Abstract
Playback experiments have been a useful tool for studying the function of sounds and the relevance of different sound characteristics in signal recognition in many different species of vertebrates. However, successful playback experiments in sound-producing fish remain rare, and few studies have investigated the role of particular sound features in the encoding of information. In this study, we set-up an apparatus in order to test the relevance of acoustic signals in males of the cichlid Metriaclima zebra. We found that territorial males responded more to playbacks by increasing their territorial activity and approaching the loudspeaker during and after playbacks. If sounds are used to indicate the presence of a competitor, we modified two sound characteristics, that is, the pulse period and the number of pulses, in order to investigate whether the observed behavioural response was modulated by the temporal structure of sounds recorded during aggressive interactions. Modified sounds yielded little or no effect on the behavioural response they elicited in territorial males, suggesting a high tolerance for variations in pulse period and number of pulses. The biological function of sounds in M. zebra and the lack of responsiveness to our temporal modifications are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several fish species are known to emit sounds in different social contexts, in particular during agonistic interactions and courtship (Ladich and Myrberg 2006; Myrberg and Lugli 2006). These sounds demonstrate a large inter- and intra-specific diversity, and one particular interest has been to study the potential function of these acoustic signals in communication (Amorim 2006). In cichlids, inter-specific differences found among closely related species emphasize the important role acoustic signals may have played in the spectacular rate of speciation witnessed in this group (Verzijden et al. 2010). Amorim et al. (2004, 2008) showed that courtship calls of five closely related species of cichlids differed in some temporal features, that is, number of pulses, pulse period and duration, and in peak frequency. Thus, acoustic signals could play a role in species recognition.
Previous studies also suggest that sounds of fish include individual characteristics that can enable sender recognition and assessment at the intra-specific level (Amorim et al. 2010). One of the most widespread differences is an inverse relationship between the frequency of sound and the size of the emitter (e.g. Ladich et al. 1992; Crawford et al. 1997; De Jong et al. 2007; Amorim et al. 2008; Colleye et al. 2009; Bertucci et al. 2012). Since bigger individuals are usually dominant and territorial, sound characteristics may be used by a receiver to assess the size of a potential rival. For example, beside the fact that sounds increased the chance of winning a fight in croaking gouramis (Trichopsis vittata) (Ladich et al. 1992), some size-related acoustic features may predict the outcome of a fight, that is, winners produce sounds with a lower dominant frequency and a higher sound pressure level (Ladich 1998). Acoustic signals could therefore encode social status and/or motivation. Moreover, in some species, sounds also show individual characteristics. Most examples come from Batrachoidids in which several studies have reported individuality of male courtship calls (e.g. Edds-Walton et al. 2002; Amorim and Vasconcelos 2008) and in male aggressive sounds (e.g. Thorson and Fine 2002).
If sounds seem to carry a wide range of information from the specific to the individual level, we can then ask whether sounds have context-specific characteristics. In birds and mammals, sounds of aggressive interactions are usually low in frequency and short, whereas courtship or affiliative sounds are purer and of higher frequency (Morton 1977). These differences seem to exist in fish. In the midshipman (Porichthys notatus), male advertisement calls directed to females are long and tonal while aggressive sounds are short and broadband (Bass and McKibben 2003). More recently, Parmentier et al. (2010) described the acoustic repertoire of a damselfish (Dacyllus flavicaudus). Sounds could be classified in three categories, that is, fighting sounds, mating sounds and chasing sounds. Differences between signals were found in the number of pulses, the pulse period and their relative intensities. For example, sounds associated with aggressive behaviours had a smaller number of pulses than courtship sounds. In cichlids, several studies have reported context-related differences. In Oreochromis mossambicus, pulse period and number of pulses of the male courtship sounds are significantly different depending on the behaviours they are associated with (Amorim et al. 2003). The structure of these sounds is also affected by recent social experience of males as winners produced more sounds with longer pulses and lower peak frequencies than losers (Amorim and Almada 2005).
