Abstract
Elaborate manual skills of food processing are known in several species of great ape; but their manner of acquisition is controversial. Local, “cultural” traditions show the influence of social learning, but it is uncertain whether this includes the ability to imitate the organization of behavior. Dispute has centered on whether program-level imitation contributes to the acquisition of feeding techniques in gorillas. Here, we show that captive western gorillas at Port Lympne, Kent, have developed a group-wide habit of feeding on nettles, using two techniques. We compare their nettle processing behavior with that of wild mountain gorillas in Rwanda. Both populations are similar in their repertoires of action elements, and in developing multi-step techniques for food processing, with coordinated asymmetric actions of the hands and iteration of parts of a process as “subroutines”. Crucially, however, the two populations deal in different ways with the special challenges presented by nettle stings, with consistently different organizations of action elements. We conclude that, while an elaborate repertoire of manual actions and the ability to develop complex manual skills are natural characteristics of gorillas, the inter-site differences in nettle-eating technique are best explained as a consequence of social transmission. According to this explanation, gorillas can copy aspects of program organization from the behavior of others and they use this ability when learning how to eat nettles, resulting in consistent styles of processing by most individuals at each different site; like other great apes, gorillas have the precursor abilities for developing culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been intense recent interest in “ape cultures” (Whiten 2005), in the sense of locally varying traditions of behavior. That excitement stems from the potential relevance to two important issues: firstly, the evolutionary origins of human technology by cumulative cultural evolution of complex skills, including ones involving tool manufacture; and secondly, the phylogenetic distribution among animals of specialized mechanisms of skill acquisition, including imitative learning, teaching, and the tendency to conform. Experiments with human subjects, however, have shown that merely seeing the products of skilled construction can be as effective, for generating a cumulative tradition of skill, as explicit instruction or the opportunity to watch the process of construction (Caldwell and Millen 2009). Indeed, animal “culture” per se has been recorded in a much wider range of animals, including fish and rats (Galef 2003; Laland and Janik 2006), with no suggestion that these species possess special learning mechanisms that might be relevant in the evolution of human technology. For these reasons, particular interest attaches to cases where apparently cultural traits involve skills that are technically challenging (Byrne 2007), because in these cases the relevance to human evolution and advanced cognitive abilities is most clear.
With growing evidence for cultural transmission of technically challenging traits in two genera of great ape, Pan and Pongo (chimpanzees: Boesch and Tomasello 1998; McGrew 1992; Whiten et al. 1999; orangutans: van Schaik et al. 2003), the absence of similar evidence from Gorilla may seem surprising. However, the only two gorilla study sites at which individuals can be observed at very close range throughout the day, Karisoke in Rwanda and Bwindi in Uganda, involve gorillas that were part of a single local population until relatively recently and still inhabit widely overlapping ecological zones—with a consequently similar range of plant problems—so cultural differences might be expected to be minimal (and see Sawyer and Robbins 2009). The food processing techniques of these mountain gorillas have, however, been argued to reflect culturally-transmitted skills that depend on imitative learning, for three reasons. Firstly, the techniques are elaborate and inherently improbable of discovery; this is not a matter of the individual manual actions (“elements”) used, which are no doubt part of the natural gorilla repertoire, but applies to the hierarchically organized processes (“techniques”) that are constructed from them (Byrne and Byrne 1993; Byrne et al. 2001a, b). Secondly, variations in technique are minimal within the local population, in contrast to the form and manner of execution of elements which show considerable variation both within and across individuals (Byrne and Byrne 1993). Thirdly, even in the “natural experiment” of individual great apes with maimed hands, a consequence of snare injuries in infancy (Stokes et al. 1999), the techniques they acquire show the same organizational structure as those used by the able-bodied (Byrne and Stokes 2002). These factors point to program-level imitation (Byrne and Russon 1998), in which it is the organization of elements into new programs that is acquired by observation, not the elements themselves. However, several commentators have pointed out that the evidence base could be stronger (Bauer 1998; de Waal 1998; Tomasello 1998; Vereijken and Whiting 1998). In particular, they suggested, it would be more compelling if another population of gorillas had developed a different group technique for dealing with the same plant problems: but none was known.
Tennie et al. (2008) attempted to remedy this deficiency by artificially presenting captive western gorillas with plants of a European nettle, Urtica dioica. This nettle species, they pointed out, is “highly similar” in structural design to the Rwandan one. Thus, if their subjects consumed nettles at all, their technique for doing so could be fairly compared to that known from wild mountain gorillas. Since nettles are absent from the range of the western gorilla species (Gorilla gorilla), it would be implausible to suggest the technique of nettle processing was genetically encoded in this species (compare the bamboo-processing techniques of Hapalemur (Stafford et al. 1993), where that could be argued).
Tennie et al. reported that “twelve gorillas in three different groups (including one nettle-naïve gorilla) used the same program level technique as wild mountain gorillas (with differences mainly at the action level)” (Tennie et al. 2008, p. 584); and “10 of 12 gorillas, including the naïve gorilla, used the same feeding program as mountain gorillas do” (Tennie et al. 2009). They therefore concluded that “at the program level gorilla nettle feeding derives mostly from genetic predispositions and individual learning of plant affordances”. Clearly, if captive western gorillas spontaneously acquire the same organizational program for processing Urtica as wild mountain gorillas use for processing Laportea, cultural transmission by imitation becomes an unnecessary part of the explanation.
