Abstract
Humans and other animals often find it difficult to choose a delayed reward over an immediate one, even when the delay leads to increased pay-offs. Using a visible incremental reward procedure, we tested the ability of three grey parrots to maintain delay of gratification for an increasingly valuable food pay-off. Up to five sunflower seeds were placed within the parrot’s reach, one at a time, at a rate of one seed per second. When the parrot took a seed the trial was ended and the birds consumed the accumulated seeds. Parrots were first tested in daily sessions of ten trials and then with single daily trials. For multiple trial sessions, all three parrots showed some limited improvement across 30 sessions. For single trial sessions, only one parrot showed any increase in seed acquisition across trials. This parrot was also able to consistently obtain two or more seeds per trial (across both multiple and single trial conditions) but was unable to able to wait 5 s to obtain the maximum number of seeds. This parrot was also tested on a slower rate of seed presentation, and this significantly reduced her mean seed acquisition in both multiple and single trial conditions, suggesting that both value of reward available and delay duration impact upon self-control. Further manipulation of both the visibility and proximity of seeds during delay maintenance had little impact upon tolerance of delays for both parrots tested in this condition. This task demanded not just a choice of delayed reward but the maintenance of delayed gratification and was clearly difficult for the parrots to learn; additional training or alternative paradigms are required to better understand the capacity for self-control in this and other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans and other animals often find it difficult to suppress the urge for immediate gratification, even if waiting will lead to greater overall rewards. Making such inter-temporal decisions may be difficult because time is often related to probability; there is some uncertainty about future outcomes as compared to opting for immediate gratification (see Kalenscher and Pennantz 2008). Rewards may become subjectively less valuable the longer the delay, and this temporal discounting means that rewards are not be maximized. Of course, an animal’s ecology may largely determine the ability to delay gratification and plan for the future, as shown most dramatically in caching birds (e.g. Raby et al. 2007). In many species and contexts, impulsivity may be the best strategy and it has been argued that in more ecologically valid contexts (outside the laboratory setting) a preference for short term rewards may be adaptive (Kalenscher and Pennantz 2008). Nonetheless, the ability to monitor and switch responses would contribute to increased behavioural flexibility in relation to resource exploitation and competition (Murray et al. 2005). For example, chimpanzees have been shown to engage in more hunting, a high-risk strategy with potentially high pay-offs, when fruit is more abundant; this may seem counterintuitive, but when a hunt is unsuccessful the costs are offset by a readily available alternative (Gilby and Wrangham 2007).
A key aspect of making these types of decisions is the need to inhibit response biases, such as taking immediate rewards (Ainslie 1974), or consistently choosing the largest reward visible (Boysen and Berntson 1995). Evidence of reward optimization on experimental tasks, such as delay of gratification (choosing to wait for a larger reward) or reversed contingency (choosing the smaller amount of two presented in order to receive the larger reward), allows us some insight into the cognitive control of impulsivity (Logue 1988). In most delay of gratification tasks, once an initial choice is made the outcome is fixed; depending on their response, the individual either receives a small reward immediately or a larger (or preferred) reward after a delay (delay-choice tasks; Ainslie 1974). As the duration of the delay increases, the value of the preferred reward diminishes in relation to the immediate or less preferred reward; when a delay threshold is reached, individuals opt for the smaller or less preferred proximal reward over distal rewards (Abeyesinghe et al. 2005; Rosati et al. 2007; Stevens et al. 2005).
An alternative approach focuses upon the ability to maintain delay of gratification (Mischel 1974); the subject can respond at any point, and the delayed reward’s value may increase with the passing of time (delay maintenance tasks). This seems to be a more ecologically valid approach, as it requires not only to a choice of strategy, but also the maintenance of self-control over time. For example, human children were offered a preferred reward after a fixed delay (15 min) or an immediate but less preferred reward at any point; they were more likely to wait for the preferred reward when either given a distraction or the rewards were not visible during delays (Mischel 1974). Pigeons tested on a variant of the Mischel paradigm waited for access to preferred food, by resisting pecking a key that allowed immediate access to a less valued food; performance improved when the food items were not visible, or a distractor key was provided, whereas waiting was reduced when the salience of the food items was enhanced (Grosch and Neuringer 1981). However, although the pigeons could opt for the less preferred reward at any time in the trial, the reward value for both pecking and waiting was fixed at 3 s of access to grain, resulting in a dichotomous choice task with fixed rewards (as in a standard delay-choice task).