One way of studying the function of sounds and the relevance of different sound characteristics in signal recognition is to use playback experiments. Whereas playbacks have been widely used in many vertebrates (McGregor 1992), only a small number of such experiments have been performed in vocal fish. One possible limitation to the use of this technique would be the ability of current loudspeakers to accurately broadcast sounds with low frequencies (Ladich and Myrberg 2006). However, early experiments showed that the responsiveness to conspecific sounds was higher than to heterospecific sounds (e.g. Delco 1960; Myrberg and Spires 1972). In damselfishes, in the oyster toadfish and in gobies, playback experiments have revealed that male sounds attract females and may provoke courtship displays in neighbours (Winn 1967, 1972; Gerald 1971; Ibara et al. 1983; Lugli et al. 1996; Rollo and Higgs 2008). In damselfish, a series of experiments have shown that the higher response to conspecific sounds depends on the number of pulses and pulse rate of ‘chirps’ (Myrberg et al. 1978; Spanier 1979). Moreover, playbacks of large male are more attractive than playbacks of small males (Myrberg et al. 1986; McKibben and Bass 1998) and may play a role in male–male interaction as a ‘nesting male present’ signal (McKibben and Bass 1998). In a subsequent study, McKibben and Bass (2001) were interested in the temporal pattern of sounds emitted by male P. notatus and used playbacks in choice test experiments in which sounds were altered in terms of pulse duration, the gap between pulses and beat of sounds. Playbacks to gravid females revealed that longer pulses and greater pulse-to-gap ratios resulted in more attractive signals and that pure tones were more attractive than beats. Overall, these playback experiments show that temporal pattern of sounds seems to be important in fish acoustic communication.
In cichlids, Verzijden et al. (2010) showed that female Pundamilia nyererei prefer conspecific males associated with playbacks of courtship sounds, and sounds emitted during aggressive interactions reduce the level of aggressiveness witnessed during a fight between males of the Malawian cichlid Metriaclima zebra (Bertucci et al. 2010). However, Bertucci et al. (2010) found no behavioural response to playbacks of sounds when they were not associated with other (visual) stimuli. The same result was found in male gobies exposed to aggressive sounds (Lugli 1997). Given this tight interaction of acoustic cues with other modalities in fish (Lugli 1997; Lugli et al. 2004; Ladich and Myrberg 2006), the results of previous experiments may be confusing. Furthermore, playback experiments focusing on the role of sounds at the intra-specific level remain rare (Stout 1963; Schwarz 1974; Rigley and Muir 1979). Hence, no study comparable to McKibben and Bass (2001) has been performed to investigate the role of, for example, temporal structure of sounds in cichlids.
Metriaclima (formerly Pseudotropheus) zebra is a rock-dwelling cichlid from Lake Malawi. Males produce sounds while defending territories against other males and while courting females (Amorim et al. 2004; Simões et al. 2008). During agonistic postures like quivering or lateral displays, males produce low-frequency sounds consisting in a train of short pulses. Simões et al. (2008) highlighted differences between sounds produced by males and females and differences in characteristics of males’ sounds emitted during agonistic interactions and courtship, as agonistic sounds were longer in duration and pulse period than courtship sounds.
In this study, we attempt to set-up an apparatus allowing us to elicit a behavioural response to acoustic playback, with no other associated stimuli, in male cichlid M. zebra. In order to test whether the observed behavioural response of males could be modulated, we then tested the relevance of acoustic cues by modifying two temporal characteristics that could be good candidates for encoding information, that is, the number of pulses and the sound duration, and broadcasting these modified stimuli to territorial males. We aimed to determine the possible function of temporal variability of sounds in coding aggressiveness in this species.
Materials and methods
Fish
Metriaclima zebra were purchased from N’Guyen International (Kingersheim, France) and stored for 2 months on a 12-h light/dark cycle, in mixed sex groups, in holding tanks (60 × 120 × 50 cm) containing 8–10 individuals with a male/female sex ratio of 1:2. They were 4-years old and sexually mature at the time of the experiment. Each tank was equipped with an external filter (Rena Filstar xP3, Rena France, Annecy, France), an aeration device, sand substrate, terracotta pots and bricks as shelters. The temperature was maintained at 25 ± 2 °C by an internal heater (RenaCal 200, Rena France, Annecy, France) and the pH remained at 8.0. Fish were fed daily with commercial cichlid food (JBL NovoRift, JBL GmbH & Co. KG, Neuhofen, Germany). Once a week, this diet was complemented with a frozen mixture of mussels, shrimps and spinach.
Twelve male individuals were involved in the experiments (mean ± SE total length—from the tip of the head to the tip of the caudal fin—of 137.08 ± 4.47 mm, standard length—from the tip of the head to the caudal peduncle—of 112.17 ± 5.48 mm, and a weight of 34.30 ± 1.66 g). The fish were identified by the number and the position of egg-spots on their anal fin, any marks on their body, in combination with VIE tags (Visible Implant Elastomer, Northwest Marine Technology, Shaw Island, WA, USA) implanted under the skin.
