Abstract
There is controversy in comparative psychology about whether on the one hand non-symbolic number estimation of small (≤4) and large numbers involves a single mechanism (an approximate number system), or whether on the other hand enumeration of the numbers 1–4 is accomplished by a separate mechanism, an object tracking system. To date, support for the latter hypothesis has come only from the different ratio-dependency of performance seen in the two numerical ranges, a reading that has been criticized on several grounds. In humans, the two-system hypothesis is supported by evidence showing that manipulation of the physical properties of the stimuli (e.g., the motion of the items) has dissimilar effects on small- and large-number discrimination. In this research, we studied this effect on guppies. Initially, fish were trained to simultaneously discriminate two numerical contrasts having the same easy ratio (0.50): one in the small-number (2 vs. 4) range and one in the large-number (6 vs. 12) range. Half of the fish were presented with moving items; the other half were shown the same stimuli without motion. Fish were then subjected to non-reinforced probe trials in the presence of a more difficult ratio (0.75: 3 vs. 4 and 9 vs. 12). Under both static and moving conditions, the fish significantly discriminated 6 versus 12, but not 9 versus 12 items. As regards small numbers, both groups learned to discriminate a 0.50 ratio, but only fish tested with moving stimuli also discriminated 3 and 4 items. This differential effect suggests that fish may possess two separate systems for small- and large-number discrimination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies in cognitive psychology have demonstrated that humans use three types of processes—counting, estimating, and subitizing—to establish which group of items is more numerous (Chesney and Haladjian 2011). Counting (Gelman and Gallistel 1978) assesses numerosity by assigning each item an exact number label from an ordered count list. This process, uniquely human, is very accurate but relatively slow, with response time becoming longer as the number of items in the set increases. Estimating (Kaufman et al. 1949) assesses the numerosity of a set quickly, instead, without a serial count. This process is supposed to be supported by an approximate number system (ANS) that is fast but poorly accurate (Nieder and Dehaene 2009). For example, by using the ANS, we can quickly estimate that there are about 40 colleagues in the conference room or roughly 100 visitors queued at the Eiffel Tower. Subitizing (Jevons 1871; Revkin et al. 2008) is another way to estimate the numerosity of a set without a serial count. This process is fast and accurate but limited to up to 3–4 items. While for numbers greater than 4 discrimination between two quantities is dependent on their ratio (e.g., 8 vs. 12 is easier than 8 vs. 10), the numerical ratio is usually irrelevant in the range of 1–4 (e.g., performance is similar for 1 vs. 4 and 3 vs. 4). Several authors hypothesize that subitizing is linked to a system for representing and tracking individual objects (Chesney and Haladjian 2011; Feigenson et al. 2004; Trick and Pylyshyn 1994), known as the object tracking system (OTS). OTS is believed to be an evolutionarily ancient process that allows individuals to track up to 4 objects in parallel, even if these are moving in space and undergo brief periods of occlusion (Assad and Maunsell 1995; Scholl and Pylyshyn 1999; Trick and Pylyshyn 1994).
Other authors, however, argue that it is not necessary to invoke two distinct numerical systems and that ANS suffices to account for discrimination in the whole numerical range (Gallistel and Gelman 1992; van Oeffelen and Vos 1982; Vetter et al. 2008). As noted by Gallistel and Gelman (1992), the representation of larger numbers is more variable and, as a consequence, representation of nearest values may overlap in the large number range, leading to lower accuracy. In contrast, in the subitizing range (1–4), ANS representations would have lower variability and there would be almost no overlap; thus, correct values would be consistently produced, leading to very accurate performance. In addition, while adults can discriminate even a 0.9 ratio (Halberda et al. 2008), the most difficult ratio in the subitizing range is 0.75 (3 vs. 4 items); therefore, ratio dependence might not appear, due to a ceiling effect in performance (Ross 2003). Others have suggested that the different performance results in the subitizing range might be due to pattern recognition rather than to numerical processes per se. In bi-dimensional displays, small sets of items are typically arranged in recognizable geometric patterns (i.e., 1 item = a dot; 2 items = a line; 3 items = a triangle), which is not possible for sets of more than 4 items (Mandler and Shebo 1982; Neisser 1967; Woodworth and Schlosberg 1954).
One way to test the above-mentioned alternatives is by looking at factors other than ratio dependency. For example a potential prediction of the one-system hypothesis is that manipulation of physical properties of the stimuli should never have opposed effects on the estimation of small and large numbers, while the hypothesis for the existence of separate systems would allow for this possibility. Trick (2008) tested whether item heterogeneity affects performance differently in the subitizing and estimation ranges in adult humans. The results showed that heterogeneity slowed enumeration in the subitizing range and sped-up enumeration in the estimation range, a dissociation that would seem to negate the one-system hypothesis. Moving versus static items represent another variable that seems to affect numerical estimation differentially in humans. Trick et al. (2003) observed that even very slow motion reduced enumeration speed for stimuli containing 6–9 items, while the enumeration of 1–4 items was not affected when items were in motion. Similarly, Alston and Humphreys (2004) presented static and moving items, finding that faster and more accurate enumeration occurred in the subitizing range given the presence of moving items. Again these results tend to support the existence of separate systems for small- and large-number enumeration.