On closer examination, however, Tennie et al.’s conclusion is not robust, because the “grain” of their analysis may have been too coarse to detect important differences in behavior. Tennie et al. analyzed nettle processing into only four categories: (1) procure, (2) gather, (3) process, (4) insert. It was the sequence of these four stages that they discovered to be “the same” in captive and wild gorillas. Their paper illustrates that, when eating nettles, the behavior of mountain gorillas and captive western gorillas, whether naïve to nettle or not, all follow the same 4-step flowchart (Tennie et al. 2008, Fig. 1a, b, d). Collapsed to this coarse level of detail, it is quite difficult to see how else nettles could be processed. Indeed, Tennie et al. (2008, p. 590) accept this assessment, stating that, given a tendency to eat nettles and the problem space of the task, “the sequence “procure-gather-process-insert” is practically inevitable”. Indeed so.
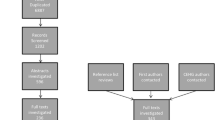
The Karisoke technique of mountain gorillas for processing nettles. The technique involves six stages (Byrne 1999b, 2001; Byrne and Byrne 1993): (1) pulling a nettle stem into range, holding the tip of the stem where stings of the young leaves are not yet active; (2) stripping up the stem with cupped hand, supporting the stem if necessary with the other hand, thus detaching the leaves; (3) detaching the leaf petioles, by levering or twisting them off with the other hand; (4) pulling the leaf blades, loosely held in the hand, slightly out, and then folding them over the thumb, using a precision grip of the other hand; (5) pulling out the thumb and using it to regrasp the folded nettle parcel; (6) placing the folded leaf parcel carefully through the open mouth, generally avoiding contact with the lips. A gorilla may repeat part of this program, thus accumulating larger bundles of leaves for subsequent processing, by treating sections of the whole program as subroutines; in addition, inedible debris if any is present can be removed at several points, most commonly before leaf folding. In the flowchart, the sequence of actions starts at the top and moves down: rectangular boxes show action elements, described by the words in them; optional processes are given in brackets. Definitions of all elements are given in ESM_3. Dotted lines indicate bilateral coordination between separate movements of the two hands; branched lines represent alternative methods for achieving the same end; loops indicate where a process may repeat or iterate until an appropriate size of handful is reached
The appropriate behavioral grain, at which to seek possible similarity or difference in technique, is that of structural organization. There is no dispute that a whole range of factors may play significant roles in acquisition of any primate manual skill: the natural endowment of manual abilities, individual trial-and-error learning, affordances of the hands, task demands, and constraints, etc. Where social learning might be especially important is in “getting the gist” of what to do, which is more likely to be revealed at a level of analysis below that of Tennie et al.’s four “practically inevitable” steps, but above the details of the particular action elements used, manual laterality, forcefulness, and extent of movements. At this intermediate, structural level of analysis, Fig. 1 shows the technique known from mountain gorillas (see video in ESM_1).
Unfortunately, Tennie et al. (2008) did not report the structural organization of nettle processing in the gorillas they studied, whether matching that developed among wild gorillas in form or not. However, Claudio Tennie has kindly allowed us the use of two videos depicting typical behavior of the gorillas in their experiment (see video in ESM_2). Certain organizational features appear the same as those of wild mountain gorillas; others are different or missing. Asymmetric bimanual coordination of the hands, and the particular stripping movement that detaches leaves from the stem, for instance, are visible in both videos. Both are similar to the way Gombe chimpanzees accumulate ants in ant-dipping (McGrew 1974), suggesting this element is part of the ape-typical repertoire. Popping leaves into the mouth in such a way that the lips are avoided seems another common feature, perhaps learnt by painful trial-and-error. However, other characteristic features of mountain gorilla nettle feeding are missing. The bimanual levering or twisting, used to detach petioles from leaves is missing; and since the petioles of any nettle are tougher than the leaf blades and well endowed with stings, it cannot be optimal to leave them attached to the leaves and eat them. Most strikingly, absent is the elaborate two-step fold process, that mountain gorillas use to reduce sting contact with the lips and mouth; instead, the captive gorillas can be seen to squash the bundle of leaves into the palm or roll them against the opposing arm. Finally, no iteration is used to build up larger bundles of leaves from several plants before consumption (nor was this seen at all in the study: C Tennie, personal communication). Contra the original conclusions of Tennie et al., it seems that major differences in technique exist between the two populations studied.
It must be remembered, however, that the gorillas in this experiment had very little experience with nettle eating compared to those in the wild population of Karisoke, Rwanda. Thus, the fact that their method was different in several ways, apparently making it less efficient and more painful, needs to be interpreted circumspectly: it might be that with sufficient individual experience, these gorillas’ behaviour would gradually converge on the same technique as that used at Karisoke. Rather than showing two social traditions, the differences in technique might reflect merely two stages in the same process of individual learning.
Here, we present new data, describing a consistent technique for eating nettles Urtica dioica that has developed in captive western gorillas at Port Lympne, Kent. These data on Lympne gorillas offer a credible comparison for Karisoke gorilla nettle-eating techniques because the technique has been used there over several years by many individuals. In order to compare the behaviour of Port Lympne and Karisoke gorillas when consuming nettle leaves, we needed to represent the recurring features of each individual’s approach. In particular, since the focus of interest was to discover whether some aspects of an individual’s method were acquired culturally by observation, it was critical to represent the most challenging or “surprising” features of a method. In contrast, because it has been generally accepted that most, if not all, action elements shown by the species are part of the species-typical or “innate” repertoire, detailed analysis of elements was less relevant. Dispute has focussed upon the organization of elements into an effective technique, which includes: the specific choice of elements; their sequential organization and coordination with other elements and whether this might be dictated by affordances of the task; and overall aspects of program organization, such as use of subroutines for iteration or substitution of methods. We examine the nettle-eating behaviour of Port Lympne gorillas specifically for these characteristics in order to compare our findings with those on Karisoke gorillas as described in the literature.