Experimental set-up
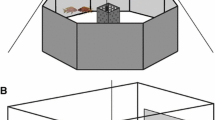
The experiment was conducted in an aquarium (60 × 30 × 30 cm) placed on a vibration-insulated shelf and located in an acoustically insulated room to reduce background noise. Additionally, three walls of the aquarium were covered with bubble wrap in order to reduce sound reverberation within the aquarium. The experimental tank contained a filter, an aeration device, an internal heater, a sand substrate and a terracotta pot in the middle in order to provide a shelter for the fish. During the experimental sessions, the filter and the aeration device were switched off to allow recordings of fish sound. On one side of the aquarium, we placed an underwater loudspeaker (University sound, Electrovoice, UW–30) (Fig. 1) connected to an amplifier (Denon PMA–100 M) and a Marantz PMD670 solid-state recorder.
A video camera (BUL520, Active Media Concept, Vallauris, France) was placed in front of the apparatus in order to record behaviour during trials. To record acoustic signals, a hydrophone (Aquarian Audio Products H2a-XLR, sensitivity: −180 dB re 1 V/μPa, flat frequency response: ±4 dB in the range 20–4.5 kHz) was placed in the centre of the aquarium, that is, above the shelter. The hydrophone was connected to a preamplifier (Yamaha MLA8, Yamaha Music France, Marne-la-Vallée, France) and a video capture card (Osprey-450e) of a PC, which synchronized audio and video signals.
In order to broadcast sounds at a ‘natural’ level, we placed the loudspeaker at a distance from the hydrophone corresponding to the distance at which the sounds were originally recorded. We then adjusted the intensity of playbacks to get the same level of recording. We confirmed that this apparatus did not alter played back sounds in a previous experiment (Bertucci et al. 2010) by comparing two features of the sound, that is, mean frequency and pulse period: these sound features did not differ before and after a playback (see Online Resource 1 for results of the comparison tests and a figure showing oscillograms of a recorded sound and of the same sound after transmission through the broadcasting apparatus).
Sounds to be used as acoustic stimuli were obtained from recordings made in our laboratory. Two unfamiliar size-matched males were introduced into adjacent tanks, separated by opaque partitions and were given 24 h to acclimatize and become territorial. A hydrophone was placed in each tank, between the shelter and the walls separating the aquaria, that is, where individuals would spend most of their time displaying; details of the hydrophone and its connections were as same as for the recording of the stimulus sounds. At the start of a recording session, the opaque partition was removed, allowing the two fish to interact visually for 20 min before the partition was replaced. We performed a maximum of three recording sessions per male to collect a sufficient number of sounds. We only considered sounds consisting of more than two pulses and recorded within a distance of 1–2 body lengths from hydrophones. Sounds recorded from four individuals with a mean ± SE total length of 115.00 ± 7.99 mm, a standard length of 98.00 ± 6.38 mm and a weight of 25.20 ± 4.25 g were used and digitized at 44.1 kHz (16-bit resolution). None of the recorded fish were involved in the present experiment, and none of the fish tested had experience with any of the individuals from which the sounds were recorded.
Analysis of behavioural responses
Behavioural data were collected from the videos recorded during the trials. For each period in the two sessions (intruder and resident), we quantified the number of aggressive behaviours performed, that is, lateral display, quiver, sound production and bite attempts, using the behavioural transcription software EthoLog 2.2.5 (Ottoni 1995–1999). We also quantified behaviours defining space occupancy, that is, time spent swimming (s) in the zone of the loudspeaker, which was one half of the aquarium, number of territory maintenance behaviours (when the fish was moving–digging—sand within the aquarium while becoming territorial, for example Oliveira and Almada 1996; Simões et al. 2008), number of times that the tested fish visited the shelter and number of times it went close (less than one body length) to the loudspeaker.
We analysed the number of lateral displays, quivers, sounds produced, bite attempts, the number of territory maintenance acts, visits to the shelter, number of positions close to the loudspeaker and time spent in the zone of the loudspeaker by means of either a repeated measures analysis of variance when the data were normally distributed or a Friedman analysis of variance if this was not the case. The analysis was performed using STATISTICA 6.0 (Statsoft Inc 2004).
Control experiment: how do male M. zebra respond to playback of conspecific sounds?
The first step of our study was to set-up an efficient playback apparatus that would allow us to study the behavioural response of M. zebra to played back signals. As we were also interested in the effect of social status on the behavioural response to playback, each fish was tested in the experimental tank in two successive playback sessions, separated by 24 h of rest. This apparatus allows testing fish in two different social conditions. During the first session, the fish was considered as an intruder, whereas it was considered as resident during the second session.