Using similar fixed reward values, chimpanzees were able to wait several minutes to exchange a small piece of cookie for a larger piece, waiting longer for much larger pieces (Dufour et al. 2007), long-tailed macaques (Macaca fascicularis) were able to wait up to 10 min (Pelé et al. 2009), but capuchins tested with a similar task were only able to wait around 20 s (Ramseyer et al. 2005). However, chimpanzees, and to a lesser extent macaques, can also refrain from taking an increasingly valuable reward; desirable food items were added to an accessible container until the primate takes the accumulated food items and the trial ends. Delays of up to 30 s were tolerated by rhesus macaques (Evans and Beran 2007a), lion tailed macaques waited for up to 1 min (Pelé et al. 2009), but chimpanzees were able to wait for up to 11 min (Beran and Evans 2006). This task is cognitively demanding, as the amount available increases so should the difficulty of inhibiting behavioural responses. We know little about species differences on this measure of impulsivity and self-control, or how effective these methods are with non-primates; it is not clear what mechanisms underlie performance or how these relate to socio-ecological factors and species typical patterns of self-control.
Among avian species, corvids and parrots have received particular attention concerning their learning and cognition (Pepperberg 1999; Emery 2006; Clayton and Emery 2005). Having a large cortical area, relative to both the rest of the brain and to body size, is associated with increased cognitive abilities. Relative brain size and cognitive abilities in birds have been examined in relation to both social and mating systems, though it remains unclear which selection pressures best explain the observed differences between species (Emery et al. 2007). A larger cortical area allows for cognitive flexibility which is adaptive in terms of the capacity to better respond to environmental challenges. For example, birds with relatively larger brains have been shown to have reduced adult mortality (controlling for factors such as body size, habitat, migratory behaviours, mating strategies and parental care, Sol et al. 2007). At the neuroanatomical level, executive functions which underlie the inhibitory control of behaviour are served by the mammalian pre-frontal cortex (PFC). It has been proposed that the nidopallium caudolaterale (NCL) area in the bird brain has a similar relative size to the primate forebrain, and it appears to serve analogous functions (Emery 2006; Güntürkün 2005; Jarvis et al. 2005; Reiner 1986). For example, the activity of single neurons in the pigeon NCL was mediated by both reward delay and size of rewards (Kalenscher and Pennantz 2008, but see also Izawa et al. 2005).
African grey parrots (Psittacus erithacus) are known to have advanced cognitive abilities, including impressive use of labels and the flexible categorization of objects according to different characteristics (reviewed in Pepperberg 1999) and relatively large brains, suggesting a repertoire characterized by behavioural flexibility. They have previously shown an ability to discriminate between amounts (continuous and discrete arrays including up to six items, Pepperberg and Gordon 2005; Pepperberg 2006; see also Al Aïn et al. 2009). However, they have never been assessed on a task which requires the ability to delay of gratification, a task which requires the discrimination of differing amounts over time.
Methods
Subjects
The subjects were Léo (male, 47 m), Shango (male, 24 m) and Zoé (female, 47 m). All three parrots were captive bred and hand reared (by DB) from 3 months of age. Shango is dominant over Zoé but subordinate to Léo, while there is a less clear dominance relationship between Zoé and Léo. They have been trained and tested on a variety of cognitive tasks, including label acquisition (N. Giret et al. unpublished data), object-permanence tasks, counting, and use of experimenter given cues in an object choice task (Giret et al. 2009).