In recent years, numerical abilities have been reported in numerous animal species. Comparative psychology is explicitly focused on those processes that do not require symbolic number labels (estimation and subitizing). Accumulating evidence indicates that non-symbolic numerical abilities are present in mammals (Beran et al. 2012, 2013; Bonanni et al. 2011; Evans et al. 2010), in birds (Garland et al. 2012; Hunt et al. 2008), in fish (Agrillo et al. 2008a, 2011; Buckingham et al. 2007; Gómez-Laplaza and Gerlai 2012) and in insects (Dacke and Srinivasan 2008; Gross et al. 2009; Pahl et al. 2013).
The similar performance reported in the literature for human and non-human species has led several authors to hypothesize the existence of the same numerical abilities among vertebrates (Beran 2008a; Feigenson et al. 2004). As in cognitive psychology, there is an open debate as to whether non-human animals display a single ANS for discrimination over the whole numerical range (Evans et al. 2009; Perdue et al. 2012; Ward and Smuts 2007), or a distinct OTS over the small-number range (Bonanni et al. 2011; Cutini and Bonato 2012; Hauser et al. 2000; Hunt et al. 2008). To date, empirical studies have focused on comparing ratio dependency on accuracy and reaction times in small- and large-number discrimination. For instance, Agrillo et al. (2012a) compared the performance of humans and guppies, presenting the same ratios for small (<4) and large quantities and finding that accuracy for both species was affected by the ratio in the large- but not in the small-number range. Yet these results are subject to the same criticism raised for human studies, and different ratio sensitivity in the range of 1–4 does not automatically imply the existence of two distinct numerical systems (Gallistel and Gelman 1992; Ross 2003).
In the present study, we adopted a different approach to this issue. We tested whether, as in humans, the motion of the items for enumeration had a different influence on large- and small-number discrimination in fish. For this purpose, we trained guppies to discriminate between groups containing different quantities of bi-dimensional geometric figures, using either static or moving objects.
Methods
Subjects
Twelve adult guppies (Poecilia reticulata) were tested. Subjects were maintained at the Department of General Psychology in 150 one-stock aquaria containing mixed-sex groups (15 individuals with approximately a 1:1 sex ratio). Aquaria were provided with natural gravel, an air filter, and live plants. Both stock aquaria and experimental tanks were maintained at a constant temperature of 25 ± 1 °C and a 14:10 h light:dark (L:D) photoperiod with an 18 W fluorescent light. Before the experiment, fish were fed twice daily to satiation with commercial food flakes and live brine shrimp (Artemia salina).
Apparatus and stimuli
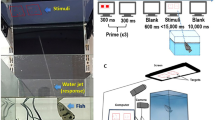
The experimental apparatus was previously used to investigate visual perception in guppies (Gori et al. in preparation). It was composed of a 50 × 19 × 32 cm tank filled with gravel and 25 cm of water. The long walls were covered with green plastic material. To reduce the potential effects of social isolation (Miletto Petrazzini et al. 2012), two mirrors (29 × 5 cm) were placed in the middle of the tank, 3 cm away on either side from the long walls. At the two short ends of the tank, two identical monitors (19 inch) were used to present the stimuli (Fig. 1).
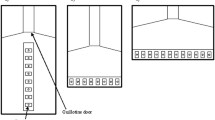
The stimuli employed in the training phase were pairs of geometrical figures (13 × 13 cm each array) differing in numerosity: 2 versus 4 (small-number range) and 6 versus 12 (large-number range). In the test phase, we presented the following numerical contrast: 3 versus 4 (small-number range) and 9 versus 12 (large-number range). Each array could contain circles, ovals, triangles, crosses, stars, squares, and rectangles (black figures on a white background, Fig. 2). It is known that numerosity co-varies with other physical attributes, such as cumulative surface area, overall space encompassed by the stimuli, and density of the elements; as well, it is known that human and non-human animals can use these non-numerical cues to estimate which group is larger/smaller (Gebuis and Reynvoet 2012; Kilian et al. 2003; Feigenson et al. 2002b). Cumulative surface area was controlled to reduce the possibility of fish using non-numerical cues. In particular, for one-third of the stimuli, the two numerosities were 100 % equated for cumulative surface area. However, a by-product of equating the cumulative surface area was that smaller-than-average figures would be more frequent in the larger groups and fish might use this cue instead of number. To reduce this possibility, cumulative surface area was controlled to 85 % in another third of the stimuli during the training phase, and, in the remaining one-third of the stimuli, it was controlled to 70 %. As a consequence, in the 70 % condition the biggest figure within each pair was shown in the larger set; in the 85 % condition the biggest figure was shown in the larger set in half of the trials and in the smaller set in the other half of trials. In probe trials, cumulative surface area was always matched to 100 %. In this way, should the fish discriminate between the matched quantities in probe trials, neither cumulative surface area—matched to 100 %—nor the individual figure size—an unreliable cue in the training phase—could have played a key-role. In addition, given that density (inter-individual distance) is negatively correlated to overall space encompassed by the most lateral figures, half of the set was controlled for overall space, whereas the second half was controlled for density. Cumulative surface area, density, and overall space represent the non-numerical variables most frequently controlled in numerical cognition studies (Durgin 1995; Kilian et al. 2003; Pisa and Agrillo 2009; Vos et al. 1988). Such a procedure was adopted in previous studies of fish (Agrillo et al. 2012b, c).