Methods
Subjects
The group of western gorillas (Gorilla gorilla) at Port Lympne, Kent, during the study comprised 7 adults and their 7 offspring, as follows: 1 adult male (Djala, est. 25 years), 6 adult females (Mumba, 20 years; Kishi, 19 years; Tamki, 18 years; Emmie, 16 years; Foufou and Kibi, both 15 years), 5 juveniles (Jaja, 8 years, son of Mumba; Dishi, 7 years, son of Kishi; Kouni, 6 years, son of Kibi; Yeni, 6 years, Mpassa, 4 years, daughter and son, respectively, of Foufou), and 2 infants (Mbwambe, 9 mo, daughter of Kishi; Djongo, 7 mo, son of Kibi). All but two of the females had successfully reared offspring; neither of those two females, Tamki and Emmie, had fully socially integrated into the group. Both were initially hand-reared and subsequently kept with other hand-reared youngsters till c. 6 years old. All juveniles and infants were sired by the current adult male, Djala, who was wild caught at a few years old. All other group members were born in captivity; Kibi and Mumba are sisters.
The gorilla housing consisted of an enclosed 5 m high indoor area, 28 m × 11 m, with individual sleeping rooms of 3 m × 2 m, 2.5 m high; a large outdoor 5 m high caged area, 30 m × 12 m; and a large open-air garden area, irregular in shape and varying from 62 m to 112 m in length, and between 22 m and 39 m in width. The gorilla group had access to all areas at all times, except that the sleeping area was briefly closed off during the morning clean and the garden was closed off at night. The areas of garden most often used by the gorillas were heavily overgrown with common nettle (Urtica dioica), only partly trimmed by the keepers and frequently exploited by the gorillas.
Each day at approximately 8.30 a.m., a feed of mixed fruit and vegetables was scattered throughout the caged area; foraging for these items represented the major part of the group’s morning activity, and they rarely ventured out of the caged area before 11 a.m. At midday and again in mid-afternoon, however, a top-up feed of highly preferred foods was scattered in the garden to encourage the whole group outside in view of the public. In general, the periods 30 min prior to and 1 h following these top-up feeds were times of peak outdoor use, and therefore of nettle feeding.
Procedure
Filming took place each day from the 5th to the 14th June, 2007, between 9.30 a.m. and 5.30 p.m. Over this period, most nettles were well-grown and in flower. As there was no existing record of the frequency with which nettle processing occurred in the group, we initially filmed all observed instances of nettle processing (focal behavior sampling: Altmann 1974). Once it became apparent that nettles were processed on an almost-daily basis, the protocol was adapted to achieve a more consistent quantity of nettle processing footage for each individual. We prioritized individuals with the least footage, and augmented filming opportunities by provisioning some individuals at the end of the day: a large bunch of approximately 20 nettle stems was placed in the individual’s room.
Analysis
As with the analyses of nettle processing in mountain gorillas (Gorilla b. beringei) at Karisoke, Rwanda (Byrne and Byrne 1991, 1993), we viewed a session of plant feeding as the processing of a series of handfuls: accumulations of edible material that were ready to eat. To produce a handful, a sequence of action elements is applied, converting raw natural plant matter into a bundle of material suitable for putting in the mouth. When a consistent sequence of elements is applied repeatedly in this way it is said to represent a technique. Only complete handfuls were coded: handfuls were discarded from analysis if their processing had been interrupted, or where visibility was such that action elements could not be discerned. Actions performed by each hand were recorded separately, as were the actions of any other body parts, e.g., teeth. Where possible, we used the same definitions for the action elements as those recorded in the processing of Laportea alatipes or Galium ruwenzoriense by Byrne and Byrne (1993); see ESM_3 for full definitions used. In order to describe the techniques used regularly by each individual, we developed a systematic representation of techniques as flowcharts (see ESM_4). Once each individual’s technique was represented, we went on to examine the commonality between them in case it was possible to construct a group flowchart.
In order to assess the reliability of coding, a second rater, familiar with recording great ape manual actions from video, was provided with video footage of 27 handfuls of nettle processing (7% of total coded) which contained 163 actions. The second rater was provided with the full list of 16 action elements and their definitions, although only 14 were displayed within the examples provided. Cohen’s kappa was calculated from the agreement between the first and second raters at the level of individual action elements: agreement with the primary coder was high (Cohen’s Kappa = 0.77). The main source of difference was the question of whether small movements of the fingers were considered to be independent actions.
Results
Of the 12 gorillas that were over a year old, we videotaped 11 engaged in processing nettle, giving 196 min of video record, from which we were able to code the steps of processing for 401 handfuls of material. Neither of the two hand-reared adult females regularly processed nettle. Emmie was never observed to eat nettles, and while Tamki gathered nettle on three occasions she only processed two handfuls in view of the camera. Each of the 2 <1-year-old infants would occasionally try to play with its mother’s bundle of pre-gathered stems, but the mother would quickly move the stems away. Similarly, when the youngest juvenile, 4-years-old Mpassa, approached his mother Foufou’s pre-gathered stems she would usually move them out of his way. Perhaps as a result, Mpassa was only observed to process four handfuls of nettles. Provisioning was impractical, since these young gorillas were never isolated from their mothers.
