Abstract
We conducted three studies to examine whether the four great ape species (chimpanzees, bonobos, gorillas, and orangutans) are able to use behavioral experimenter-given cues in an object-choice task. In the subsequent experimental conditions subjects were presented with two eggs, one of which contained food and the other did not. In Study 1 the experimenter examined both eggs by smelling or shaking them, but only made a failed attempt to open (via biting) the egg containing food. In a control condition, the experimenter examined and attempted to open both eggs, but in reverse order to control for stimulus enhancement. The apes significantly preferred the egg that was first examined and then bitten, but had no preference in a baseline condition in which there were no cues. In Study 2, we investigated whether the apes could extend this ability to cues not observed in apes so far (i.e., attempting to pull apart the egg), as well as whether they made this discrimination based on the function of the action the experimenter performed. Subjects significantly preferred eggs presented with this novel cue, but did not prefer eggs presented with a novel but functionally irrelevant action. In Study 3, apes did not interpret human actions as cues to food-location when they already knew that the eggs were empty. Thus, great apes were able to use a variety of experimenter-given cues associated with foraging actions to locate hidden food and thereby were partially sensitive to the general purpose underlying these actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, much research has been done investigating the types of cues various non-human species can use to locate hidden rewards. In some cases, these cues are due to the effect that a reward has on the environment, such as a noise that a container makes when shaken because a reward is inside, or the orientation or shape of the container due to the reward’s presence (e.g., Baillargeon 1995; Call in press). In other cases, these cues are actions performed by experimenters with the intent to inform the receiver about the presence of a reward inside one of two identical containers. The most well-studied of these communicative cues are pointing or head orientation, which are often accompanied by gaze alternation between the subject and the target object to reinforce the communicative nature of the action.
Human children appear to use communicative cues to solve object-choice tasks from an early age (Behne et al. 2005). Even some domesticated species such as puppies, without human contact, can spontaneously use communicative cues like pointing (Hare et al. 2002). In contrast, non-human primates rarely demonstrate such abilities. Multiple species of monkeys seem unable to understand head- or eye-orientation spontaneously, although they can learn to use these kinds of cues correctly after training (Anderson et al. 1995; Itakura and Anderson 1996; Vick and Anderson 2000; Anderson et al. 1996; but see also Vick and Anderson (2003) for positive results in a competitive task). Great apes show more variable performance. Some studies indicate that, despite their ability to follow a human’s gaze (Call et al. 1998), chimpanzees still fail to use human pointing or gazing cues in object-choice paradigms (Call and Tomasello 1994; Tomasello et al. 1997; Call et al. 1998, 2000; Itakura et al. 1999). Other studies, however, indicate that chimpanzees, orangutans, gorillas, and a white-handed gibbon are all successful at using at least some communicative cues like pointing and head and eye orientation to locate hidden food (Barth et al. 2005; Byrnit 2004; Inoue et al. 2004; Itakura and Tanaka 1998; Peignot and Anderson 1999; Povinelli et al. 1999; Miklósi and Soproni 2006). However, it is notable that in most cases when subjects did use these cues successfully, they have already had extensive training wherefrom they might have learnt the cues. Therefore there is currently no evidence that great apes can spontaneously use communicative experimenter-given cues.
Several things, however, might account for apes’ failures in these tasks. Most prominently, communicative experimenter-given cues such as pointing, tapping the container, or gazing are common behaviors for humans, but may not normally be used between conspecifics in other species. For instance, chimpanzees do not manually point to indicate the location of monopolizable food or the presence of predators to other chimpanzees. Yet primates regularly observe their conspecifics’ non-communicative behavior, which in some cases may be used to infer potential food locations. Thus, individuals may be able to use behavioral cues associated with food acquisition and food processing. Unlike communicative cues, pure behavioral cues consist of fully functional behaviors and do not reflect the actor’s intent to communicate specific information to other individuals. Considering an actor’s intent, in most situations observers obtain information from behavioral cues that are not given consciously by the actor, since his goal is to achieve some benefit for himself rather than providing others with information that might lead to their benefit (as it is the case with most communicative cues). In addition, in some cases the information that can be extracted from pure behavioral cues (as foraging actions shown by a conspecific) can exceed the amount of information extracted from communicative cues (as gazing or pointing) by giving information on the nature of the item (food or not) or how to obtain it. Because of this fact and given that behavioral cues are both ubiquitous in non-human life and may be more ecologically valid, apes may find it easier to use such cues compared to communicative cues.