Schematic representation of the stimuli. Stimuli consisted of two groups of geometric figures differing in numerosity (either static or in motion). Here we depicted an example of a 9 versus 12 contrast, with cumulative surface area controlled to 100 % (a), to 85 % (b) and to 70 % (c). In a and c stimuli are controlled for the overall space encompassed by the most lateral figures, whereas in (b) they are controlled for density
For each numerical contrast, we set up two different conditions: in the first (‘moving stimuli’), the items were moving on the screen; in the second, the same figures were presented without any motion (‘static stimuli’). As the overall quantity of motion could be another non-numerical cue used by animals to discriminate between two numerosities (Agrillo et al. 2008b; Krusche et al. 2010; Gómez-Laplaza and Gerlai 2012), two different sub-conditions were created with the moving stimuli. In the first sub-condition (controlled motion), the overall quantity of motion was equated between the larger and the smaller group. For instance in the 2 versus 4 contrast, the figures included in the smaller group moved twice as fast as those included in the larger group. However, a by-product of controlling for total motion was that faster-than-average items would appear more frequently in the smaller group, and fish might use this cue instead of number. As a consequence, we set up another sub-condition (non-controlled motion) where number and quantity of motion were simultaneously congruent. In this case, in the 2 versus 4 contrast, the quantity of motion for the larger group was double what it was for the smaller group. For each numerical contrast, half of the stimuli were controlled for motion, and the other half were not.
Moving and static stimuli were created and presented using Adobe Flash CS4®. For each numerical contrast, a total of 20 different pairs of stimuli were employed and alternated in pseudo-random order.
Six identical experimental tanks were employed. They were placed close to each other, on the same table, and lit by two fluorescent lamps (36 W). A video camera was suspended about 1 meter above the experimental tanks and used to record the position of the subjects during the tests.
Procedure
A modification of the procedure that has been adopted recently for investigating numerical competence in fish (Agrillo et al. 2012c) was employed here. The procedure diverges from standard operant conditioning procedures: in this case discrimination is inferred from the proportion of time spent near the trained stimulus during probe trials instead of from individual learning criterion (Agrillo et al. 2012b).
In details, the experiment was divided into three different steps: pre-training, training, and test. Pre-training was set up to familiarize the fish with the experimental apparatus. Subsequently, all fish were singly trained to discriminate an easy numerical ratio (0.50), both within (2 vs. 4) and outside (6 vs. 12) the subitizing range: half of the fish were tested in the presence of moving stimuli; the other half was tested in the presence of static stimuli. In the test phase, we assessed whether they could generalize the numerical rule to a more difficult numerical ratio (0.75) in both the small- (3 vs. 4) and the large-number (9 vs. 12) ranges.
Pre-training
To familiarize fish with the experimental environment, we allowed a two-day habituation period, starting 10 days before the beginning of the experiment. On the first day of habituation, a group of 6 fish was introduced to a slightly larger version of the tank (60 × 40 × 35 cm); on the second day, fish were grouped in three pairs and each pair was introduced to one of three experimental tanks (6 h each day). On both days, subjects were exposed to continuous changes in the background colour of the monitors (alternating between black and white).
After a five-day interval, when they were kept undisturbed in their home tank, we administered another identical two-day habituation treatment. The day before the beginning of the experiment, each fish was individually housed in one of the six experimental tanks.
Training
Fish were randomly assigned to one of two conditions: half of the fish were trained in the presence of moving stimuli; the other half in the presence of static stimuli. During this phase, they were subjected to four trials per day (three consecutive days, for a total of 12 trials). Soon after the stimuli appeared on the screens, the experimenter used a Pasteur pipette to release the food reward (Artemia nauplii) in correspondence with the reinforced numerosity; an identical syringe was used to simultaneously insert pure water close to the opposite stimulus. Subjects were left free to feed for 7 min. After this time, stimuli disappeared from the screen. The inter-trial interval lasted 3 h. Stimuli were presented in a pseudo-random sequence and the left–right positions of the stimuli were counterbalanced over the course of the trials. For each condition, half of the subjects were trained to move toward the larger numbers, whereas the other half was trained toward the smaller numbers as positive.
In order to measure learning to discriminate between reinforced and non-reinforced numerosity, on days 4 and 5, two probe trials were alternated each day with two reinforced trials (four overall probe trials for both days). In the probe trials, stimuli appeared on the screen for 4 min; no reinforcement was provided (extinction procedure) and the time spent by fish within a 12-cm distance (‘choice area’) from the monitors showing the stimuli was recorded as a measure of their capacity to discriminate between the two numerosities. Reinforced trials were identical to those described for days 1 to 3. To avoid the possibility of fish using local/spatial cues in their tank, each subject was moved from one tank to another at the end of each day.
Test
After a two-day interval, three probe trials were presented each day for four consecutive days (days 8–11). Novel numerical contrasts with higher ratios were presented at this time: 3 versus 4 (within the subitizing range) and 9 versus 12 (large-number range). Fish previously trained with moving stimuli were presented with moving stimuli; fish trained with static stimuli were shown static stimuli. The inter-trial interval lasted 3 h. Two reinforced trials with the same stimuli as presented in the training phase were alternated with the probe trials. As a dependent variable, we considered the proportion of time spent in the ‘choice areas’ (accuracy) during probe trials. Proportions were arcsine square-root transformed (Sokal and Rohlf 1995). Mean ± standard deviations were provided. Statistical tests were carried out using SPSS 20.0.