We excluded all individuals with less than 10 handfuls recorded in a technique, leaving 9 gorillas whose data allowed detailed analysis (see Table 1). These individuals contributed a median of 35 handfuls each (range, 6–106 for specific techniques analyzed; grand total of 395 handfuls). The four adult females always assembled a bundle of nettle stems and then retreated with them away from the nettle patch before processing and consuming them. The silverback male and the four juveniles also gathered stems before processing, but rarely left the area of the growing nettles; and all but Yeni also sometimes processed growing stems without first assembling a bundle (Yeni was only recorded processing nettle when provisioned, and thus had no opportunity to demonstrate processing of growing nettles). As shown in Table 1, the majority of handfuls were processed by dealing with the stem and leaves separately; the alternative of processing both together was less frequent overall, but used more by adult females and some juveniles.
For the leaves-with-stem technique, six individuals were observed to process more than 10 handfuls of leaves still attached to stems, eating both together; their techniques were described as flowcharts (see Table 1 and ESM_4, ESM_6). The techniques of all six individuals consisted of at least three stages and up to six: procuring a leafy stem, perhaps picking off inedible debris, optionally removing the dirty root end, optionally collapsing a long stem, adjusting the leaves so they wrapped around the stem, and consuming the result (the group flowchart is shown in Fig. 2).
The Port Lympne “leaves-with-stem” technique for processing nettles. Key generally as Fig. 1; diamonds indicate cases where some (unknown) criterion determines the choice of one of two separate processing routes
For the leaves-separate technique, five individuals were observed to remove more than 10 handfuls of leaves from stems; their techniques were represented as flowcharts (see Table 1 and ESM_4, ESM_6). The techniques of all five individuals consisted of four stages: procuring a leafy stem (omitted when material processed directly from the ground), removing leaves from it, adjusting the handful (occasionally omitted, apparently when the bundle was already tidy), and consumption (videos of four of these individuals, and one other, using the leaves-separate technique are shown in ESM_7 to ESM_11; the group flowchart is shown in Fig. 3).
The Port Lympne “leaves-separate” technique for processing nettles. Key as Fig. 2
Sixteen action elements were used for nettle processing by the 9 gorillas whose behavior was studied in detail (see ESM_5), with individual repertoires ranging between 7 and 13 elements. The repertoire used for each technique, however, was remarkably similar. Eleven elements were recorded used in leaves-separate processing (range, 7–10 for each individual); 14 elements were recorded used for processing leaves-with-stem (range, 7–12 for each individual). The variation across individuals may reflect preferences in size of stems dealt with: those tackling a larger range of plant sizes necessarily needing to use more different elements.
Comparing Port Lympne and Karisoke techniques
Before examining whether Port Lympne and Karisoke gorillas have converged on the same program-level organization, we checked whether their techniques are comparable in other ways, such that a comparison of program-level organization would be meaningful: the repertoire of elements used, the length of the sequence of processing steps, functionality in terms of their adjustment to dealing with the plant’s defences, and the use of coordination between the two hands and within a single hand. In all these aspects, we found no evidence of a fundamental difference between western gorillas processing Urtica dioica in Kent and mountain gorillas processing Laportea alatipes in Rwanda (Table 2); and see ESM_5.
To summarize those aspects on which no differences were seen, the gorillas of Port Lympne showed (a) an elaborate repertoire of action elements, most of which were identical with those of wild mountain gorillas, (b) the ability to build up a consistent, multi-step technique that was goal-directed, in the sense that it is adjusted to solving the particular problems presented by the plant food, (c) the ability to coordinate the two hands, each carrying out different functions, to achieve a single result, (d) the ability to retain part-processed material in the hand while simultaneously carrying out further operations, thus building up a more substantial handful of food by iterative use of a “subroutine” of the main technique.
Specific organization of techniques
All 9 Port Lympne gorillas whose nettle-eating techniques could be analyzed used two different techniques for eating nettle leaves (leaves-separate, leaves-with-stem; see ESM_6 for details); and all 9 individuals used both techniques at least sometimes, whereas Byrne and Byrne (1993) at Karisoke reported only a single technique. The leaves-with-stem technique (Fig. 2) differs radically from the Karisoke technique because Urtica stems are consumed as well as leaves. In fact, neither of the Port Lympne techniques exactly matched the Karisoke one, but the leaves-separate technique is closer. We therefore, focus on this technique for comparison with Karisoke data (see Table 2; and compare ESM_1 with ESM_7 to ESM_11).
Like that used at Karisoke, the Port Lympne technique of leaves-separate (Fig. 3) can involve stripping the leaves off a stem, and also culminates in placing a neat bundle of leaves carefully into the mouth. It differs, however, in three major ways: (1) individuals often use an alternative to stripping off leaves, picking them from a leafy stem individually, often two leaves at a time; (2) leaf petioles are not detached; and (3) no folding of the leaf-bundle is seen, instead the bundle is “neatened” only by using a range of adjustment, reorientation, squeezing, tucking in leaves, etc.
The first difference may not be significant, as mountain gorilla infants have been seen picking individual nettle leaves (RWB personal observation), and accumulation of small items in the hand is used for other purposes in many mountain gorilla feeding techniques, including nettle processing. Thus, it is possible that unweaned juveniles, whose food processing has not been studied in the wild, might alternate between the same two ways of acquiring leaves from a nettle plant (see Corp and Byrne 2002 for evidence of more varied processing techniques in young chimpanzees).