They were housed together in a well-furnished aviary (340 × 330 × 300 cm, maintained at 25°C). Fruit, vegetables and parrot formula (Nutribird A21) were given to the parrots once a day. The sunflower seeds used for testing were highly preferred dietary treats; during training and testing the parrots had ad libitum access to their regular food (Nutribird P15) and water.
Apparatus
The apparatus was a laminated cardboard tray (28 cm × 40 cm). For the 10-trial sessions, each parrot was videotaped during one session per each week using a Canon mini-DV camera.
Training
Parrots can be neophobic and so they were first habituated to accepting seeds presented on the tray (this took the following number of 10-min sessions for each parrot: Léo = 1, Shango = 1, Zoé = 7). For training sessions, the experimenter stood in front of the parrot’s perch and placed five seeds in the centre of the tray and then visibly moved each seed forward, to near the front edge of the tray, at a rate of approximately one seed per second. The tray was held about 5 cm beneath the parrot’s perch. Once all seeds were in place, the tray was raised level with the perch and the parrot was allowed to eat the seeds. The parrots were given demonstration sessions (4–10 trials each) until they were able to wait on the perch for the tray to be raised, rather than moving away by climbing to another part pf their perch (sessions: Léo = 4, Shango = 6, Zoé = 2).
Testing
The birds sat upon a perch (1.5 m high and 1 m from the door to their aviary). The other parrots were either moved to a large holding cage in another area of the aviary, or taken into another familiar room. During testing, the parrots were free to fly around the aviary, so their participation was voluntary.
In the first condition, parrots were tested in daily 10-trial testing sessions before subsequently being tested on a single daily test trial. In the latter trials the cost of acting impulsively was greater as there were no further opportunities to gain seeds. Finally, we modified our procedures to examine the impact of both food visibility and proximity on self-control.
For multiple trial sessions, each session started with a demonstration trial, as described for training trials above with seeds placed before the tray was raised to be within reach, followed by 5-test trials, a mid-session demonstration trial (omitted for Zoé after session 8), five more test trials, and a final demonstration trial. Demonstrations were used to facilitate learning of the number of seeds potentially available for each trial but also served to assess motivation to gain seeds during each session. Test trials started with five seeds in the centre of the tray, which was held just below the perch and within the parrot’s reach. The seeds were out of reach until they were individually moved forward and placed at the front of the tray. The experimenter moved the seeds forward one by one, stopping the trial as soon as the parrot took a seed. The experimenter then waited (using her hand to shield any remaining seeds) until the parrot had removed the presented seeds, before leaving the testing area for a 30-s inter-trial interval. As the number of trials and inter-trial intervals were fixed; the failure to delay gratification led to reduced seed intake across the session. Léo participated in fewer than five trials in two test sessions and these data are excluded from analyses.
Given that even without waiting the parrots would obtain one seed on each of the 10 trials and 15 more on the demonstration trials, following testing with the 10-test trial sessions described above, we subsequently presented the parrots with a single daily trial so that the relative cost of taking the first seed was higher (six seeds obtained in total instead of the maximum of ten available). The same general procedure was used for the single trial session, with the test trial preceded by a single demonstration trial. This new testing regime commenced 6 months after the completion of the 10-trial sessions.
In order to examine whether food visibility and proximity impacted on ability to maintain delay of gratification, the procedure was then modified in two ways. To reduce the visibility and salience of all five seeds being visible on the tray from the start of each trial, the seeds were kept out of view in a small dish held under the presentation tray. Seeds were then individually placed as before, at a rate of approximately one seed per second. To reduce the proximity of the seeds to the parrot during the delay, we introduced a small bell, which the parrots had previously been trained to ring in order to gain seeds. There were ten trials in addition to the initial demonstration trial within a session and one session was conducted daily. The tray was held out of reach (approximately 20 cm beneath the perch) until the bell was rung; the parrots needed to refrain from ringing the bell, rather than refrain from taking seeds within reach as in previous test trials. The parrots were still able to gain access to the seeds at any point within the trial.