Results
Training
Moving stimuli
Fish spent significantly more time near the reinforced numerosity in both the 2 versus 4 (one sample t test, t(5) = 6.184, P = 0.002) and the 6 versus 12 (one sample t test, t(5) = 2.655, P = 0.045, Fig. 3) contrasts. No difference between the two numerical contrasts was found (paired t test, t(5) = 0.535, P = 0.616). When we contrasted the controlled and non-controlled motion condition, we found no difference for either 2 versus 4 (controlled motion, mean ± SD: 0.730 ± 0.302; non-controlled motion: 0.762 ± 0.217; paired t test t(5) = 0.123, P = 0.907) or 6 versus 12 (controlled motion: 0.655 ± 0.258; non-controlled motion: 0.833 ± 0.258; paired t test t(5) = 1.332, P = 0.240).
Results of the training phase. Accuracy (proportion of time spent near the reinforced numerosity) is plotted against numerical contrasts, for moving (dark grey columns) and static (white grey) stimuli. Fish learned to discriminate the two numerical contrasts, with both static and moving stimuli. Asterisks denote a significant departure from chance level (P < 0.05). Bars represent the standard error
On the whole, we found no difference in accuracy between fish reinforced with the larger or smaller numerosities as positive (larger numbers as positive: 0.708 ± 0.105; smaller numbers as positive, 0.809 ± 0.128; independent t test, t(4) = 1.052, P = 0.352).
Static stimuli
Fish spent significantly more time near the reinforced numerosity in 2 versus 4 (one sample t test, t(5) = 2.643, P = 0.046) and 6 versus 12 (one sample t test, t(5) = 4.752, P = 0.005, Fig. 3). No difference between the two numerical contrasts was found (paired t test, t(5) = 0.702, P = 0.514). On the whole we found no difference in the accuracy between fish trained with the larger or smaller numerosities as positive (larger numbers as positive, 0.779 ± 0.083; smaller numbers as positive, 0.639 ± 0.054; independent t test, t(4) = 2.443, P = 0.071). We also found no difference in accuracy between trials controlled for density (0.651 ± 0.329) and those controlled for overall space (0.744 ± 0.195, paired t test t(5) = 0.405, P = 0.702).
When we compared the two conditions (moving vs. static stimuli), we did not find any difference between fish trained with moving stimuli and those trained with static stimuli (2 vs. 4: independent t test, t(10) = 0.885, P = 0.397; 6 vs. 12: t(10) = 0.610, P = 0.556).
Test
To assess whether the ability to discriminate a 0.75 ratio varies as a function of movement and the numerical range, we performed a repeated measures ANOVA with Condition (Moving/Static stimuli) as the between subjects factor and Numerical contrast (3 vs. 4/9 vs. 12) as the within subjects factor. Main effects of Condition (F(1, 10) = 10.668, P = 0.008) and Numerical contrast (F(1, 10) = 5.460, P = 0.042) were found. The interaction was also significant (F(1, 10) = 5.054, P = 0.048), suggesting that fish succeeded with items in motion only in the small number range (Fig. 4). Because of the significant interaction, in subsequent analyses we examined the effect of moving and static stimuli separately.
Results of the test phase. In the presence of moving stimuli (dark grey columns), fish successfully discriminated 3 versus 4, but not 9 versus 12. No discrimination for either numerical contrast was found in the presence of static stimuli (white grey columns). Asterisks denote a significant departure from chance level (P < 0.05)
Moving stimuli
Fish spent more time near the reinforced numerosity in the 3 versus 4 contrast (one sample t test, t(5) = 5.639, P = 0.002), while no significant discrimination was found in the 9 versus 12 contrast (t(5) = 0.363, P = 0.731). Paired t tests showed a significant difference between the two numerical contrasts (t(5) = 2.655, P = 0.045). When we contrasted the controlled and the non-controlled motion conditions, we found no difference for 3 versus 4 (controlled motion: 0.843 ± 0.100; non-controlled motion: 0.770 ± 0.123; paired t test, t(5) = 1.550, P = 0.182), while we did find a significant difference for 9 versus 12 (controlled motion: 0.396 ± 0.155; non-controlled motion: 0.623 ± 0.291; t(5) = 2.733, P = 0.041).
Static stimuli
Fish did not discriminate either 3 versus 4 (one sample t test, t(5) = 0.089, P = 0.933) or 9 versus 12 (t(5) = 0.251, P = 0.812). Paired t tests showed no difference between the two numerical contrasts (t(5) = 0.054, P = 0.959).
Lastly, we analyzed whether the overall accuracy differed between moving and static stimuli for each numerical contrast. Independent t tests showed that fish were more accurate in the presence of moving stimuli for 3 versus 4 (t(10) = 3.511, P = 0.006) but not 9 versus 12 (t(10) = 0.440, P = 0.670).
Discussion
Our first finding is that the influence of motion differs as a function of the numerical range examined. Fish trained with the 0.50 ratio showed the same performance for small and large numbers, whether they were tested with static or moving objects. In contrast, when the ratio became more difficult (0.75), items in motion were successfully discriminated in the subitizing range while no effect of motion was found on large-number discrimination.