The test fish was introduced to the experimental set-up and was given 10–15 min to acclimatize. The first session was then divided into three periods of 10 min. During the first 10 min, no sounds were played back (pre-playback control). At the end of this first period, we started to play back aggressive sounds to the subject every time it entered in the half of the aquarium containing the loudspeaker (playback period, see Fig. 1). The fish then received no playback for 10 min, until the end of the first session (post-playback period). We then switched the electric devices back on—filter and aeration—and left the fish in the tank for 24 h, so that they could become resident. During the second session, we repeated the procedure with the 10 min pre-playback, 10 min playback and 10 min post-playback periods playing back the same sounds as the day before. At the end of the trial, the fish was returned to its home tank and 2/3 of the water in the test aquarium were renewed and allowed to stand for 24 h before the introduction of a new fish.
Experiment with modified signals: do modifications of sound temporal pattern alter the behavioural response to playbacks?
In a second step, we wanted to test whether modifications of a sound’s temporal pattern could elicit differential behavioural responses in the tested fish. Sounds of M. zebra consist of trains of a variable number of short and low-frequency pulses. Male sounds show a range of variation in the period of pulses from 40 to 180 ms and sound duration varies from 250 ms to 1 s with 5–15 pulses per sound (Simões et al. 2008; Bertucci et al. 2012). We altered temporal features of these sounds using the PRAAT software version 5.0.35 (Boersma and Weenink 1992–2008) (Fig. 2). To test the significance of the pulse period, we built sounds for which we retained the number of pulses of the original sounds (Fig. 2a), but modified pulse period corresponding either to twice the maximum peak-to-peak distance observed for each individual (PP Max, Fig. 2b) or to half the minimum peak-to-peak distance (PP Min, Fig. 2c). These two modifications, respectively, resulted in a series of slow sounds and fast sounds. To test the significance of the number of pulses, we built sounds with no alteration of the pulse period but we either deleted the second half of the original sounds (half pulses, Fig. 2d) or pasted a copy of the original sound at its end (double pulses, Fig. 2e). For the latter modification, the two parts were separated by the mean peak-to-peak interval of the original sounds. These two modifications, respectively, resulted in a series of short sounds and long sounds.
Temporal modifications of male M. zebra sounds recorded during aggressive interactions. a Oscillogram of an unmodified control sound. b Oscillogram of the same sound with an increased pulse period (PP), resulting in a slow sound (PP Max). c Oscillogram of a sound with a decreased pulse period, resulting in a fast sound (PP Min). d Oscillogram of a sound with only the first half of pulses resulting in a short sound (half pulses). e Oscillogram of a sound with a double number of pulses, resulting in a long sound (double pulses). Oscillograms are shown to scale
Prior to the experiment, fish were put into the aquarium for 24 h to let them become resident. Resident fish were then tested with playbacks of modified sounds following the same procedure as previously described for the control experiment. We used a repeated measures design with five different treatments corresponding to the five categories of sounds that we created, that is, normal sounds, PP Max, PP Min, Double pulses and Half pulses. We tested two fish a day for six consecutive days over a period of 5 weeks. Each individual thus received a new treatment every 7 days in a balanced order to avoid order effects. Since we broadcast sounds when a fish was located in the half part of the aquarium containing the loudspeaker, the number of playbacks received by each individual depended on its motivation to approach the loudspeaker, which explains why the number of stimulations varied between individuals and treatments (Table 1).
Results
How do male M. zebra respond to playback of conspecific sounds?
Numbers of aggressive displays (lateral displays, quivers and sounds) were too low to be analysed.
We found significant differences in the number of territory maintenance behaviours (Friedman ANOVA, n = 12, F 4,44 = 39.59, P < 10−3) with no difference in intruders and a significant increase of this number in residents during the playback and after the playback as revealed by a Fisher’s least significant difference (LSD) post hoc test (Fig. 3a). Residents visited significantly less the shelter than intruders after the playback (Friedman ANOVA, n = 12, F 4,44 = 16.31, P = 0.006) (Fig. 3b). Residents came close to the loudspeaker significantly more often after the playback than intruders during the same period (Friedman ANOVA, n = 12, F 4,44 = 16.26, P = 0.006, followed by a Fisher’s LSD post hoc test) (Fig. 3c). We also found that residents tended to spend more time in the zone of the loudspeaker than intruders after the playback (Friedman ANOVA, n = 12, F 4,44 = 10.58, P = 0.06).