Indeed, there is some recent evidence supporting this idea. Hare and Tomasello (2004) presented chimpanzees with both communicative and behavioral cues in a cooperative and a competitive situation, respectively. Chimpanzees could successfully retrieve the cup with food when it was indicated by a behavioral cue, namely a human or conspecific reaching for the baited cup but not paying attention to the subject. Confirming previous researches, the subjects failed to use a very similar communicative cue, i.e., a human intentionally signaling the baited cup by pointing at it. Although the models were performing very similar arm movements, subjects differentiated these two cues. Bräuer et al. (2006) presented chimpanzees and bonobos with these same cues (as well as others), but subjects failed to locate the hidden food using the two behavioral cues: one cue in which the experimenter attempted to remove the lid from the baited cup, and the reaching cue from Hare and Tomasello (2004). One important difference between the two studies may account for the subjects’ failures in the reaching condition: in Hare and Tomasello’s study the experimenter first established a competitive relationship with the subject before the cue was given; such a relationship was not established in the Bräuer study. This competitive context may have especially motivated the subjects.
Nonhuman primates routinely observe conspecifics eating, and may partially adopt the foraging techniques or eat the same food as others (e.g., King 1994; Rijksen 1978; Whitehead 1986). It is therefore possible that individuals may be able to use behavioral cues involved in foraging to decide which of two alternatives is more likely to yield food. For example, some evidence suggests that rhesus macaques find behavioral cues useful in choosing between edible and non-edible items. Santos et al. (2001) presented monkeys with two novel objects, one of which the experimenter pretended to eat. Subjects preferred the “edible” object over the one the experimenter had performed an irrelevant action on.
Thus, great apes’ and monkeys’ mixed results on the use of communicative experimenter-given cues may contrast with their ability to spontaneously “eavesdrop” and extract relevant information from behavioral cues that others provide (Santos et al. 2001; Hare and Tomasello 2004). The aim of this study was to systematically examine the extent to which great apes can use such behavioral cues. In particular, we investigated their ability to extract information about food location based on the differential food-processing behavior that an experimenter directed toward identical pairs of objects (one of which was baited). After the experimenter had manipulated both objects, subjects were allowed to select one. Except in control tests, the experimenter always directed behaviors toward the baited object that were consistent with the attempt to extract its contents. The types of actions used to indicate this intent varied in their topography and sequence across conditions. We first presented subjects with behaviors that they had previously used during foraging, and later we examined how flexibly they could use behavioral cues by presenting them with uncommon foraging behaviors. Before presenting the subjects with the actual test, we exposed them to the plastic eggs later used in the studies for observing the apes’ typical foraging behavior when they encountered those objects. This allowed us to determine what behaviors may be uncommon to the subjects in this foraging situation.
Study 1: cues belonging to the subjects’ typical repertoire
This study tested whether witnessing an experimenter trying (but failing) to open a plastic egg led great apes to select it over an alternative. We presented the apes different sets of behavioral cues across three conditions. The Familiar behavior condition included very familiar behaviors both for checking and trying to open the egg. In the Novel checking behavior condition we used an uncommon checking action and a familiar opening attempt action. In the Reversed sequence condition we applied the same checking and opening attempt action on each egg but we varied their sequences. For one egg the experimenter first checked and then made an attempt as if to open it, and for the other the experimenter first attempted to open it and then checked it.
Methods
Subjects and housing
Twelve chimpanzees (Pan troglodytes), four bonobos (Pan paniscus), six gorillas (Gorilla gorilla gorilla), and seven orangutans (Pongo pygmaeus abelii) participated in this study. The bonobos ranged in age from 7 to 21 years, the gorillas from 6 to 26 years, the orangutans from 6 to 33 years, and the chimpanzees from 4 to 28 years. The subjects were socially housed in groups of at least five individuals (separated by species) at the Wolfgang Köhler Primate Research Center (WKPRC) in the Leipzig Zoo, Germany. Each species had access to an indoor area (230–430 m2) and an outdoor area (1,680–4,000 m2) furnished with various climbing structures, shelter, and natural vegetation. At night, the apes slept in several series of cages (40–50 m2). In addition to experiments, the animals were provided with a special enrichment program, including different kinds of tools and foraging containers. Several times per day, the apes were fed a diet consisting primarily of vegetables, fruits, and cereals with regular additions of eggs and meat for the chimpanzees.