When the bell was rung, the tray was immediately raised and the parrot was allowed to consume the seeds which had accumulated. The experimenter then left the test room for a 30 s inter-trial interval as before. If the parrot rang the bell before any seeds were placed (this sometimes occurred, especially during initial sessions), the empty tray was raised for 5 s and then lowered before the trial continued as above. If the parrot failed to ring the bell within 5 s of all five seeds being placed, the trial was ended and there was an ITI of 30 s before the trial was repeated (this occurred on only seven trials in total). The first trial in each session was a demonstration trial, with all five seeds in place before the tray was presented, the tray was held 20 cm below the perch and it was raised as soon as the parrot rang the bell; the parrot was then allowed to consume the accumulated seeds. Shango completed 23 sessions and Zoé 28 sessions, but Léo failed to learn to use the bell so he was not tested in this new condition.
Results
Multiple trial sessions
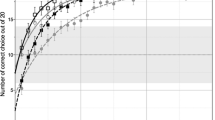
The parrots consumed most of the seeds available on the demonstration trials (mean number of seeds: Léo = 4.95, Shango = 4.98, Zoé = 4.99) indicating good motivation during testing. Léo particpated in 29 sessions, Shango in 32 and Zoé in 26 with an additional 5 sessions at a slower rate of seed presentation. There was considerable individual variation in performance on test trials (see Fig. 1). In order to examine improvement across sessions, the number of seeds obtained was correlated with session number (Pearson correlation, two tailed). All three parrots obtained more seeds in later sessions: for Zoé, r = 0.67, n = 26, p < 0.001; Léo r = 0.74, n = 29, p < 0.001; Shango, r = 0.51, n = 32, p = 0.003. In their last five sessions, the mean number of seeds obtained per trial was 2.62 for Zoé, 1.12 for Shango and 1.43 for Léo; paired t tests compared first five and last five trials and showed that more seeds were obtained in later trials by all three parrots (Zoé t 4 = 7.06 p = 0.002; Léo t 4 = 3.2, p = 0.03; Shango t 4 3.67 p = 0.02). However, Shango took the first seed presented on the majority of trials (272/320 = 85%), as did Léo (180/290 trials, 62%). In contrast, Zoé took the first seed on only a small proportion of trials, mostly within the first three sessions (43/260 = 16.5%). Although Zoé was usually able to wait for more than one seed to be placed, she rarely waited for all five seeds (8/260 trials, 3.1%). For Zoé’s final five sessions, seeds were placed at the slower rate of one seed every 2 s, in order to determine whether number of seeds or duration of the delay acted as a constraint on her performance. There was a significant difference between the mean number of seeds obtained on the final five regular rate sessions (mean = 3.2) compared to the 5 slower sessions (mean = 2.6, t 4 = 3.34, p = 0.029).
Single trial sessions
During initial sessions with the single daily trial (sessions 1–14) neither Zoé nor Léo took all the seeds available on the demonstration trial, suggesting that they were uncertain of the procedure after the break of several months between conditions. Trials 1–14 were therefore excluded from their analyses (as were three other trials in which the seeds were not taken during demonstration trial, see Fig. 2b, c). There was a significant correlation between trial number and number of seeds obtained for Zoé (r = 0.66, n = 43, p < 0.001) but there was no improvement across sessions for Léo (r = −0.19, n = 44, p = 0.21) or Shango (r = −0.031, n = 60 p = 0.81). The mean number of seeds obtained on these single test trials was 1.1 for Shango, 1.1 for Léo and 2.1 for Zoé (see Fig. 2a–c). Paired sample t tests comparing the first and last five trials again showed that Zoé obtained significantly more seeds in the final sessions (t 4 = −6.53, p = 0.003; t tests could not be conducted for Shango and Léo as both took the first seed on all ten trials). Zoé was again tested at a slower presentation rate (1 seed every 2 s for the final 12 trials) and her mean was 1.8 seeds per trial, significantly less than her final 12 one-seed per-second trials, mean = 2.5 (t 11 = 2.96, p = 0.01).