The other two differences, however, make the technique very different (compare ESM_1 with any of ESM_7 to ESM_11). The stinging hairs of any stinging-nettle species are most abundant on the main stem and the leaf stems, or petioles (see ESM_12 for Laportea alatipes and ESM_13 for Urtica dioica). Moreover, as with almost any herbaceous plant, nettle petioles are tougher and less digestible than leaf blades. For Karisoke gorillas, using the movement of twisting one hand grasping leaf blades against the other hand grasping the petioles, levering one hand against the other fist, or simply pulling apart a handful of nettle leaves with the petioles grasped in one hand and the leaf blades in the other, allows this unrewarding part of the nettle to be discarded. We know that pull-apart, lever-apart and/or twist-apart are elements within the repertoire of Port Lympne gorillas; but they were never used to detach petioles during this study, by any individual on any occasion. In the Karisoke fold process, a bundle of leaves, held loosely by the thumb against the palm and fingers in one hand after the petioles have been detached and dropped, is pulled partly out with the fingers of the other hand which are then used to fold the bundle over the thumb of the retaining hand; then the thumb is deftly pulled out from the folded bundle and used to regrasp it so that only a single leaf underside is showing. This coordinated sequence is apparently unique to Karisoke, and was entirely missing from the behavior of Port Lympne gorillas.
Discussion
Western gorillas in captivity at Port Lympne, Kent, have developed a local habit of processing Urtica nettle leaves to eat. They process nettle leaves in two ways: one in which the leaves are detached before consumption, as is done by wild mountain gorillas at Karisoke, Rwanda, when consuming Laportea nettles; and one in which leaves and stems are eaten together, as Rwandan gorillas do when consuming Carduus thistle leaves. Each Port Lympne method shows the level of complexity in organization that justifies the use of the term “techniques” for the skilled food processing sequences of wild gorillas. In all Port Lympne gorillas whose behavior could be examined quantitatively, nettle preparation was consistently a several-step process, in which highly specific manual actions were applied in a regular sequence. In some of these stages, actions were coordinated between the two hands: each hand carried out a different action, synchronized with the other toward a common goal. Other stages relied on the ability of great apes to carry out two independent actions with the same hand at once (Byrne et al. 2001b; Connolly and Elliott 1972): for instance retaining some items in part of the hand while processing new items to add to the bundle, thus allowing a gorilla to use part of a process as an iterated subroutine.
These techniques did not spring into existence in response to experimental provisioning with nettles. Neither could they have been transferred directly from Africa: all but one of the adult Port Lympne gorillas were born in captivity. The exception, Djala, was wild caught as a juvenile, but in any case no stinging nettle occurs within the natural range of the western gorilla. In contrast, nettle leaf consumption has been noticed over several years at Port Lympne. The head keeper recalled that the 20-years-old female Mumba’s mother had been fond of nettles at Howletts Wild Animal Park, where she was born; Howletts gorillas did not have access to growing nettles, but keepers would push them into her cage as a form of diet enrichment. Within the Port Lympne group, it is also possible that vertical as well as horizontal transfer may have contributed to the stable techniques we have documented. Thus, there has been ample chance for local traditions to develop within this group.
The alternative, to such “cultural” transfer of knowledge augmenting the information and skills that gorillas acquire on their own, is that each individual gorilla has developed every aspect of the two techniques on its own, by individual exploration and experiment. On this hypothesis, the consistent group-wide techniques result from the characteristics—affordances and constraints—of the nettle plant and the gorilla hand, as proposed by Tomasello and Call (1997). Tennie et al. (2008) have argued that Urtica and Laportea nettles—since they are of similar size, are both defended by stinging hairs distributed in the same way over the plant, and both are edible and nutritious—would allow a fair test between these hypotheses. At Port Lympne, gorillas detach bundles of Urtica leaves for consumption, as at Karisoke gorillas do with Laportea: but these two techniques, despite sharing many similarities in the way gorillas use their hands, clearly differed in organization. These differences are difficult to explain, on the Tomasello and Call proposal that similar techniques are generated by from individual learning guided by nettle and gorilla constraints and affordances.
The two techniques often began in the same way, stripping leaves from a stem with asymmetric, bimanual coordination (although Port Lympne gorillas often used a second process to accumulate a bundle: repeated, individual picking of leaves); and they finished in the same way, placing the handful of leaves neatly into the mouth, avoiding lip contact. Between these stages, the processes were different. Port Lympne gorillas never detached the sting-infested leaf petioles, an invariable part of the Karisoke technique; yet the specific actions needed to do so were present in their repertoire and were indeed used when consuming leaves still attached to stems (twist-off, lever-off, and pull-off were used commonly in tearing off the dirty, root-ends of large stems before consumption). Moreover, the Port Lympne technique entirely lacked the distinctive and deft bimanual manner of folding the leaf-bundle; yet the manual actions used in Karisoke folding are simple ones well within their repertoire. Folding the bundle serves to “wrap” several nettle leaves in a single leaf underside and thus reduces sting contact with the mouth, and appears highly efficient. Presumably to achieve the same result, i.e., minimizing stings of the mouth, Port Lympne gorillas instead used a variety of unimanual and bimanual adjustments and squeezing actions that certainly made the leaf-bundle less untidy.
According to the logic used by Tennie et al. (2008), then, the striking organizational differences we found, between the techniques found in Rwanda and in Kent, imply that social learning must have a role in acquisition at one or both sites. As emphasized in the introduction, there is no dispute that there are other contributory influences on the acquisition of manual plant-processing in apes: affordances of the hand and the plant, individual exploration, stimulus enhancement, and other learning mechanisms. The many similarities between the behavior at the two sites, and individual differences between subjects, do not therefore argue against the involvement of social learning.
