Abstract
Beaconing to rewarded locations is typically achieved by visual recognition of the actual goal. Spatial recognition, on the other hand, can occur in the absence of the goal itself, relying instead on the landmarks surrounding the goal location. Although the duration or frequency of experiences that an animal needs to learn the landmarks surrounding a goal have been extensively studied with a variety of laboratory tasks, little is known about the way in which wild vertebrates use them in their natural environment. Here, we allowed hummingbirds to feed once only from a rewarding flower (goal) before it was removed. When we presented a similar flower at a different height in another location, birds frequently returned to the location the flower had previously occupied (spatial recognition) before flying to the flower itself (beaconing). After experiencing three rewarded flowers, each in a different location, they were more likely to beacon to the current visible flower than they were to return to previously rewarded locations (without a visible flower). These data show that hummingbirds can encode a rewarded location on the basis of the surrounding landmarks after a single visit. After multiple goal location manipulations, however, the birds changed their strategy to beaconing presumably because they had learned that the flower itself reliably signalled reward.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Successful small-scale navigation relies on animals being able to remember a goal’s features plus proximal and distal landmarks and to use them either as a single beacon or as associative cues in order to relocate that goal (Gould et al. 2010). In order to relocate a goal, animals may form a memory either of the visual characteristics of the goal itself (goal recognition, beaconing) or of the landmarks surrounding that goal (spatial recognition), that is, the goal need not be visible. In instances where the goal is very conspicuous, the animal does not need to learn the surrounding landmarks to reach the goal but when it is less conspicuous it may be more efficient for the animal to encode routes to the goal by learning those landmarks. In some cases, beacons are used in establishing the spatial recognition system. For example, wood ants Formica rufa use beacons as a ‘scaffold’ when learning a route such that when it is learned, removing the beacons has no impact and the ants follow exactly the same route as they had when the beacons were present (Graham et al. 2003). The ants’ use of landmarks is context specific and depends on their foraging round-trip stage: they use panoramic landmarks cues when experimentally displaced while departing from the nest or from the artificial feeder, whereas they rely on local landmark cues for arrival at either location (Fukushi and Wehner 2004). Rats, too, may use landmarks in a kind of scaffolding: when trained to find an escape platform signalled by a landmark at a constant orientation and distance from the platform they can relocate it in the absence of that landmark (Pearce et al. 1998). Beacons appear to facilitate learning about spatial information in pigeons (Kelly and Spetch 2004a) and in humans (Kelly and Spetch 2004b) as they do in ants and rats (Pearce et al. 2001, 2006). Pigeons trained to a single rewarded corner in a rectangular arena with a distinct feature at each corner learn not only the feature associated with reward but also the geometrical properties, even though these are unnecessary for successful completion of the task (Kelly and Spetch 2004a). Finally, Clark’s nutcrackers Nucifraga columbiana, trained to a single rewarded location in a similar rectangular arena required that location to be identifiable with a visually unique landmark: when all of the objects were identical, the birds did not learn the rewarded location. Furthermore, when the landmark information was put in conflict with the geometric information, the birds used the landmark (Kelly 2010).
This facilitation of location learning by the features of the goal itself (used as a beacon) is also seen in wild, free-living hummingbirds Selasphorus rufus, which learn the location of a reward faster when it is marked by a location-specific colour than when it is not (Hurly and Healy 2002; Hurly et al. 2010). However, since birds return to the location of the reward regardless of a change in the colour of the flower, it appears that birds encode a goal’s location using landmarks in addition to the beacon (Hurly and Healy 2002). This is seen most strikingly when the rewarded flower at which a bird has fed on multiple occasions need not be present for the bird to fly to its location (Hurly et al. 2010). Examination of the birds’ flight paths shows that even when the flower is displaced only 2 m from the original rewarded location the birds flew first to the original location of the now absent flower before flying to the flower that was present. Furthermore, rufous hummingbirds’ accuracy in relocating a rewarded location without being guided by a beacon is related to the size of the flower used for training: accuracy of relocation in three dimensions increases as the flower gets smaller.