Behavioural responses to playback of intruders and residents, during the three successive observation periods. Pre-PB:10 min before playback, PB: playback period (duration: 10 min; sound played back each time the fish approached the loudspeaker, Post-PB: 10 min after playback. a Number of territory maintenance behaviours, b number of visits in the shelter, c number of positions close to the loudspeaker. The boxes represent the first and third quartiles, points (filled circle) are the median (second quartile) and whiskers correspond to the range (min–max) of the distributions. Different letters indicate significant differences (P < 0.05, Fisher post hoc test)
In summary, playback of conspecific sounds does not alter the behaviour of fish that have just entered the experimental set-up (intruders), whereas it elicits a territorial response by fish that have been present for 24 h (residents). This territorial response is well characterized by territory maintenance behaviour.
Do modifications of the temporal pattern of sounds alter the response?
For the territory maintenance activity, we found a significant effect of temporal modifications in the post-playback period (Repeated measures ANOVA, n = 11, F 4,40 = 3.15, P = 0.02), with a higher number of territory maintenance acts in response to sounds with a high pulse period (PP Max) compared to other modifications as revealed by a Fisher’ LSD post hoc test. However, none of the treatments differed from the control playback (Table 2; Fig. 4).
Behavioural response (number of territory maintenance behaviours) elicited by sounds with modified time pattern. Fish are tested when resident (i.e. after 24 h in the aquarium, see text for details). Pre-PB: 10 min before playback, PB: playback period (duration: 10 min; sound played back each time the fish approached the loudspeaker, Post-PB: 10 min after playback. Different letters indicate significant differences (P < 0.05, Fisher post hoc test)
We found no differences between modifications for the number of visits in the shelter (repeated measures ANOVA, n = 11, F 4,40 = 0.96, P = 0.44), the number of positions close to the loudspeaker (repeated measures ANOVA, n = 11, F 4,40 = 0.79, P = 0.54) or the time spent in the zone of the loudspeaker (s) (Repeated measures ANOVA, n = 11, F 4,40 = 1.12, P = 0.36) (Table 2).
Discussion
This study used playbacks of sounds produced by males during agonistic interactions in order to investigate the role of these signals in M. zebra. Playbacks have been successfully applied when investigating the biological function of advertisement sounds of insects, frogs, birds or mammals (McGregor 1992). In comparison, however, most of the studies using playback experiments in fishes have been unsuccessful, leading to confusing results. Such studies therefore remain rare. One explanation comes from the close-range feature of fish sounds and the requirement of additional stimuli to elicit a behavioural response (Stout 1963; Schwarz 1974; Rigley and Muir 1979; Ladich 1997; Bertucci et al. 2010).
Control experiment: only resident males respond to acoustic playbacks
We showed in the first part of this experiment that acoustic playbacks of sounds could trigger a behavioural response in residents, with an increased number of maintenance acts (or digging) during and after the playback periods. Substrate plays an important social role in cichlids especially during the breeding season, when males build nests, that is, a depression in the substrate, representing their territory in which females spawn (Oliveira and Almada 1996; Galhardo et al. 2008, 2009). Hence, our results suggest that aggressive sounds are relevant to a resident (territorial) male and lead him to defend or at least advertise his territory against a potential competitor. While the increased number of territory maintenance behaviours may also be due to the fact that fish became habituated to the experimental tank and start exhibit some behaviours, the occurrence of this response during and after the playbacks clearly indicates that sounds are the source of this reaction. Aggressive sounds might then play the role of a ‘territorial male present’ signal in resident individuals, similar to the ‘nesting male present’ signal proposed by McKibben and Bass (1998) in the midshipman. At the same time, males reduced their visits in their shelter and came more often close to the loudspeaker. Such phonotaxic responding is comparable to that found in previous experiments conducted in fishes (e.g. Myrberg and Spires 1972; Winn 1972; Ibara et al. 1983). Thus, besides attracting mates or being used during fights (Simões et al. 2008), acoustic signals of cichlids may be used by territorial males to detect potential rivals interacting with conspecifics in their vicinity.
This response is robust against modifications of the temporal pattern of sounds
In contrast to previous findings that the number of pulses and pulse rate were important sound characteristics in attracting a mate or neighbour’s attention and for species discrimination in damselfishes (Myrberg et al. 1978; Ibara et al. 1983; Amorim 2006), most of the modifications of temporal features here had little or no effect on the behaviour of resident male M. zebra. Only a massive increase in the pulse period (PP Max signal) resulted in a stronger behavioural response implying a bigger threat in the receiver. A longer pulse period is generally associated with agonistic interactions in M. zebra (Simões et al. 2008) and with larger males (Bertucci et al. 2012) and might thus promote the observed reaction.