It remains possible to argue that the differences in technique reflect only the results of individual learning, however, because the species of plant and the species of gorilla are not identical. However similar in anatomy and manual behavior are individuals of G. beringei and G.gorilla, the populations are genetically distinct; however similar are their physical challenges, Laportea alatipes and Urtica dioica are different plants. It will therefore always be arguable that some subtle and unremarked feature has channeled individuals’ behavior along different paths at the two sites during learning. How plausible might this account be?
Certain aspects of our data would present little difficulty for a non-social explanation. The less-efficient approach of Port Lympne gorillas to leaf detachment, often using blade-by-blade accumulation, might simply reflect motivational differences, or the fact that these gorillas get less practice since nettles are highly seasonal in Kent. The existence of the second technique at Port Lympne, in which stems and leaves are consumed together, might only indicate that the stems of Urtica dioica are better food than those of Laportea alatipes, worth eating from at least some plants. Other features present real difficulty for a non-social account.
Any explanation based on individual learning relies on the power of trial-and-error learning to “hill climb” to an optimal solution. To be consistent with acquisition by individual learning, then, the skill acquired should be optimal for the job. But the critical organizational differences (Table 2) mean that the Karisoke technique for eating leaves after detachment from stems is superior for dealing with any stinging-nettle species, whereas the Port Lympne technique is inferior. This follows because Urtica, like Laportea, has stings more abundantly on the petiole than the leaf blade, and more abundantly on the edges of the leaf than the underside; yet, the Port Lympne technique lacks the processes for minimizing these problems, by detaching petioles and by deftly wrapping the bundle in a single leaf before consumption (compare ESM_1 with ESM_9, for instance). It might be argued that Urtica is overall less virulent—although there is no evidence to support this claim, and one of us (RWB) has had much personal experience of being stung by both species and did not notice any consistent difference. But even so, it must make sense to reduce the ratio of tough/indigestible to tender/nutritious material, and it must make sense to reduce the contact between potentially painful stings and sensitive lips. Moreover, since Port Lympne gorillas are resident in a well-managed zoo, their nutrition is presumably good and small effort is needed to obtain it: differences as a consequence of motivation would be plausible, but only if Port Lympne gorillas were the ones more careful to maximize quality and minimize pain from nettles consumed, not less so. And, unlike the limited experience of the subjects in the Tennie et al. (2008) experiment, Port Lympne gorillas have been eating nettles for years: if individual exploration were all that were needed for every individual to develop a technique that detached petioles and for folded over and regrasped leaf blades, Port Lympne gorillas should have developed it.
However, if gorillas are able to parse and thus imitate the “program-level” of behavior (Bates and Byrne 2010; Byrne and Russon 1998; Hobaiter and Byrne 2010), then these results are entirely as expected. By repeated casual observation of the behavior of a skilled practitioner—and as infants, gorillas can readily observe the efforts of their mother or a silverback, since close approach is tolerated—the organizational “gist” can be parsed from the superficial variability of behavior. Then, when it comes to tackling the task itself, the young gorilla’s exploration will be guided into the same overall technique, while the details of how it achieves each step may be learnt individually. (No doubt parts of any technique will be co-opted from existing skills or discovered by individual exploration: but some critical features may not be possible to acquire this way, and those must rely on social learning.) The failure of two hand-reared females at Port Lympne to show nettle-eating technique is therefore predictable, since they joined the group when too old to be allowed such opportunities for causal observation of adult skills. Similarly, one gorilla at Karisoke, Picasso, who transferred into Group 5 when adolescent from a natal range below the distribution of Laportea nettle, lacked those opportunities (Byrne 1999a). Although she did learn to eat nettles, she consumed much less than other adult females, and neither she nor her juvenile acquired the sequence of actions for folding the leaf blade bundle.
We conclude, therefore, that the systematic differences between food processing techniques, used in Kent and Rwanda for consuming nettle leaves, must derive in part from social learning. Because these differences concern organizational “gist” rather than fine detail in how the behavioral programs are implemented, we suggest that the gorilla social learning relies on behavior parsing rather than action-by-action copying (Bates and Byrne 2010; Byrne 2003). Behavior parsing picks out the consistent aspects of multiply-viewed efforts at the same process; if these efforts are skilled, those aspects will almost never be a matter of specific actions, unless the fine detail is critical for the skill. With ape feeding, the level of organization most useful for learning the underlying skill, the gist, will be parsed out automatically. Tennie et al. (2008) argued the reverse: i.e., that while nettle-eating technique was acquired solely by individual learning, “certain actions (i.e., single actions) of this complex skill may be owing to social learning”. However, what we have found in the current study is that the inter-population differences do not concern single elements of manual action—which seem most likely to be part of a species-typical repertoire. Rather, it is the overall manner in which these elements can be organized together, when building a program for attaining particular ends, which is most likely acquired imitatively.