Different performance for static and moving items has been previously reported in the literature. Humans proved to be faster (Trick et al. 2003) and more accurate (Alston and Humphreys 2004) at enumerating the number of moving items in the range of 1–4. As concerns comparative psychology, the picture is less clear: it has been shown that old world (rhesus monkeys) and new world (capuchin monkeys) monkeys can discriminate the larger group of items in motion, apparently with the same effort required to discriminate static patterns (Beran 2008b). However, the performance was not analyzed separately for small and large numbers in that study, making it impossible to assess whether items in motion were better discriminated in the small-number range. Instead, a recent study found that black bears are better able to enumerate static rather than moving stimuli (Vonk and Beran 2012). The authors referred to the object tracking system (OTS) as a potential explanation for their findings: bears are known not to live in social groups and hence they might not have been subject to strong selective pressures in favor of the ability to track individual members of a group. In this sense, their performance would be worse for items in motion. On the contrary, for social species (including guppies), it would be important to track the presence of individual members of a moving group, and the precision of their OTS would be crucial for survival.
A second interesting finding is that, under some conditions, fish can discriminate up to a 0.75 ratio, a performance similar to that reported for most non-human primates (Beran 2004; Beran et al. 2008; Cantlon and Brannon 2007; Hauser et al. 2000). Previously, discrimination of a 0.75 ratio was reported when fish had to choose between two groups of social companions. However, the latter paradigm did not allow an accurate control for non-numerical continuous variables that co-vary with number; indeed, it was suggested that, in choosing the largest shoal, fish could have used primarily a proxy for number, such as the cumulative area occupied by stimuli (Agrillo et al. 2008b).
For both numerical ranges, the items to be enumerated were identical; the numerical ratio was the same, as well as the way we controlled for the continuous variables. How can we then explain the different effect of the items in motion on the performance for small and large numerosities? Many authors have suggested that the discrimination of a small number of items is generally accomplished by an object tracking system. This is believed to be a visual indexing mechanism that can individuate and follow several items within view (normally up to 4 items), keeping track of these items as they move or undergo brief periods of occlusion. The system, shared by human and non-human animals, would primarily serve to keep track of objects present in the perceptual space, such as social companions or prey (Bisazza et al. 2010; Bonanni et al. 2011; Hauser and Spelke 2004), but it could be also co-opted to enumerate precisely small quantities of objects producing the subitizing effect. The OTS should respond maximally to moving objects. Indeed it was recently shown that the accuracy of the OTS in keeping track of objects is higher in human and non-human primates for moving items (Matsuno and Tomonaga 2006). It was suggested that “motion” would be a basic feature in the primate visual system, and that the presence of that basic feature would be more easily detected by the OTS than its absence. Regardless of the exact reason underlying this difference, the better performance of the OTS with respect to items in motion aligns with the hypothesis according to which the activation of the OTS would facilitate better performance in numerical tasks involving small numbers.
While the existence of an ANS in non-human vertebrates is generally accepted, an open debate surrounds the possibility that non-human vertebrates are endowed with a distinct mechanism for enumerating small quantities. To date, evidence supporting the two-system hypothesis comes principally from empirical studies that have compared ratio dependency in small- and large-number discrimination. For instance, Agrillo et al. (2012a) showed that adult guppies are particularly accurate when required to differentiate the larger shoal from the smaller one in the range of 1–4. Fish successfully discriminated 1 versus 4 (ratio 0.25), 1 versus 3 (0.33), 1 versus 2 (0.50), 2 versus 3 (0.67), and 3 versus 4 fish (0.75) with the same accuracy, thus showing no influence of numerical ratio for small quantities. In contrast, their ability with larger quantities (>4) for the same ratios depended on numerical ratio, with their accuracy decreasing as numerical ratios increased between the small and the larger groups. Similarly, data in line with the two-system hypothesis have been reported for macaques (Flombaum et al. 2005; Hauser et al. 2000), dogs (Bonanni et al. 2011), New Zealand robins (Hunt et al. 2008), and angelfish (Gómez-Laplaza and Gerlai 2011). Nonetheless, as several authors noted, the different ratio sensitivity for small and large numbers may have alternative explanations. Our results provide further support to the idea of separate numerical systems as a function of numerical range. In addition, the fact that numerical acuity with items in motion is improved only with small numbers suggests the automatic activation of an OTS in fish similar to that described in adult humans.
In our species the automatic engagement of the OTS by items in motion may not be present at birth. Infants fail when comparing sets near the boundary of the OTS range in both moving (Feigenson and Carey 2005; Feigenson et al. 2002a; van Marle 2013) and static conditions (Cordes and Brannon, 2009; Xu et al. 2005) and the possibility exists that the automatic activation of the OTS varies across development. This debate extends far beyond the scope of this study. However, future studies on newborn guppies may help us to test this hypothesis, at least in non-human species. It is worth noting that the present method is relatively rapid compared to standard operant conditioning procedures, thus making it possible to study the development of numerical abilities in a rapidly growing species, such as guppies. Indeed previous studies showed that newborn guppies can be trained to discriminate between groups of static figures differing in numerosities in only 2 weeks (Miletto Petrazzini et al. 2013; Piffer et al. 2013). The influence of motion in the OTS range could now be investigated in newborn and juvenile fish.