If the total sound duration was responsible for the observed response to playbacks, we would have expected the double pulse signal to show the same or a greater effect as PPmax sounds on the number of territory maintenance behaviours. This was not the case, which suggests that the increased pulse period, not the total sound duration, may be the cause of the increased territorial activity induced by the PP Max signal. Increasing the sound duration by playing the same sound twice successively not only creates a twofold longer sound, but also alters the sound envelope. Indeed, most cichlid sounds start with pulses of low amplitude; pulse amplitude then increases and declines at the end (e.g. Lobel 2001; Amorim et al. 2008). Repetition of the same sound twice may affect the dynamic pattern of pulses. Pulse amplitude would therefore be a good candidate parameter to be modified in further playback experiments.
Apart from the PPMax modification, and in relation to the previous point, playbacks of only the first half of sounds provided the same results as fast sounds (PPMin) and long sounds (Double pulses), with a decreased number of territory maintenance behaviours. This decrease was not significantly different from the control, but it suggests that during the first half of a sound (which usually corresponds to an increase in pulse amplitude), a fast sound or a long sound do not provide the same amount or quality of information regarding territorial behaviour as unmodified sounds. The question whether information is carried by the entire sound or in other (decreasing) parts of the sound thus deserves more investigation.
Only two temporal acoustic features were considered here, and we cannot exclude the possibility that the key characteristics of our stimuli were preserved despite our drastic modifications and that other cues might also be involved in the encoding process affecting, in particular, the phonotaxic response. Sound pressure level and dominant frequency of sounds may play an especially important role in conspecifics’ assessment (Myrberg et al. 1986; Ladich 1998; McKibben and Bass 1998) and deserve further investigations. On present data, the system seems to be able to sustain and tolerate a wide range of variations in term of pulse period and sound duration before the behavioural response of receivers is altered.
The association of the territorial and the phonotaxic response found in the control experiment validates the efficiency and reliability of our results and represents one of the first convincing playback experiments conducted in a cichlid fish. We also provide good evidence of the important role of acoustic communication at the intra-specific level in M. zebra.
There is a major gap in the fish literature compared to insects and anurans in particular. While our drastic modifications may have resulted in some supernormal stimuli, that is, very slow signals or unnatural stimuli, that is, with a modified amplitude envelope, the results of the second part of our study have to be considered as a first attempt at deciphering the encoding mechanisms of this fish. Numerous studies have shown that fish auditory system is well suited for temporal processing (e.g. Suzuki et al. 2002; Wysocki and Ladich 2003; Vasconcelos et al. 2011). However, to our knowledge, no neurophysiological studies have been performed to know how pulse interval and other temporal features are represented in the auditory system of this species. Further playback experiments focusing on other acoustic cues like frequency, amplitude of pulses or spectral properties of sounds, in association with neurophysiological experiments, will be necessary to understand more thoroughly information processing in fish acoustic communication.
References
Amorim MCP (2006) Diversity of sound production in fish. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes. Science, Enfield, pp 71–105
Amorim MCP, Almada VC (2005) The outcome of male–male encounters affects subsequent sound production during courtship in the cichlid fish Oreochromis mossambicus. Anim Behav 69:595–601
Amorim MCP, Vasconcelos RO (2008) Variability in the mating calls of the Lusitanian toadfish Halobatrachus didactylus: cues for potential individual recognition. J Fish Biol 73:1267–1283
Amorim MCP, Fonseca PJ, Almada VC (2003) Sound production during courtship and spawning of Oreochromis mossambicus: male–female and male–male interactions. J Fish Biol 62:658–672
Amorim MCP, Knight ME, Stratoudakis Y, Turner GF (2004) Differences in sounds made by courting males of three closely related Lake Malawi cichlid species. J Fish Biol 65:1358–1371