All individuals living at the WKPRC were tested in all three studies, except for three female chimpanzees (aged 25, 28, and 29 years) that were not comfortable touching the plastic materials used in the experiments. The male gorilla “Gorgo” only participated in the first experimental and the Baseline condition because of motivational reasons. All subjects had participated in numerous cognitive testing (including object choice paradigms, e.g., Bräuer et al. 2006; Call 2006; Hare et al. 2002; Hare and Tomasello 2004). According to keepers’ knowledge and the study archive of the WKPRC, our studies were the first to use plastic eggs. Test sessions took place in one of two familiar test cages that were ∼15 m2. The subjects were used to being separated from their group members and kept in adjacent enclosures for testing. They were not food-deprived for testing, and water was available throughout all testing times. They were not distressed and were free to stop participating at any time.
Materials and setup
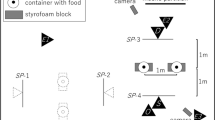
In all experiments the experimenter sat behind a wooden table that was 100 cm wide, 40 cm long, and 50 cm tall. This table was fixed at the animal’s cage in front of a plexiglass panel. Subjects sat behind this panel during the test; three holes (left, middle, and right; ∅ 5 cm) in the panel allowed subjects to reach through the panel and indicate their choices.
A movable platform was attached onto the top of the table. Two small, round plastic containers (2 cm high) were affixed to the left and the right corners of the platform, opposite the experimenter. In each trial, choice objects (plastic eggs) were placed in these containers.
In the present study, we used an object-choice-design in which the subjects chose between two blue plastic eggs (6 × 4 cm) that had been filled by the experimenter before each test session. In every trial, one of the eggs was filled with wood wool and a grape (baited egg), while the other contained wood wool only (distractor egg). There was no visual difference between the two eggs, but the experimenter always knew the contents of each egg.
To make sure that the subject could only choose one side in every trial, a piece of plexiglass (Sliding Plexi, 80 × 10 cm) was attached to the platform on the subject’s side of the table; the Plexiglas was mounted on rails so that it could be moved to the left or right by the subject to indicate its choice. Once the Plexiglas was moved to allow access to one side of the table, it blocked the access to the other. This way the subjects’ choice was explicit. All tests were videotaped.
Procedure
Pretest
Although all 29 subjects had previously experienced studies with object-choice-designs, none of them had been deliberately exposed to plastic eggs before this study. Therefore, each subject completed a two-part pretest before the experiment began. First, seven plastic eggs, each containing one grape, were given to the ape; the eggs were presented one after the other with an 1 min delay in between. Three of the chimpanzees were unwilling to touch the eggs, and consequently were dropped from this study. All other apes did not hesitate to take the eggs and open them in one of the three following ways: in 5.9% of the trials subjects stepped on the egg to break it; in 6.4% of the trials they pushed it open by scrunching both hands together and in most of the trials (87.7%) the subjects bit the egg open.
The second pretest was designed to show the subjects that the egg containers did not always contain food. Every subject was given eight eggs in the way described above, but half of them were filled with a grape and wood wool (to avoid auditory cues), and the other half with wood wool only. The apes opened the eggs in a manner similar to first pretest, biting open most of the eggs (93.1%). As such, it seems that biting is the most typical way for the tested apes to open a small plastic ovaliform container.
Test
At the beginning of each trial, the experimenter occluded the plexiglass window with a solid opaque occluder (100 × 50 cm) to ensure that the subject could not watch him while he placed an egg in each of the two plastic containers. To prevent auditory cues, he always baited the left container first, the right one second. After aligning the Sliding Plexi in the center of the table, the occluder was removed. The subject now had an unrestricted view of the person and the platform. To draw the ape’s attention to the testing setup, the experimenter called the subject by her name and waited until she took a seat in front of the plexiglass window. Once the subject was looking at the experimenter, the experimenter began a specified foraging behavior (see conditions below). According to a randomly determined order he took one egg and performed specific behavioral cues that varied depending on whether the egg contained food, or was just a distractor. The side on which the egg with food was placed and the egg on which the demonstrator first performed the cues were both counterbalanced. During the cue period, the experimenter attended only to the action he was performing, looking either at the egg or at the platform. All his demonstrations were done with his right hand. Once the behavioral cue was completed, the experimenter then placed the egg back in its respective gourd, and began another set of cues with the remaining egg. The experimenter focused on each egg for the same amount of time.