As an alternative explanation for the different ratio dependency seen in the two numerical ranges, some authors have suggested that small sets of items usually generate recognizable geometric patterns—the so-called ‘pattern recognition’ hypothesis (Mandler and Shebo 1982; Neisser 1967). Our study does not support this view, as we found that items in motion, whose general configuration is continuously dynamic and for which no stable pattern can be easily recognized hence, are better discriminated by comparison to static patterns. One may also argue that better performance with items in motion might reflect the use of a non-numerical cue, namely the total quantity of motion, as a proxy for number (Gómez-Laplaza and Gerlai 2012). However, this is not the case, as we did not find any difference in the range of 1–4 (2 vs. 4 and 3 vs. 4) and in 6 versus 12, whether stimuli were controlled or not controlled for the total quantity of motion. In contrast, it is interesting to note that the accuracy in discrimination of 9 versus 12 varies as a function of the type of motion control: when stimuli were controlled for the total quantity of motion, fish performance was significantly lower compared to that observed for the condition in which both number and total motion were congruent and simultaneously available. It is worth noting that several fish species, including guppies, appear to be unable to use numerical information to discriminate a 0.75 ratio in the ANS range (Agrillo et al. 2012b, c). In this sense, guppies seem to use the quantity of motion cue when the numerical ratio exceeds their capacity to discriminate by using numerical information only.
Previous evidence has found that in some circumstances human infants are sensitive to continuous quantities in the OTS range (Feigenson et al. 2002a; Wood and Spelke 2005; Xu 2003). On the other hand infants repeatedly proved able to discriminate between large quantities by using numerical information only (Lipton and Spelke 2003; Xu and Spelke 2000). In our study the performance of guppies was not affected by any of static continuous quantities in both small and large number discrimination. However, as regards the quantity of motion cue, we found a reverse pattern from what would be predicted from the infant data, that is guppies use quantity of motion cue in the ANS range. Despite several similarities have been reported in large number discrimination between the numerical systems of fish and the pre-verbal systems of humans (Agrillo et al. 2010, 2012a; Piffer et al. 2012), the possibility remains that the perceptual/cognitive mechanisms involved in the ANS range are at least partially different in the two species.
To summarize, although the present work does not represent direct evidence for the existence of an OTS in fish, it reinforces the idea of separate cognitive systems for small and large numbers. Besides different responses to movement found in the present study and a ratio-insensitivity in the range of 1–4 reported by Agrillo et al. (2012a), several other lines of evidence support the hypothesis for multiple numerical systems in fish. In spontaneous shoal choice, mosquitofish make use of different continuous quantities for small- and large-number discrimination (Agrillo et al. 2008b); as well, the continuous quantities used by trained mosquitofish to discriminate between sets of bi-dimensional figures differed in the two numerical ranges (Agrillo et al. 2009, 2010). In guppies, the developmental trajectory was found to differ for small- and large-number discrimination: the spontaneous ability to discriminate between small quantities of conspecifics is displayed at birth, while this ability appears at 20–40 days old for quantities beyond 4 units (Bisazza et al. 2010). Recently, differences in ontogeny between small and large numbers were reported in newborn guppies trained to discriminate between sets of bi-dimensional geometric figures (Miletto Petrazzini et al. 2013; Piffer et al. 2013). Human infants show an apparent inability to compare quantities across the small- and the large-number boundary, for example, an inability to discriminate 1 from 4 items (the OTS in infants seems to be limited to 3 items, see Feigenson and Carey 2005). According to some authors, a direct comparison between small numbers (which are supposed to be processed by the OTS) and large numbers (processed by the ANS) would determine a conflict between the two types of representations, leading to poor performance (Cordes and Brannon 2009). A recent study found that fish may also suffer from the same inability to discriminate across the small-number/large-number divide. In shoal choice experiments, guppies were able to discriminate 3 versus 4 fish, but not 3 versus 5 fish, although the latter had a more favorable numerical ratio (Piffer et al. 2012).
Further investigation manipulating other physical features of the stimuli will help us to shed light on this issue. In the meantime, given the absence of alternative theoretical frameworks that might enable us to explain all of the differences reported below and above 4 units, we must admit the possibility that teleost fish, like humans, display the use of different numerical systems for small and large quantities.