We do not know whether the hummingbirds in the field require multiple experiences to learn a rewarded location, as do the ants and species tested in laboratory experiments. The aim of this study, then, was to determine whether rufous hummingbirds could relocate a location after one rewarded visit. Birds were allowed to feed from an artificial flower only once before we moved that flower to a novel nearby location. If the birds returned to the previously rewarded location, from which the flower was now absent, it would appear that the birds had learned the goal’s location (spatial recognition) without needing to use the goal’s visual features (beaconing) after a single experience.
Methods
Study species and site
The subjects used in this experiment were 14 free-living male rufous hummingbirds, Selasphorus rufus. The experiment was run from 08:00 to 19:00 h Mountain Standard Time in May and June in 2009 in a valley in the eastern Rocky Mountains, 20 km southwest of Beaver Mines, Alberta, Canada, (49º20′56.61′′N 114º24′38.49′′W). On return from overwintering in Mexico, males set up territories along this valley centred on artificial feeders at an approximate height of 2–3 m from the ground containing 14 % sucrose. Territorial males were caught, banded and colour marked (with nontoxic waterproof ink on the breast) for individual identification.
Description of experimental arena
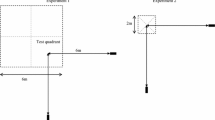
The experiments were conducted within the birds’ territories, which were about 1 ha in size (Hurly et al. 2001) and largely forested with multiple open, grassy spaces (Fig. 1).
Initial training
To train a bird to feed from artificial flowers containing sucrose, his feeder was lowered in three steps of approximately 1 m, until the feeder reached the height of the training flower 60 cm (2 ft) which was always placed immediately below (at the exact ground position of) the feeder. The feeder was removed either once the bird fed from the training flower or when the bird had fed from the feeder beside the training flower. The experimental flower was made of a 6 cm (2.4 in) diameter disc of orange cardboard mounted vertically on a wooden stake. This stake was one of three different heights (short: 60 cm (2 ft) = S; medium: 120 cm (4 ft) = M; tall: 180 cm (6 ft) = T). In the centre of the flower, a syringe tip was filled with 200 μl of 25 % sucrose solution. As hummingbirds fly through three dimensions, we used flowers of different heights so as to change the flower’s location within three-dimensional space and not only in the horizontal plane. In this way, we could determine whether the birds revisited locations at the correct height as well as the correct x–y location. The bird was allowed to feed twice from the training flower in the same location before the experiment began. Therefore, whenever a bird hovered at the training flower location this was considered a visit based on two trials. Training took no longer than 2 h.
Experimental procedure
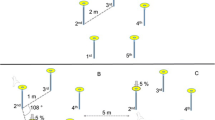
Once the bird had fed twice from the training flower (TF), it experienced three successive one-trial learning presentations. First, the training flower was removed and the first experimental flower (F1) was placed 2.74 m (9 ft) from the location of the training flower (Stage 1). After the subject had fed once from F1, that flower was removed and the second experimental flower (F2) was placed 2.74 m (9 ft) away from the location F1 had occupied (Stage 2). The bird was allowed to feed once from F2, that flower was removed and the third (F3) and last experimental flower was placed 2.74 m (9 ft) from the location F2 had occupied (Stage 3). All birds experienced the three different flower heights in a pseudo-randomized order. The placement of flowers was either linear, in the shape of an ‘L’ or a diamond (Fig. 2) so that successive flowers were not simply further from the location that had been occupied by the training flower.
When a bird flew into feed from a nearby perch, he could fly directly to the visible flower or to any of the locations previously occupied by flowers. The approximate location of the visits to absent flowers could be recorded when a bird hovered in the air in the vicinity of a flower’s previous location. All visits as well as their order were recorded. All of male’s visits to his meadow ended by his feeding at the visible flower. After feeding from a flower, the male perched high up in a tree in his territory, chased other males or displayed to females. Once the bird had fed from F3, the feeder was returned to its original location and the bird’s behaviour was recorded for a further foraging bout.