Modified visibility and proximity sessions
On trials in which seeds remained out of view until placement and which also required the ringing of a bell for access to placed seeds, Zoé obtained a mean of 1.9 seeds over 29 sessions and Shango obtained a mean of 1.7 seeds over 27 sessions. There was no correlation between number of seeds and session for Zoé (r = −0.27, n = 28, p = 0.162) or Shango (r = −0.249, n = 26, p = 0.251), that is, performance did not improve across sessions.
Discussion
The results of this study indicate that parrots found maintaining a delay of even a few seconds difficult, at least on this specific task. There was considerable individual variation in their ability with one parrot (Zoé) consistently able to wait 2 or 3 s to gain more than one seed, regardless of testing conditions. Although both Léo and Shango showed a slight improvement during multiple trial sessions they were unable to reach a mean of two seeds per trial within a session. Unlike Zoé, these two did not show any improvement across the single trial condition and manipulating the visibility and proximity of the seeds did not enhance tolerance of delays across sessions for the two parrots tested in this condition. It is important to note that all three parrots had opportunities to learn that a short delay led to more seeds, for example, all three experienced test trials on which they obtained more than the first seed due to brief inattention (due to self-grooming or some distraction); while Zoé seemed to quickly learn from these ‘errors’ how to gain additional seeds, the two males did not.
Although the delays used here were only a few seconds in duration, this method of assessing self-control is particularly challenging for a number of reasons. In the first two conditions, the potential rewards were visible throughout the trials, operant responses were not used to indicate choices (e.g. key peck, Grosch and Neuringer 1981), and the birds could access an increasingly attractive reward at any point within a trial. In addition to the duration of delays, the visibility of rewards can affect the prepotency of the stimuli, as in reverse-contingency tasks (e.g. Kralik et al. 2002), and impact upon delay maintenance (Grosch and Neuringer 1981; Mischel 1974). While reducing proximity and visibility had little impact upon Zoé’s performance, Shango did seem to show some improvement, although not across testing sessions. The time needed to reach and ring the bell may have been sufficient to slow down his response times and furthermore, there were no behavioural indications that he was trying to maintain delays; indeed he often rang the bell before any seeds were in place. Overall, the manipulation of visibility and proximity did not serve to enhance delay of gratification in these two parrots.
The parrots always gained the first seed, so that the relative cost of impulsivity was reduced as compared to the all-or-none contingencies often used in reverse contingency tasks (e.g. Silberberg and Fujita 1996). However, presenting only one trial a day did not increase motivation to wait (Beran and Evans 2006; Mischel 1974); results were comparable across conditions. This indicates that the failure to wait was not due to a lack of motivation due to the overall number of rewards available within a session, but instead indicates a failure to inhibit responses when presented with desirable food items. The outcome of slowing down rate of presentation (in Zoé’s final sessions with both multiple and single trials) suggests that although delay length is a limiting factor, the value of the reward also influences delay maintenance; Zoé’s mean seed acquisition was not reduced in direct relation to the temporal delay for each seed presentation.
All conditions, even single trial sessions, were preceded by a demonstration trial on which the parrots obtained five seeds. The aim of the demonstration trials was to allow the parrots to learn that the amount of food available increased steadily on each trial but on these trials. These trials did not allow the birds to remove any seeds until the tray was raised and as a result may have instead encouraged them to take seeds as soon as the tray was presented on test trials too; removal of these demonstration trials may have facilitated improved performance. However, demonstration trials were also important in assessing motivation during test sessions and it could also be argued that gaining five seeds immediately prior to test trials should have reduced arousal in response to seed presentation and enabled greater tolerance of delays. Finally, the demonstration trials also showed that these parrots are capable of quickly taking a few seeds in their beaks; indicating that the time needed to take and consume seeds should not have had a negative impact on their ability to gain more than the minimum of one seed during test trials. Demonstrating the task with another person in the same role the parrot (e.g. triadic method, Pepperberg 1999) or with a rival parrot (for example, allowing Léo and Shango to observe Zoé) might be a more effective may of informing the parrots about the nature of this task. However, it is unclear how social relationships and the more competitive dimension (e.g. Hare 2001) would impact upon the task; competition may instead facilitate impulsive responses rather than increasing the motivation to maintain the delay of gratification.