References
Agrillo C, Dadda M, Serena G (2008a) Choice of female groups by male mosquitofish (Gambusia holbrooki). Ethology 114(5):479–488
Agrillo C, Dadda M, Serena G, Bisazza A (2008b) Do fish count? Spontaneous discrimination of quantity in female mosquitofish. Anim Cogn 11:495–503
Agrillo C, Dadda M, Serena G, Bisazza A (2009) Use of number by fish. PLoS ONE 4(3):e4786
Agrillo C, Piffer L, Bisazza A (2010) Large number discrimination by fish. PLoS ONE 5(12):e15232
Agrillo C, Piffer L, Bisazza A (2011) Number versus continuous quantity in numerosity judgments by fish. Cognition 119:281–287
Agrillo C, Piffer L, Bisazza A, Butterworth B (2012a) Evidence for two numerical systems that are similar in humans and guppies. PLoS ONE 7(2):e31923
Agrillo C, Miletto Petrazzini ME, Piffer L, Dadda M, Bisazza A (2012b) A new training procedure for studying discrimination learning in fishes. Behav Brain Res 230:343–348
Agrillo C, Miletto Petrazzini ME, Tagliapietra C, Bisazza A (2012c) Inter-specific differences in numerical abilities among teleost fish. Front Psychol 3:483. doi:10.3389/fpsyg.2012.00483
Alston L, Humphreys GW (2004) Subitization and attentional engagement by transient stimuli. Spat Vis 17:17–50
Assad JA, Maunsell JHR (1995) Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature 373:518–521
Beran MJ (2004) Chimpanzees (Pan troglodytes) respond to nonvisible sets after one-by-one addition and removal of items. J Comp Psychol 118:25–36
Beran MJ (2008a) The evolutionary and developmental foundations of mathematics. PLoS Biol 6:221–223
Beran MJ (2008b) Monkeys (Macaca mulatta and Cebus apella) track, enumerate, and compare multiple sets of moving items. J Exp Psych Anim Behav Proc 34:63–74
Beran MJ, Evans TA, Leighty KA, Harris EH, Rice D (2008) Summation and quantity judgments of sequentially presented sets by capuchin monkeys (Cebus apella). Am J Primatol 70:191–194
Beran MJ, Perdue BM, Parrish AE, Evans TA (2012) Do social conditions affect capuchin monkeys’ (Cebus apella) choices in a quantity judgment task? Front Psychol 3:492. doi:10.3389/fpsyg.2012.00492
Beran MJ, McIntyre JM, Garland A, Evans TA (2013) What counts for “counting”? Chimpanzees (Pan troglodytes) respond appropriately to relevant and irrelevant information in a quantity judgment task. Anim Behav 85:987–993
Bisazza A, Serena G, Piffer L, Agrillo C (2010) Ontogeny of numerical abilities in guppies. PLoS ONE 5(11):e15516
Bonanni R, Natoli E, Cafazzo S, Valsecchi P (2011) Free ranging dogs assess the quantity of opponents in intergroup conflicts. Anim Cogn 14:103–115
Buckingham JN, Wong BBM, Rosenthal GG (2007) Shoaling decision in female swordtails: how do fish gauge group size? Behaviour 144:1333–1346
Cantlon JF, Brannon EM (2007) Basic math in monkeys and college students. PLoS Biol 5(12):e328
Chesney DL, Haladjian H (2011) Evidence for a shared mechanism used in multiple-object tracking and subitizing. Atten Percept Psychophys 73:2457–2480
Cordes S, Brannon EM (2009) Crossing the divide: infants discriminate small from large numerosities. Dev Psychol 45:1583–1594
Cutini S, Bonato M (2012) Subitizing and visual short term memory in human and non-human species: a common shared system? Front Psychol 3:469. doi:10.3389/fpsyg.2012.00469
Dacke M, Srinivasan MV (2008) Evidence for counting in insects. Anim Cogn 11:683–689
Durgin FH (1995) Texture density adaptation and the perceived numerosity and distribution of texture. J Exp Psychol Hum Percept Perform 21:149–169
Evans TA, Beran MJ, Harris EH, Rice D (2009) Quantity judgments of sequentially presented food items by capuchin monkeys (Cebus apella). Anim Cogn 12:97–105
Evans TA, Beran MJ, Addessi E (2010) Can nonhuman primates use tokens to represent and sum quantities? J Comp Psychol 124:369–380
Feigenson L, Carey S (2005) On the limits of infants’ quantification of small object arrays. Cognition 97:295–313
Feigenson L, Carey S, Hauser MD (2002a) The representations underlying infants’ choice of more: object files versus analog magnitudes. Psychol Sci 13:150–156
Feigenson L, Carey S, Spelke ES (2002b) Infants’ discrimination of number vs. continuous extent. Cogn Psychol 44:33–66
Feigenson L, Dehaene S, Spelke ES (2004) Core systems of number. Trends Cogn Sci 8(7):307–314
Flombaum JI, Junge JA, Hauser MD (2005) Rhesus monkeys (Macaca mulatta) spontaneously compute addition operations over large numbers. Cognition 97:315–325
Gallistel CR, Gelman R (1992) Preverbal and verbal counting and computation. Cognition 44:43–74
Garland A, Low J, Burns KC (2012) Large quantity discrimination by North Island robins (Petroica longipes). Anim Cogn 15:1129–1140
Gebuis T, Reynvoet B (2012) The role of visual information in numerosity estimation. PLoS ONE 7(5):e37426
Gelman R, Gallistel C (1978) The child’s understanding of number. Harvard University Press, Cambridge
Gómez-Laplaza LM, Gerlai R (2011) Spontaneous discrimination of small quantities: shoaling preferences in angelfish (Pterophyllum scalare). Anim Cogn 14(4):565–574
Gómez-Laplaza LM, Gerlai R (2012) Activity counts: the effect of swimming activity on quantity discrimination in fish. Front Psychol 3:484. doi:10.3389/fpsyg.2012.00484
Gross HJ, Pahl M, Si A, Zhu H, Tautz J, Zhang S (2009) Number-based visual generalisation in the honeybee. PLoSONE 4:e4263
Halberda J, Mazzocco M, Feigenson L (2008) Individual differences in nonverbal number acuity predict maths achievement. Nature 455:665–668