Results
All the birds returned to previously rewarded locations, hovering for 1–3 s, in one or more experimental stages. None of the birds hovered at locations other than those at which they had fed at some point in this experiment (the feeder, TF, F1 and F2). Most of them (10/14) required a single visit to relocate a relevant location, returning to it after a single rewarded visit in at least one experimental stage, that is, F1 and F2, having previously visited each of these just once to feed. The interval between foraging bouts was 15.0 ± 0.5 (SE) min.
Spatial recognition
A bird could make two kinds of visits when he returned to forage in the experimental area: either to the present flower or to an empty space where there used to be a rewarded flower. We designated all those occasions on which the birds hovered very near (approximately 20 cm of the flower’s previous location) to the location of an absent flower, spatial recognition, while flying directly to a flower we designated as beaconing. The flower could have been the training flower (TF) or any of the subsequent three experimental flowers (F1, F2 or F3). Hovering at the locations of absent flowers always ended (in less than 10 s) with the bird feeding from the present flower.
When F1 was present, 10/14 birds also visited the training flower’s location (Fig. 3a). When F2 was present, 12/14 birds visited the locations of either the training flower only (two-trial learning: 3/12 birds) or F1 (with or without visiting TF, single-trial learning: 9/12 birds; Fig. 3b). When F3 was present, 8/13 birds visited the locations of either only TF (1/8) or F1 and/or F2 (with or without visiting TF, single-trial learning: 7/8; Fig. 3c). The proportion of birds that performed spatial recognition (regardless of being its 1st choice or not) at any time during a trial was similar throughout the experiment (Fig. 4).
a Locations visited during the first experimental stage (when F1 is the visible flower). The light grey bar represents the number of birds that used spatial recognition on their first choice (after two visits to TF), The dark grey bar represents the number of birds that beaconed for their first choice and the striped bar represents spatial recognition used after visiting F1. TF = Training flower, F1 = Flower 1, n = 14. b Locations visited during the second experimental stage (when F2 was the visible flower). The light grey bar represents the number of birds that used spatial recognition as their first strategy after two visits to TF, while the white bars represent the number of birds that used spatial recognition after a single experience. The dark grey bar represents the number of birds that beaconed for their first choice. The horizonal striped bars represent visits to both TF and F1 before beaconing was used to visit F2 while the grey diagonal stripes represent visits to F1 and/or TF after visiting F2. TF = Training flower, F1 = Flower 1, F2 = Flower 2, n = 14. c Locations visited during the third experimental stage (when F3 was the visible flower). The light grey bar represents the number of birds that used spatial recognition as their first strategy after two visits to TF, while the white bars represent the number of birds that used spatial recognition after a single experience. The dark grey bar represents the number of birds that beaconed for their first choice. The horizonal striped bars represent visits to two of TF, F1 and F2 before beaconing to visit F3, while the grey diagonal stripes represent visits to F2, F1 and/or TF after visiting F3. TF = Training flower, F1 = Flower 1, F2 = Flower 2, F3 = Flower 3, N = 13
To evaluate the birds’ strategy for relocating flowers, we compared the proportion of birds that hovered at one of the flower’s previous locations (spatial recognition) as their first choice before going on to the actual flower with a chance expectation of the same probability of a visit to each flower location. We did this for each of the three stages. The probability of spatial recognition increases throughout the experiment from 0.5 in Stage 1 to 0.67 and 0.75 in Stages 2 and 3, respectively, due to the increase in flower locations (i.e., during Stage 3, chance would be 25 % as there were then four possible locations: TF, F1, F2, F3). The number of beaconing visits (as a 1st choice) was significantly higher than chance only at Stage 3 (Stage 1: χ2 = 0.286, df = 1, p = 0.593; Stage 2: χ2 = 0.986, df = 1, p = 0.321; Stage 3: χ2 = 5.770, df = 1, p = 0.016; see Fig. 5). In addition, a significant proportion of birds (7/11) switched from spatial recognition during Stage 2 to beaconing in Stage 3 (χ2 = 8.757, df = 1, p = 0.004).