As we have noted, our evidence is not conclusive. However, that reservation applies also to other evidence for the involvement of social learning in acquisition of technologically complex skills by great apes. Experimental research on the transmission of cultural traditions within great ape groups has so far been restricted to simple actions, such as pushing or pulling a cover, twisting or pulling a handle (Custance et al. 1999; Stoinski et al. 2001; Whiten et al. 2005). Similarly, most of the inter-population differences described as traditions in chimpanzees or orangutans involve simple actions (van Schaik et al. 2003; Whiten et al. 2001). To pass on the choice of a simple action, no more than a tendency to simple social learning by local enhancement or response facilitation (Hoppitt and Laland 2008) may be needed. Where population differences concern technically complex skills, that explanation would not do. But even there, interpretation as a result of imitation is tricky, because ecological influences are difficult to rule out: the behavior generally involves feeding on locally-available plants and/or insects, or use of locally-made tools (Byrne 2007), and ecological interactions are known to have subtle effects on ape feeding (Byrne et al. 2004; Laland and Janik 2006). Some researchers have pointed to inter-site differences in style as implying cultural learning (e.g., Boesch 1996; McGrew 1974); but in the best-studied case these proved to relate to differences in the nature of the problem solved (Humle and Matsuzawa 2002; although see Mobius et al. 2008). In a few cases, the geographical distribution of a technically complex skill points to cultural transmission rather than ecology (e.g., chimpanzee hammer-and-anvil use: Boesch et al. 1994; McGrew et al. 1997); orangutan tool-use for eating Neesia fruit (Fox et al. 1999; van Schaik et al. 2003). But even then, an alternative explanation remains: that of genetical transmission (Langergraber et al. 2010).
For the site-specific traditions of nettle eating we have examined, the basic task is similar at both sites and the techniques at both sites are consistent, multi-step sequences; yet they differ in organization of their behavioral program. We therefore, suggest that our gorilla data give some of the strongest evidence yet to come from any great ape that observational learning of a skilled conspecific can allow a novel behavioral program to be built up from simpler components.
References
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49:227–265
Bates LA, Byrne RW (2010) Imitation: what animal imitation tells us about animal cognition. Wiley Interdisciplinary Rev Cogn Sci doi:10.1002/wcs.77
Bauer PJ (1998) If it is inevitable, it need not be imitated. Behav Brain Sci 21:684–685
Boesch C (1996) The emergence of cultures among wild chimpanzees. In: Runciman WG, Maynard-Smith J, Dunbar RIM (eds) Evolution of social behaviour patterns in monkeys and man. The British Academy, London, pp 251–268
Boesch C, Tomasello M (1998) Chimpanzee and human cultures. Curr Anthropol 39:591–614
Boesch C, Marchesi P, Marchesi N, Fruth B, Joulian F (1994) Is nut cracking in wild chimpanzees a cultural behaviour? J Hum Evol 26:325–338
Byrne RW (1999a) Cognition in great ape ecology. Skill-learning ability opens up foraging opportunities. Symp Zool Soc Lond 72:333–350
Byrne RW (1999b) Object manipulation and skill organization in the complex food preparation of mountain gorillas. In: Parker ST, Mitchell RW, Miles HL (eds) The mentality of gorillas and orangutans. Cambridge University Press, Cambridge, pp 147–159
Byrne RW (2001) Clever hands: the food processing skills of mountain gorillas. In: Robbins MM, Sicotte P, Stewart KJ (eds) Mountain gorillas. Three decades of research at Karisoke. Cambridge University Press, Cambridge
Byrne RW (2003) Imitation as behaviour parsing. Philos Trans R Soc Lond B 358:529–536
Byrne RW (2007) Culture in great apes: using intricate complexity in feeding skills to trace the evolutionary origin of human technical prowess. Philos Trans R Soc B 362:577–585
Byrne RW, Byrne JME (1991) Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla g. beringei). Cortex 27:521–546
Byrne RW, Byrne JME (1993) Complex leaf-gathering skills of mountain gorillas (Gorilla g. beringei): variability and standardization. Am J Primatol 31:241–261
Byrne RW, Russon AE (1998) Learning by imitation: a hierarchical approach. Behav Brain Sci 21:667–721
Byrne RW, Stokes EJ (2002) Effects of manual disability on feeding skills in gorillas and chimpanzees: a cognitive analysis. Int J Primatol 23:539–554
Byrne RW, Corp N, Byrne JME (2001a) Estimating the complexity of animal behaviour: how mountain gorillas eat thistles. Behaviour 138:525–557
Byrne RW, Corp N, Byrne JME (2001b) Manual dexterity in the gorilla: bimanual and digit role differentiation in a natural task. Anim Cogn 4:347–361
Byrne RW, Barnard PJ, Davidson I, Janik VM, McGrew WC, Miklósi Á, Wiessner P (2004) Understanding culture across species. Trends Cogn Sci 8:341–346
Caldwell CA, Millen AE (2009) Social learning mechanisms and cumulative cultural evolution: is imitation necessary? Psychol Sci 20:1478–1483
Connolly K, Elliott JM (1972) The evolution and ontogeny of hand function. In: Blurton-Jones N (ed) Ethological studies of child behaviour. Cambridge University Press, Cambridge, pp 329–383
Corp N, Byrne RW (2002) The ontogeny of manual skill in wild chimpanzees: evidence from feeding on the fruit of Saba florida. Behaviour 139:137–168
Custance D, Whiten A, Fredman T (1999) Social learning of an artificial fruit task in capuchin monkeys (Cebus apella). J Comp Psychol 113:13–23
de Waal FBM (1998) No imitation without identification. Behav Brain Sci 21:689