Hauser MD, Spelke ES (2004) Evolutionary and developmental foundations of human knowledge: a case study of mathematics. In: Gazzaniga M (ed) The cognitive neurosciences, vol 3. MIT Press, Cambridge
Hauser MD, Carey S, Hauser LB (2000) Spontaneous number representation in semi-free-ranging rhesus monkeys. Proc R Soc Lond B 267:829–833
Hunt S, Low J, Burns KC (2008) Adaptive numerical competency in a food-hoarding songbird. Proc R Soc Lond B 275:2373–2379
Jevons WS (1871) The power of numerical discrimination. Nature 3(67):281–282
Kaufman EL, Lord MW, Reese TW, Volkmann J (1949) The discrimination of visual number. Am J Psychol 62(4):498–525
Kilian A, Yaman S, Fersen L, Güntürkün O (2003) A bottlenose dolphin (Tursiops truncatus) discriminates visual stimuli differing in numerosity. Learn Behav 31:133–142
Krusche P, Uller C, Ursula D (2010) Quantity discrimination in salamanders. J Exp Biol 213:1822–1828
Lipton J, Spelke ES (2003) Origins of number sense: large number discrimination in human infants. Psychol Sci 14:396–401
Mandler G, Shebo BJ (1982) Subitizing: an analysis of its component processes. J Exp Psychol Gen 111:1–22
Matsuno T, Tomonaga M (2006) Visual search for moving and stationary items in chimpanzees (Pan troglodytes) and humans (Homo sapiens). Behav Brain Res 172:219–232
Miletto Petrazzini ME, Agrillo C, Piffer L, Dadda M, Bisazza A (2012) Development and application of a new method to investigate cognition in newborn guppies. Behav Brain Res 233:443–449
Miletto Petrazzini ME, Agrillo C, Piffer L, Bisazza A (2013) Ontogeny of the capacity to compare discrete quantities in fish. Dev Psychobiol, online first, doi:10.1002/dev.21122
Neisser U (1967) Cognitive psychology. Prentice-Hall, Englewood Cliffs
Nieder A, Dehaene S (2009) Representation of number in the brain. Annu Rev Neurosci 32:185–208
Pahl M, Si A, Zhang S (2013) Numerical cognition in bees and other insects. Front Psychol 4:162. doi:10.3389/fpsyg.2013.00162
Perdue BM, Talbot CF, Stone A, Beran MJ (2012) Putting the elephant back in the herd: elephant relative quantity judgments match those of other species. Anim Cogn 15:955–961
Piffer L, Agrillo C, Hyde CD (2012) Small and large number discrimination in guppies. Anim Cogn 15:215–221
Piffer L, Miletto Petrazzini ME, Agrillo C (2013) Large number discrimination in newborn fish. PLoS ONE 8(4):e62466
Pisa PE, Agrillo C (2009) Quantity discrimination in felines: a preliminary investigation of the domestic cat (Felis silvestris catus). J Ethol 27:289–293
Revkin SK, Piazza M, Izard V, Cohen L, Dehaene S (2008) Does subitizing reflect numerical estimation? Psychol Sci 19:607–614
Ross J (2003) Visual discrimination of number without counting. Percept 32:867–870
Scholl BJ, Pylyshyn ZW (1999) Tracking multiple items through occlusion: clues to visual objecthood. Cogn Psychol 38:259–290
Sokal RR, Rohlf FJ (1995) Biometry. Freeman and Company, New York
Trick LM (2008) More than superstition: differential effects of featural heterogeneity and change on subitizing and counting. Percept Psychophys 70:743–760
Trick LM, Pylyshyn ZW (1994) Why are small and large number enumerated differently: a limited-capacity preattentive stage in vision. Psychol Rev 101(1):80–102
Trick LM, Audet D, Dales L (2003) Age differences in enumerating things that move: implications for the development of multiple-object tracking. Mem Cogn 31(8):1229–1237
Van Marle K (2013) Infants use different mechanisms to make small and large number ordinal judgments. J Exp Child Psych 114(1):102–110
Van Oeffelen MP, Vos PG (1982) A probabilistic model for the discrimination of visual number. Percept Psychophys 32:163–170
Vetter P, Butterworth B, Bahrami B (2008) Modulating attentional load affects numerosity estimation: evidence against a pre-attentive subitizing mechanism. PLoS ONE 3(9):e3269
Vonk J, Beran MJ (2012) Bears “count” too: quantity estimation and comparison in black bears (Ursus americanus). Anim Behav 84:231–238
Vos PG, Van Oeffelen MP, Tibosch HJ, Allik J (1988) Area-numerosity interactions. Psychol Res 50:148–150
Ward C, Smuts BB (2007) Quantity-based judgments in the domestic dog (Canis lupus familiaris). Anim Cogn 10:71–80
Wood JN, Spelke ES (2005) Infants’ enumeration of actions: numerical discrimination and its signature limits. Dev Sci 8(2):173–181
Woodworth RS, Schlosberg H (1954) Experimental psychology. Holt, New York
Xu F (2003) Numerosity discrimination in infants: evidence for two systems of representations. Cognition 89(1):B15–B25
Xu F, Spelke ES (2000) Large number discrimination in 6-month-old infants. Cognition 74:B1–B11
Xu F, Spelke ES, Goddard S (2005) Number sense in human infants. Dev Sci 8(1):88–101
Acknowledgments
This study was supported by the “Progetto Giovani Studiosi 2010” (prot.: GRIC101125) research grant, given by the University of Padua to Christian Agrillo; and by PRIN 2009 (Prin (2009WZXK7T), given by MIUR to Angelo Bisazza. The reported experiments comply with all of the laws of the country (Italy) in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrillo, C., Miletto Petrazzini, M.E. & Bisazza, A. Numerical acuity of fish is improved in the presence of moving targets, but only in the subitizing range. Anim Cogn 17, 307–316 (2014). https://doi.org/10.1007/s10071-013-0663-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-013-0663-6