Amorim MCP, Simões JM, Fonseca PJ, Turner GF (2008) Species differences in courtship acoustic signals among five Lake Malawi cichlid species (Pseudotropheus spp.). J Fish Biol 72:1355–1368
Amorim MCP, Simões JM, Mendonça N, Bandarra NM, Almada VC, Fonseca PJ (2010) Lusitanian toadfish song reflects male quality. J Exp Biol 213:2997–3004
Bass AH, McKibben JR (2003) Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol 69:1–26
Bertucci F, Beauchaud M, Attia J, Mathevon N (2010) Sounds modulate males’ aggressiveness in a cichlid fish. Ethology 116:1179–1188
Bertucci F, Attia J, Beauchaud M, Mathevon N (2012) Sounds produced by the cichlid fish Metriaclima zebra allow reliable estimation of size and provide information on individual identity. J Fish Biol 80:752–766
Boersma P, Weenink D (1992–2008) PRAAT, version 5.0.35. www.praat.org
Colleye O, Frederich B, Vandewalle P, Casadevall M, Parmentier E (2009) Agonistic sounds in the skunk clownfish Amphiprion akallopisos: size-related variation in acoustic features. J Fish Biol 75:908–916
Crawford JD, Cook AP, Heberlein CD (1997) Bioacoustic behavior of African fishes (Mormyridae): potential cues for species and individual recognition in Pollimyrus. J Acoust Soc Am 102:1200–1212
De Jong K, Bouton N, Slabbekoorn H (2007) Azorean rock-pool blennies produce size-dependent calls in a courtship context. Anim Behav 74:1285–1292
Delco EA Jr (1960) Sound discrimination by males of two cyprinid fishes. Tex J Sci 12:48–54
Edds-Walton PL, Mangiamele LA, Rome LC (2002) Variations of pulse repetition rate in boatwhistle sounds from oyster toadfish Opsanus tau around Waquoit Bay, Massachusetts. Bioacoustics 13:153–173
Galhardo L, Correia J, Oliveira RF (2008) The effect of substrate availability on behavioural and physiological indicators of welfare in the African cichlid (Oreochromis mossambicus). Anim Welf 17:239–254
Galhardo L, Almeida O, Oliveira RF (2009) Preference for the presence of substrate in male cichlid fish: effects of social dominance and context. Appl Anim Behav Sci 120:224–230
Gerald JW (1971) Sound production during courtship in six species of sunfish (Centrarchidae). Evolution 25:75–87
Ibara RM, Penny LT, Ebeling AW, Van Dykhuisen G, Cailliet G (1983) The mating call of the plainfin midshipman fish, Porichthys notatus. In: Noakes DLG, Lindquist DG, Helfman GS, Ward JA (eds) Predators and preys in fishes. Dr. W. Junk, The Hague, pp 205–212
Ladich F (1997) Agonistic behaviour and significance of sounds in vocalizing fish. Mar Freshw Behav Physiol 29:87–108
Ladich F (1998) Sound characteristics and outcome of contests in male croaking gouramis (Teleostei). Ethology 104:517–529
Ladich F, Myrberg AA Jr (2006) Agonistic behavior and acoustic communication. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes. Science, Enfield, pp 121–148
Ladich F, Brittinger W, Kratochvil H (1992) Signifance of agonistic vocalization in the croaking gourami (Trichopsis vittatus, Teleostei). Ethology 90:307–314
Lobel PS (2001) Acoustic behaviour of cichlid fishes. J Aquac Aquat Sci 9:167–186
Lugli M (1997) Response of male goby, Padogobius martensii, to aggressive sound playback following pre-experimental visual stimulation. Behaviour 134:1175–1188
Lugli M, Pavan G, Torricelli P (1996) The importance of breeding vocalizations for mate attraction in a freshwater goby with a composite sound repertoire. Ethol Ecol Evol 8:343–351
Lugli A, Pavan G, Torricelli P (2004) The response of the male freshwater goby to natural and synthetic male courtship sound playback following exposure to different female sexual stimuli. Ethol Ecol Evol 16:55–70
McGregor PK (1992) Playback and studies of animal communication. Plenum Press, New York
McKibben JR, Bass AH (1998) Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J Acoust Soc Am 104:3520–3533
McKibben JR, Bass AH (2001) Effects of temporal envelope modulation on acoustic signal recognition in a vocal fish, the plainfin midshipman. J Acoust Soc Am 109:2934–2943
Morton ES (1977) On the occurrence of significance of motivation—structural rules in some birds and mammals sounds. Am Nat 111:855–869
Myrberg AA Jr, Lugli M (2006) Reproductive behavior and acoustic communication. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes. Science, Enfield, pp 149–176
Myrberg AA Jr, Spires JY (1972) Sound discrimination by the bicolor damselfish, Eupomacentrus partitus. J Exp Biol 57:727–735