Fox E, Sitompul A, Van Schaik CP (1999) Intelligent tool use in wild Sumatran orangutans. In: Parker ST, Miles HL, Mitchell RW (eds) The mentality of gorillas and orangutans. Cambridge University Press, Cambridge, pp 99–116
Galef BG (2003) “Traditional” foraging behaviours of brown and black rats (Rattus norwegicus and Rattus rattus). In: Fragaszy DM, Perry S (eds) The biology of traditions: models and evidence. Cambridge University Press, Cambridge, pp 159–186
Hobaiter C, Byrne RW (2010) Able-bodied wild chimpanzees imitate a motor procedure used by a disabled individual to overcome handicap. PLoS One 5:e11959
Hoppitt WJE, Laland KN (2008) Social processes influencing learning in animals: a review of the evidence. Adv Study Behav 38:105–165
Humle T, Matsuzawa T (2002) Ant dipping among the chimpanzees of Bossou, Guinea, and some comparisons with other sites. Am J Phys Anthropol 58:133–148
Laland KN, Janik V (2006) The animal cultures debate. Trends Evol Ecol 21:542–547
Langergraber KE, Boesch C, Inoue E, Inoue-Murayama M, Mitani JC, Nishida T, Pusey AE, Reynolds V, Schubert G, Wrangham RW, Wroblewski E, Vigilant L (2010) Genetic and ‘cultural’ similarity in wild chimpanzees. Proc R Soc B doi:10.1098/rspb.2010.1112
McGrew WC (1974) Tool use by wild chimpanzees feeding on driver ants. J Hum Evol 3:501–508
McGrew WC (1992) Chimpanzee material culture: implications for human evolution. Cambridge University Press, Cambridge
McGrew WC, Ham RM, White L, Tutin CG, Fernandez M (1997) Why don’t chimpanzees in Gabon crack nuts? Int J Primatol 18:353–374
Mobius Y, Boesch C, Koops K, Matsuzawa T, Humle T (2008) Cultural differences in army ant predation by West African chimpanzees? A comparative study of microecological variables. Anim Behav 76:37–45
Sawyer SC, Robbins MM (2009) A novel food processing technique by a wild mountain gorilla (Gorilla beringei beringei). Folia Primatol 80:83–88
Stafford DK, Milliken GW, Ward JP (1993) Patterns of hand and mouth lateral biases in bamboo leaf shoot feeding and simple food reaching in the gentle lemur (Hapalemur griseus). Am J Primatol 29:195–207
Stoinski TS, Wrate JL, Ure N, Whiten A (2001) Imitative learning by captive western lowland gorillas (Gorilla gorilla gorilla) in a simulated food-processing task. J Comp Psychol 115:272–281
Stokes EJ, Quiatt D, Reynolds V (1999) Snare injuries to chimpanzees (Pan troglodytes) at 10 study sites in East and West Africa. Am J Primatol 49:104–105
Tennie C, Hedwig D, Call J, Tomasello M (2008) An experimental study of nettle feeding in captive gorillas. Am J Primatol 70:584–593
Tennie C, Call J, Tomasello M (2009) Great ape traditions and zones of latent solutions. St Andrews, Scotland
Tomasello M (1998) Emulation learning and cultural learning. Behav Brain Sci 21:703–704
van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M (2003) Orangutan cultures and the evolution of material culture. Science 299:102–105
Vereijken B, Whiting HTA (1998) Hoist by their own petard: the constraints of hierarchical models. Behav Brain Sci 21:705
Whiten A (2005) The second inheritance system of chimpanzees and humans. Nature 437:52–55
Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C (1999) Cultures in chimpanzees. Nature 399:682–685
Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C (2001) Charting cultural variation in chimpanzees. Behaviour 138:1481–1516
Whiten A, Horner V, de Waal FBM (2005) Conformity to cultural norms of tool use in chimpanzees. Nature 437:737–740
Acknowledgments
The Port Lympne data were collected by CH, who would like to acknowledge the financial support of the European Commission Sixth Framework Program grant “Origins of Referential Communication” Contract 12787. MK acknowledges the support of the University of Stirling and Metro Toronto Zoo for her Ph.D. funding. We thank the Howletts Wild Animal Trust for permission to conduct the study at Port Lympne Wild Animal Park, and in particular we are grateful to Pippa Ducat, Mark Kingston Jones and Charlie Romer for their assistance in planning the project and gathering information. We thank all the staff members of the gorilla section for so generously sharing their invaluable knowledge and assistance. In particular head keeper Phil Ridges, along with fellow keepers Helen Roberts, Ingrid Naisby, Rachel Wood, Sharon Tremaine and Julia Betts. We thank Claudio Tennie for stimulating discussions, and for allowing us to use his video exemplars.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM_1 Video of mountain gorilla processing nettle in group-typical way (MPG 4409 kb)
ESM_2 Video of captive gorilla processing nettle; file kindly provided by Dr. Claudio Tennie, who noted that this video was “typical” of the behavior analyses in Tennie et al. (2008) (MPG 4782 kb)
ESM_7 Video of Port Lympne gorilla Djala using the leaves-separate technique for processing nettles (MPG 6862 kb)
ESM_8 Video of Port Lympne gorilla Kishi using the leaves-separate technique for processing nettles (MPG 8080 kb)
ESM_9 Video of Port Lympne gorilla Jaja using the leaves-separate technique for processing nettles (MPG 15024 kb)
ESM_10 Video of Port Lympne gorilla Dishi using the leaves-separate technique for processing nettles (MPG 8034 kb)
ESM_11 Video of Port Lympne gorilla Kibi using the leaves-separate technique for processing nettles (MPG 8230 kb)
ESM_14 Video of captive gorilla using the action described by Tennie et al. (2008) as “folding”; file kindly provided by Dr Claudio Tennie (MPG 724 kb)
Rights and permissions
About this article
Cite this article
Byrne, R.W., Hobaiter, C. & Klailova, M. Local traditions in gorilla manual skill: evidence for observational learning of behavioral organization. Anim Cogn 14, 683–693 (2011). https://doi.org/10.1007/s10071-011-0403-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-011-0403-8