The presence of distractor objects or activities may also facilitate increased self-control (Evans and Beran 2007b; Grosch and Neuringer 1981; Mischel 1974); comparative data across species on the impact of these various factors would further our understanding of self-control. Only primates have been tested with the maintenance of delayed gratification methodology used here; although chimpanzees were able to wait for minutes and long-tailed macaques around 1 min, rhesus monkeys waited for less than 30 s (Evans and Beran 2007a; Pelé et al. 2009). For comparison, on delay choice tasks, the threshold for opting for small immediate rewards is usually less than 1 min and often a matter of much less even in primates, for example, less than 20 s for marmosets and less than 10 s for tamarins (Stevens et al. 2005).
There was considerable individual variation in performance as also reported for other species tested on delay-choice (e.g. Ainslie 1974), reverse contingency (e.g. Anderson et al. 2004) and delay maintenance tasks (e.g. Evans and Beran 2007a). It is unclear whether the variation is due to temperament or other factors such as sex, age or dominance. Status is particularly interesting in relation to self-control; subordinates may be too anxious to delay gratification due to past experiences of losing resources, or they may exercise restraint more easily in the presence of dominant individuals (e.g. Menzel 1974). In these parrots, dominance does not readily explain the pattern of performance across individuals, nor does rearing, as all birds have been raised in the same manner. However, age may go some way to explain Shango’s inability to wait; he gained fewer seeds than the older parrots in all conditions. Longitudinal data would be useful to examine this possibility more fully but given that he showed little improvement across 1 year of testing it seems that age may not be the best predictor of self-control.
In addition to individual differences in seed acquisition, there were other clear behavioural differences in responses (see Electronic Supplementary Materials 1–3 for video clips of each parrot during trials). Shango leaned forward towards the tray, rather than wait upright as the seeds were presented, and he was also very active during the inter-trial interval (often flying away when the experimenter left the room before returning to the perch ready for the next trial). Léo showed no particular behavioural responses during trials. Zoé typically approached and withdrew from the tray during seed placement, indicating her difficulty in overcoming the impulse to take available seeds. Similar strategies were not seen in the two males suggesting that these behaviours correlate with delay tolerance; this could be explored further with the addition of distractor objects during testing, for example (Evans and Beran 2007b).
Social and ecological factors shape competition levels between individuals, and these may result in differences in performance on self-control tasks across species, currently masked by different testing paradigms or individual variation and small sample sizes. For example, it has been suggested that the patience necessary for gum-feeding underlies better performance on a delay of gratification task in common marmosets as compared to cotton top tamarins (Stevens et al. 2005). Recent experimental work also suggests that feeding ecology in closely related primate species can determine willingness to take risks to gain larger rewards. Bonobos rely more heavily on more spatially and temporally consistent food sources than chimpanzees; when presented with a task involving a choice between a fixed and an uncertain option (either larger or smaller than the fixed reward), chimpanzees are far less risk averse and prefer the uncertain reward (Heilbronner et al. 2008). Chimpanzees, bonobos and humans tested on a delay choice task also showed different sensitivities to delays suggesting that species differences are not task specific (Rosati et al. 2007).
Surprisingly little is known about the socio-ecology of wild African grey parrots, but there is no indication that their foraging entails any need to delay gratification. Parrots pair bond and show marked competition in their social interactions, as evidenced by the success of the triadic ‘Model/Rival’ method, in which the parrot ‘competes’ with a human during word acquisition training (Pepperberg 1999; but see N. Giret et al. submitted). In the wild, their large social aggregations suggest that resource competition is an important constraint on fitness; the costs and rewards related to impulsivity and inhibition are likely to be considerable. More evidence using different methodologies is required to better understand the precise form and function of self-control in parrots and other birds.