Myrberg AA Jr, Spanier E, Ha SJ (1978) Temporal patterning in acoustic communication. In: Reese ES, Lighter FJ (eds) Contrasts in behaviour. Wiley, New York, pp 137–179
Myrberg AA Jr, Mohler M, Catala JC (1986) Sound production by males of a coral reef fish (Pomacentrus partitus): its significance to females. Anim Behav 34:923–933
Oliveira RF, Almada VC (1996) Dominance hierarchies and social structure in captive groups of the Mozambique tilapia Oreochromis mossambicus (Teleostei Cichlidae). Ethol Ecol Evol 8:39–55
Ottoni EB (1995–1999) Etholog 2.2 (Ethological Transcription Tool), version 2.2.5. www.ip.usp.br/ebottoni/EthoLog/ethohome
Parmentier E, Kéver L, Casadevall M, Lecchini D (2010) Diversity and complexity in the acoustic behavior of Dacyllus flavicaudus (Pomacentridae). Mar Biol 157:2317–2327
Rigley L, Muir J (1979) The role of sound production by the brown bullhead Ictalurus nebulosus. Proc Pa Acad Sci 53:132–134
Rollo A, Higgs D (2008) Differential acoustic response specificity and directionality in the round goby, Neogobius melanostomus. Anim Behav 75:1903–1912
Schwarz A (1974) The inhibition of aggressive behaviour by sound in the cichlid fish, Cichlasoma centrarchus. Z Tierpsychol 35:508–517
Simões JM, Duarte IG, Fonseca PJ, Turner GF, Amorim MCP (2008) Courtship and agonistic sounds by the cichlid fish Pseudotropheus zebra. J Acoust Soc Am 124:1332–1338
Spanier E (1979) Aspects of species recognition by sounds in four species of damselfishes, genus Eupomacentrus (Pisces: Pomacentridae). Z Tierpsychol 51:301–316
StatSoft Inc. (2004) STATISTICA (data analysis software system), version 6. www.statsoft.com
Stout JF (1963) The significance of sound production during the reproductive behaviour of Notropis analostanus (Family Ciprinidae). Anim Behav 11:83–92
Suzuki A, Kozloski J, Crawford JD (2002) Temporal encoding for auditory computation: physiology of primary afferent neurons in sound-producing fish. J Neurosci 22:6290–6301
Thorson RF, Fine ML (2002) Acoustic competition in the gulf toadfish Opsanus beta: acoustic tagging. J Acoust Soc Am 111:2302–2307
Vasconcelos RO, Fonseca PJ, Amorim MCP, Ladich F (2011) Representation of complex vocalizations in the Lusitanian toadfish auditory system: evidence of fine temporal, frequency and amplitude discrimination. Proc R Soc B 278:826–834
Verzijden MN, van Heusden J, Bouton N, Witte F, ten Cate C, Slabbekoorn H (2010) Sounds of male Lake Victoria cichlids vary within and between species and affect female mate preferences. Behav Ecol 21:548–555
Winn HE (1967) Vocal facilitation and the biological significance of fish sounds. In: Tavolga WN (ed) Marine Bioacoustics, vol 2. Pergamon, Oxford, pp 213–230
Winn HE (1972) Acoustic discrimination by the toadfish with comments on signals systems. In: Winn HE, Olla BL (eds) Behavior of marine animals, volume 2: vertebrates. Plenum, New York, pp 361–385
Wysocki LE, Ladich F (2003) The representation of conspecific sounds in the auditory brainstem of teleost fishes. J Exp Biol 206:2229–2240
Acknowledgments
The authors would like to thank Nicolas Boyer and Colette Bouchut for their technical support. We are grateful to Christina Meier, Friedrich Ladich and an anonymous referee for their comments on previous versions of the manuscript. F. B. was supported by a Ph.D. fellowship from the French Ministère de l’Enseignement Supérieur et de la Recherche. This study was funded by the Institut Universitaire de France (N. M.), the Centre National de la Recherche Scientifique and the University of Saint-Etienne.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10071_2012_549_MOESM1_ESM.pdf
Supplementary material 1 (PDF 73 kb): Oscillograms of a sound produced by a male M. zebra during an agonistic interaction (a) before playing it back, and (b) once played back through our broadcasting apparatus (recorded at 15 cm from the loudspeaker, within the aquarium filled with water). For each oscillogram, the detail of a pulse is shown. Two variables (mean frequency and pulse period) of 10 randomly selected sounds were compared before and after playback. A paired t test revealed no significant differences in the mean frequency (n = 10, t = 1.91, p = 0.09) nor in the pulse period (n = 10, t = 1.25, p = 0.24)
Rights and permissions
About this article
Cite this article
Bertucci, F., Attia, J., Beauchaud, M. et al. The relevance of temporal cues in a fish sound: a first experimental investigation using modified signals in cichlids. Anim Cogn 16, 45–54 (2013). https://doi.org/10.1007/s10071-012-0549-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-012-0549-z