The strategy birds used to visit flowers. The light grey bars represent the proportion of birds that used spatial recognition as their first choice in all experimental stages. The dark grey bars represent the proportion of birds that used beaconing as their first choice. The grey line represents the chance level for stage 1 (line A), the dot-dash line the chance level for stage 2 (line B) and the dotted line the chance level for stage 3 (line C), respectively
Discussion
Wild, free-living rufous hummingbirds can relocate a goal in the absence of the goal’s visual features after a single feeding event. As far as we know this is the first time that spatial recognition, in the absence of any marker at the previously rewarded locations, after a single-trial has been demonstrated in the field. In all previous experiments, memory has been tested after considerable training or when the beacon remains in place during the one-trial spatial experiments (e.g. Healy and Krebs 1992; Brodbeck et al. 1992; Clayton and Krebs 1994; Hurly and Healy 1996, 2002; Henderson et al. 2001; Hurly et al. 2010). The hummingbirds’ ability to relocate a reward in the absence of a beacon is consistent with the evidence that they attend to other environmental spatial cues in addition to just the features of the goal (Healy and Hurly 1998, 2003; Hurly and Healy 1996, 2002). We assume that the birds used a combination of landmarks surrounding the goal to do so (e.g., the meadow’s geometry, trees, bushes, trunks, rocks, flowers, etc.), perhaps as a cognitive map, which is an allocentric representation in terms of distances and directions among items in the environment (O’Keefe and Nadel 1978; Collett and Graham 2004). However, as the experiment progressed, the birds appeared to switch from using spatial recognition to using the cues supplied by the visible flower. This finding is consistent with rats eventually learning to use the beacon signalling a goal’s location when the beacon is a more reliable indicator of reward than are the surrounding landmarks (Pearce et al. 1998).
Different brain regions appear to underpin the use of beacons and the encoding of locations with regard to the surrounding landmarks: rats with hippocampal damage appear to rely on beaconing regardless of cue reliability, as do rats with medial lesions to the dorsal striatum (Devan and White 1999). FMRI data recorded during the performance of virtual reality task support a similar spatial recognition/beaconing dichotomy in humans too, in the right posterior hippocampus and dorsal striatum, respectively, (Doeller et al. 2008).
The behaviour of the birds during experimental stage 2 shows that hummingbirds can encode and retrieve the spatial position within their environment after a single experience. In addition, the birds’ first choice at Stage 3 suggests both primacy and recency effects: more visits to both TF (primacy) and F2 (recency) locations (6/6 birds) than to F1 (0 birds) appears somewhat similar to the performance of humans on word-list learning and with the performance on spatial and non-spatial tasks by other animals (Bjork and Whitten 1974; Castro and Larsen 1992; Crystal and Shettleworth 1994).
That a rufous hummingbird, while visiting a rewarded flower for the first time, encodes the spatial location and then remembers this information to revisit the flower is a remarkable phenomenon when viewed in an ecological context. Hummingbirds visit hundreds of flowers each day, taking a tiny amount of nectar from each. Given the birds’ behaviour in our experiment, it seems possible that the spatial location of each flower is encoded for each visit, even when the flower is empty: rufous hummingbirds that empty some flowers in an array can avoid these flowers on their next visit and direct their foraging to previously unvisited flowers (Healy and Hurly 1995). If locations of numerous flowers are encoded each day, one must question how much spatial information can be retained and how out-of-date information is eliminated or discounted.
