte P (2001) The conflict between vervet monkeys and farmers at the forest edge in Entebbe, Uganda. Afr J Ecol 39:195–199
Séguinot V, Maurer R, Etienne AS (1993) Dead reckoning in a small mammal: the evaluation of distance. J Comp Physiol A 173:103–113
Shettleworth SJ (2010) Cognition, evolution, and behavior. Oxford University Press, New York
Skinner JD, Smithers RHN (1990) The mammals of the southern African subregion. University of Pretoria, Pretoria
Smith T, Rossie J (2006) Primate olfaction: anatomy and evolution. In: Brewer WJ, Castle D, Pantelis C (eds) Olfaction and the brain. Cambridge University Press, New York, pp 135–166
Sokal RR, Rohlf FJ (1981) Biometry. WH Freeman, New York
Spetch ML, Kelly DM (2006) Comparative spatial cognition: processes in landmark- and surface-based place finding. In: Wasserman EA, Zentall TR (eds) Comparative cognition: experimental explorations of animal intelligence. Oxford University Press, New York, pp 210–228
Sumner P, Mollon JD (2000) Catarrhine photopigments and optimized for detecting targets against a foliage background. J Exp Biol 203:1963–1986
Sutton JE, Olthof A, Roberts WA (2000) Landmark use by squirrel monkeys (Saimiri sciureus). Anim Lean Behav 28:28–42
Suzuki S, Augerinos G, Black AH (1980) Stimulus control of spatial behavior on the eight-arm maze in rats. Learn Motiv 11:1–18
Terborgh J (1983) Five new world primates. Princeton University Press, Princeton
Valero A, Byrne RW (2007) Spider monkey ranging patterns in Mexican subtropical forest: do travel routes reflect planning? Anim Cogn 10:305–331
Vallortigara G, Zanforlin M, Pasti G (1990) Geometric modules in animals’ spatial representations: a test with chicks (Gallus gallus domesticus). J Comp Psychol 104:248–254
Vickers NJ (2000) Mechanisms of animal navigation in odor plumes. Biol Bull 198:203–212
Waller D, Lippa Y (2007) Landmarks as beacons and associative cues: their role in route learning. Mem Cogn 35:910–924
Wang RF, Spelke ES (2002) Human spatial representation: insights from animals. Trends Cogn Sci 6:376–382
Warburton K (1990) The use of local landmarks by foraging goldfish. Anim Behav 40:500–505
Wehner R, Raeber F (1979) Visual spatial memories in desert ants, Cataglyphis bicolor (Hymenoptera: Formicidae). Experientia 35:1569–1571
Wehner R, Srinivasan MV (1981) Searching behaviour of desert ants, genus Cataglyphis. J Comp Physiol A 142:315–338
Wehner R, Lehrer M, Harvey P (1996) Navigation. J Exp Biol 199:1–261
Wrangham RW, Waterman PG (1981) Feeding behaviour of vervet monkeys on Acacia tortilis and Acacia xanthophloea: with special reference to reproductive strategies and tannin production. J Anim Ecol 50:715–731
Acknowledgments
We thank the Uganda Wildlife Authority and the Uganda National Council for Science and Technology for permission to conduct this research, and methods were approved by the McGill University Animal Care committee. We are also grateful to Dennis Twinomugisha, Patrick Omeja, Lauren Chapman, Matovu Ponsiano, Maxine White, Jan Gogarten, and Jessica Rothman, who provided assistance both in and out of the field. Paul Garber provided valuable advice at the beginning of this study, and this manuscript was improved with helpful comments from Amanda Melin. Two anonymous reviewers improved the quality of this manuscript. Funding was provided by the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teichroeb, J.A., Chapman, C.A. Sensory information and associative cues used in food detection by wild vervet monkeys. Anim Cogn 17, 517–528 (2014). https://doi.org/10.1007/s10071-013-0683-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-013-0683-2