References
Abeyesinghe SM, Nicol CJ, Hartnell SJ, Wathes CM (2005) Can domestic fowl, Gallus gallus domesticaus, show self-control? Anim Behav 70:1–11
Ainslie GW (1974) Impulse control in pigeons. J Exp Anal Beh 21(3):485–489. doi:10.1901/jeab.1974.21-485
Al Aïn S, Giret N, Grand M, Kreutzer M, Bovet D (2009) The discrimination of discrete and continuous amounts in African grey parrots (Psittacus erithacus) Anim Cogn 12:145–154. doi:10.1007/s10071-008-0178-8
Anderson JR, Awazu S, Fujita K (2004) Squirrel monkeys (Saimiri sciureus) choose smaller food arrays: long-term retention, choice and nonpreferred food, and transposition. J Comp Psych 118:58–64
Beran MJ, Evans TA (2006) Maintenance of delay of gratification by four chimpanzees (Pan troglodytes): the effects of delayed reward visibility, experimenter presence, and extended delay intervals. Behav Proc 73:315–324
Boysen ST, Berntson GG (1995) Responses to quantity: perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes). J Exp Psych: Anim Behav Proc 21:82–86
Clayton NS, Emery NJ (2005) Corvid cognition. Curr Biol 15(23):R946–R950
Dufour V, Pelé M, Sterck EHM, Thierry B (2007) Chimpanzee (Pan troglodytes) anticipation of food return: coping with waiting time in an exchange task. J Comp Psych 121:145–155
Emery NJ (2006) Cognitive ornithology: the evolution of avian intelligence. Phil Trans R Soc 361:23–43. doi:10.1098/rstb.2005.1736
Emery NJ, Seed AM, von Bayern AMP, Clayton NS (2007) Cognitive adaptations of social bonding in birds. Phil Trans R Soc 362:489–505
Evans TA, Beran MJ (2007a) Delay of gratification and delay maintenance by rhesus macaques (Macaca mulatta). J Gen Psych 134:199–216
Evans TA, Beran MJ (2007b) Chimpanzees use self-distraction to cope with impulsivity. Biol Lett 22:599–602. doi:10.1098/rsbl.2007.0399
Gilby IC, Wrangham RW (2007) Risk prone hunting by chimpanzees (Pan troglodytes schweinfurthii) increases during periods of high quality diet. Behav Ecol Sociobiol 61:1771–1779. doi:10.1007/s00265-007-0410-6
Giret N, Miklosi A, Kreutzer M, Bovet D (2009) Use of experimenter given cues in African grey parrots (Psittacus erithacus). Anim Cogn 12:1–10. doi:10.1007/s10071-008-0163-2
Grosch J, Neuringer A (1981) Self-control in pigeons under the Mischel paradigm. J Exp Anal Beh 35:3–21
Güntürkün O (2005) The avian ‘prefrontal cortex’ and cognition. Curr Opin Neurobiol 15:686–693
Hare B (2001) Can competitive paradigms increase the validity of experiments on primate social cognition? Anim Cogn 4:1435–1448. doi:10.1007/s100710100084
Heilbronner SR, Rosati AG, Stevens JR, Hare B, Hauser MD (2008) A fruit in the hand or two in the bush? Divergent risk preferences in chimpanzees and bonobos. Biol Lett 4:246–249. doi:10.1098/rsbl.2008.0081
Izawa E-I, Aoki N, Matsushima T (2005) Neural correlates of the proximity and quantity of anticipated food rewards in the ventral striatum of domestic chicks. Eu J Neurosci 22(6):1502–1512. doi:10.1111/j.1460-9568.2005.04311.x
Jarvis ED, Güntürkün O, Bruce L, Csaillag A, Karten HJ, Kuenzel W, Medina L, Paxinos G, Perke DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, Powers A, Siang C, Smulders TV, Wada K, White SA, Yamamoto K, Yu J, Reiner A, Butler AB (2005) Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci 6:151–159
Kalenscher T, Pennantz CMA (2008) Is a bird in the hand worth two in the future? The neuroeconomics of intertemporal decision-making. Progress Neurobiol 84:284–315
Kralik JD, Hauser MD, Zimilicki R (2002) The relationship between problem solving and inhibitory control: cotton top tamarim (Saguinus Oedipus) performance on a reverse contingency task. J Comp Psych 116:35–50