Our experimental design is comparable with those used to test spatial memory in the absence of the beacon, for example, delayed-matching-to-place water-maze tasks (Steele and Morris 1999) and one-trial place memory (Bast et al. 2005) as well as cued recall in rats. for example, place-odour paired-associate task (Day et al. 2003), all of which involve the animal retrieving a location after a single experience. Retrieving a single experience is one of several characteristics of episodic-like memory. Since the ground-breaking experiments with scrub jays retrieving different kinds of stored food, the evidence that birds and rodents have episodic-like memory has mounted (Clayton and Dickinson 1998, 1999; Clayton et al. 2003a, b; Day et al. 2003; Babb and Crystal 2006; Crystal 2010; Dere et al. 2006; Naqshbandi and Roberts 2006; Roberts et al. 2008; Eacott et al. 2005; Eacott and Easton 2007; Zinkivskay et al. 2009). However, all of these experiments have been carried out in the laboratory. It is not yet clear whether hummingbirds are capable of episodic-like memory but as they can learn the refill rate of flowers (Gill 1988; Henderson et al. 2006), as well as what flowers look like and their locations (Hurly and Healy 1996, 2002), it may be that they will prove to be a useful system in which to explore episodic-like memory. The fact that they show learning in a trial-unique way is a key aspect for the study of episodic memory. It is possible that if hummingbirds can encode the location of a reward from a single experience they could also distinguish between different experiences (for example, if the flower they sample was empty or full and if it contained nectar with a high or a low sugar concentration), which would imply that wild birds encoded the ‘what and where’ aspects of episodic-like memory. Such episodic-like memory is yet to be tested in wild hummingbirds.
References
Babb SJ, Crystal JD (2006) Episodic-like memory in the rat. Curr Biol 16:1317–1321
Bast T, Da Silva BM, Morris RGM (2005) Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci 25:5845–5856
Bjork RA, Whitten WB (1974) Recency-sensitive retrieval processes in long-term free recall. Cognitive Psychol 6:173–189
Brodbeck DR, Burack OR, Shettleworth SJ (1992) One-trial associative memory in black-capped chickadees. J Exp Psychol Anim Behav Process 18:12–21
Castro CA, Larsen T (1992) Primacy and recency effects in nonhuman primates. J Exp Psychol Anim Behav Process 18:335–340
Clayton NS, Dickinson A (1998) Episodic-like memory during cache recovery by scrub jays. Nature 395:272–274
Clayton NS, Dickinson A (1999) Scrub Jays (Aphelocoma coerulescens) remember the relative time of caching as well as the location and content of their caches. J Comp Psychol 113:403–416
Clayton NS, Krebs JR (1994) One-trial associative memory: comparison of food-storing and non-storing species of bird. Anim Learn Behav 22:366–372
Clayton NS, Bussey TJ, Dickinson A (2003a) Can animals recall the past and plan for the future? Nat Rev Neurosci 4:685–691
Clayton NS, Yu KS, Dickinson A (2003b) Interacting cache memories: evidence for flexible memory use by Western Scrub-Jays (Aphelocoma californica). J Exp Psychol Anim Behav Process 29:14–22
Collett TS, Graham P (2004) Animal navigation: path integration, visual landmarks and cognitive maps. Curr Biol 14:475–478
Crystal JD (2010) Episodic-like memory in animals. Behav Brain Res 215:235–243
Crystal JD, Shettleworth SJ (1994) Spatial list learning in black-capped chickadees. Anim Learn Behav 22:77–83
Day M, Langston R, Morris RGM (2003) Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature 424:205–209
Dere E, Kart-Teke E, Huston JP, De Souza Silva MA (2006) The case for episodic memory in animals. Neurosci Biobehav Rev 30:1206–1224
Devan BD, White NM (1999) Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci 19:2789–2798
Doeller CF, King JA, Burgess N (2008) Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. P Natl Acad Sci-Biol 105:5915–5920
Eacott MJ, Easton A (2007) On familiarity and recall of events by rats. Hippocampus 17:890–897
Eacott MJ, Easton A, Zinkivskay A (2005) Recollection in an episodic-like memory task in the rat. Learn Memory 12:221–223
Fukushi T, Wehner R (2004) Navigation in wood ants Formica japonica: context dependent use of landmarks. J Exp Biol 207:3431–3439
Gill FB (1988) Trapline foraging by Hermit hummingbirds: competition for an undefended, renewable resource. Ecology 69:1933–1942
Gould KL, Kelly DM, Kamil AC (2010) What scatter-hoarding animals have taught us about small-scale navigation. Phil Trans R Soc B 365:901–914
Graham P, Fauria K, Collett TS (2003) The influence of beacon-aiming on the routes of wood ants. J Exp Biol 206:535–541
Healy S, Hurly TA (1995) Spatial memory in rufous hummingbirds (Selasphorus rufus): a field test. Anim Learn Behav 23:63–68
Healy SD, Hurly TA (1998) Rufous hummingbirds’ (Selasphorus rufus) memory for flowers: patterns or actual spatial locations? J Exp Psychol Anim Behav Process 24:396–404
Healy SD, Hurly TA (2003) Cognitive ecology: foraging in hummingbirds as a model system. Adv Stud Behav 32:325–359
Healy SD, Krebs JR (1992) Comparing spatial memory in two species of tit: recalling a single positive location. Anim Learn Behav 20:121–126
Henderson J, Hurly TA, Healy SD (2001) Rufous hummingbirds’ memory for flowers. Anim Behav 61:981–986
Henderson J, Hurly T, Healy S (2006) Spatial relational learning in rufous hummingbirds (Selasphorus rufus). Anim Cogn 9:201–205
Hurly TA, Healy SD (1996) Memory for flowers in rufous hummingbirds: location or local visual cues? Anim Behav 51:1149–2257
Hurly TA, Healy SD (2002) Cue learning by rufous hummingbirds. J Exp Psychol Anim Behav Process 28:209–223
Hurly TA, Scott RD, Healy SD (2001) The function of displays of male rufous hummingbirds. Condor 103:647–651
Hurly TA, Franz S, Healy SD (2010) Do rufous hummingbirds (Selasphorus rufus) use visual beacons? Anim Cogn 13:377–383
Kelly D (2010) Features enhance the encoding of geometry. Anim Cogn 13:453–462
Kelly DM, Spetch ML (2004a) Reorientation in a two-dimensional environment: I. Do pigeons (Columba livia) encode the featural and geometric properties of a two-dimensional schematic of a room? J Comp Psychol 118:384–395
Kelly DM, Spetch ML (2004b) Reorientation in a two-dimensional environment: II. Do adults encode the featural and geometric properties of a two-dimensional schematic of a room? J Comp Psychol 118:82–94
Naqshbandi M, Roberts WA (2006) Anticipation of future events in squirrel monkeys (Saimiri sciureus) and rats (Rattus norvegicus): tests of the Bischof-Kohler hypothesis. J Comp Psychol 120:345–357
O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford
Pearce JM, Roberts ADL, Good M (1998) Hippocampal lesions disrupt navigation based on cognitive maps but not heading vectors. Nature 396:75–77
Pearce JM, Ward-Robinson J, Good M, Fussell C, Aydin A (2001) Influence of a beacon on spatial learning based on the shape of the test environment. J Exp Psychol Anim Behav Process 27:329–344
Pearce JM, Graham M, Good MA, Jones PM, McGregor A (2006) Potentiation, overshadowing, and blocking of spatial learning based on the shape of the environment. J Exp Psychol Anim Behav Process 32:201–214
Roberts WA, Feeney MC, Macpherson K, Petter M, Mcmillan N, Musolino E (2008) Episodic-like memory in rats: is it based on when or how long ago? Science 320:113–115
Steele R, Morris RGM (1999) Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus 9:118–136
Zinkivskay A, Nazir F, Smulders TV (2009) What-where-when memory in magpies (Pica pica). Anim Cogn 12:119–125
Acknowledgments
This research was supported by CONACYT (Consejo Nacional de Ciencia y Teconología), the University of Lethbridge and the Natural Sciences and Engineering Council of Canada. We thank Cam Finlay and Ida Bacon for support in the field and Rosamund Langston and three anonymous reviewers for comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flores-Abreu, I.N., Hurly, T.A. & Healy, S.D. One-trial spatial learning: wild hummingbirds relocate a reward after a single visit. Anim Cogn 15, 631–637 (2012). https://doi.org/10.1007/s10071-012-0491-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-012-0491-0