Logue AW (1988) Research on self-control: an integrating framework. Behav Brain Sci 11:665–709
Menzel EW (1974) A group of young chimpanzees in a one-acre field: leadership and communication. In: Schrier AM, Stollnitz F (eds) Behavior of nonhuman primates. Academic Press, New York, pp 83–153
Mischel W (1974) Processes in delay of gratification. In: Berkowitz L (ed) Advances in experimental social psychology. Academic Press, New York, pp 249–292
Murray EA, Kralik JD, Wise SP (2005) Learning to inhibit prepotent responses: successful performance by rhesus macaques, Macaca mulatta, on the reversed-contingency task. Anim Behav 69:991–998. doi:10.1016/j.anbehav.2004.06.034
Pelé M, Dufour V, Micheletta J, Thierry B (2009) Long-tailed macaques display unexpected waiting abilities in exchange tasks. Anim Cogn. doi:10.1007/s10071-009-0264-6
Pepperberg IM (1999) The Alex studies; cognitive and communicative abilities of grey parrots. Harvard University Press, Cambridge
Pepperberg IM (2006) Grey parrot numerical competence: a review. Anim Cogn 9:377–391
Pepperberg IM, Gordon JD (2005) Number comprehension by a grey parrot (Psittacus erithacus), including a zero-like concept. J Comp Psych 119:197–209
Raby CR, Alexis DM, Dickinson A, Clayton NS (2007) Planning for the future by western scrub-jays. Nature 445:919–921. doi:10.1038/nature05575
Ramseyer A, Pelé M, Dufiour V, Chauvin C, Thierry B (2005) The temporal limits of reciprocity in brown capuchin monkeys. Proc R Soc Lond B 273:179–184
Reiner A (1986) Is prefrontal cortex found only in mammals? Trends Neurosci 9:298–300
Rosati AG, Stevens JR, Hare B, Hauser MD (2007) The evolutionary origins of human patience: temporal preferences in chimpanzees, bonobos, and human adults. Curr Biol 17:1663–1668. doi:10.1016/j.cub.2007.08.033
Silberberg A, Fujita K (1996) Pointing at smaller amounts in an analogue of Boysen and Berntson’s (1995) procedure. J Exp Anal Behav 66:143–147
Sol D, Székely T, Liker A, Lefebvre L (2007) Big-brained birds survive better in nature. Proc R Soc B274:763–769
Stevens JR, Hallinan EV, Hauser MD (2005) The ecology and evolution of patience in two new world monkeys. Biol Lett 1:223–226. doi:10.1098/rsbl.2004.0285
Acknowledgments
This study was funded by a visiting researcher programme at the University of Nanterre and conducted during research leave granted to S-JV by the University of Stirling. We would like to thank all the staff and students at the Laboratoire d’Ethologie et de Cognition Comparées. We also thank our anonymous reviewers for their constructive comments on this manuscript. This study complies with French legislation for animal care and with the Association for the Study of Animal Behaviour guidelines (2009) for the treatment of animals in behavioural research and teaching (doi:10.1016/j.anbehav.2005.10.001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Léo showed no obvious behavioural strategy during trials (WMV 1.53 MB)
Shango’s behaviour indicated that he found waiting difficult; he would learn forward as soon as the tray was presented (WMV 1.08 MB)
During the task, Zoé would approach and withdraw from the seeds while waiting suggesting that there was conflicting motivation due to a prepotent desire to take the seeds (WMV 3.13 MB)
Rights and permissions
About this article
Cite this article
Vick, SJ., Bovet, D. & Anderson, J.R. How do African grey parrots (Psittacus erithacus) perform on a delay of gratification task?. Anim Cogn 13, 351–358 (2010). https://doi.org/10.1007/s10071-009-0284-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-009-0284-2