After the second object had been placed back in the gourd, the platform was moved closer to the plexiglass window so that the subject could make her choice. Until she had moved the Sliding Plexi, the experimenter looked at the middle of the platform. The chosen egg was given to the subject through the middle hole in the panel. Once the chosen egg was in the ape’s enclosure the trial was finished.
In every condition each subject was given either one (Baseline) or two sessions (experimental conditions) on different days. Each session consisted of six trials. We administered the four conditions in the following order.
Familiar behavior (smell vs. smell and bite)
Baited egg
The demonstrator took the egg, moved it near his nose and smelled it (three sniffs, duration: 3 s). He then bit onto the egg with his teeth visible (three times, duration: 3 s). This biting action was combined with clear signs of physical effort: a facial expression (constricted eyes caused by exertion of cheek muscles and muscular pulled down eyebrows) and for chimps and bonobos typical auditory signals (short and low pitch palatal sounds). This failed attempt was repeated once. After that he placed this egg back in the round container.
Distractor egg
The demonstrator took the egg filled with wood wool only, moved it near his nose and smelled it (three sniffs, duration: 3 s). This smelling action was repeated twice.
Baseline (control)
This condition tested whether subjects were able to use visual cues or scents to detect the hidden food. Following the completion of the second session of the previous condition, subjects had a 2-min break. Then the experimenter placed the baited and the distractor eggs in the plastic containers as in the previous condition, but gave no behavioral cues. That is, directly following the placement of the eggs, the platform was moved toward the ape so that it could make its choice.
Novel checking behavior (shake vs. shake and bite)
This condition examined how a more novel checking behavior (shaking the egg near one’s ear) that differs from the standard behavior displayed by great apes (smelling the egg) influences their selection between two objects.
Baited egg
The demonstrator took the egg, moved it to his right ear, and shook it focusing all his attention on the object (three shakings, duration: 3 s). He then bit into the egg in the same way as described in the Familiar behavior condition. The only difference in this condition was that there were no auditory signals given by the demonstrator. He also repeated this failed attempt once before he placed this egg back in the gourd.
Distractor egg
The person took the egg, moved it to the right side of his head and shook it (three shakings, duration: 3 s). He repeated this exploratory behavior twice and then put the egg back.
Reversed sequence (bite and smell vs. smell and bite)
This condition investigated whether apes simply chose the egg that was bitten and was therefore enhanced by that additional action. Here both eggs were bitten and smelled although each egg received a different order of actions. Additionally, this condition examined whether the order of the actions had an effect on the apes’ choices.
Baited egg
The demonstrator’s actions were the same as those shown in the Familiar behavior condition (baited egg).
Distractor egg
The demonstrator performed the same actions as with the baited egg, but in reverse order: he took the egg, moved it to his mouth and bit onto it (see also Baited egg) (three times, duration: 3 s). He repeated this failed attempt once, and then moved the object to his nose, smelled it (three sniffs, duration: 3 s), and then placed this egg back in the container.
Data analysis
We videotaped all trials and scored the object selected by the subjects. We did not assess inter-observer reliability because the subjects’ choices could be determined without ambiguity. First, we compared species’ performance using ANOVAs. We used the one-sample t-test to determine whether subjects selected the baited egg above chance levels. In cases where variables violated the criteria of normality (using Kolmogorov—Smirnov test), we used Wilcoxon tests to test the observed values against those expected by chance (p = 0.50). Likewise, binomial tests were used to test whether individual subjects performed above chance levels individually (p = 0.50). These same tests were used to compare the number of subjects that chose correctly in more than half of the trials to the ones that chose correctly in less than half of the trials. The effect of age was assessed with the Pearson correlations.
Results
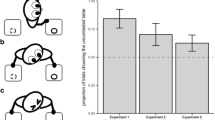
Figure 1 presents the percentage of correct trials for all conditions. Subjects as a group selected the baited eggs above chance in all experimental conditions [Familiar: (56.6% of trials), t 28 = 3.11, p = 0.004; Novel checking: (55.9% of trials), t 27 = 3.60, p = 0.001; Reversed sequence: (56.3% of trials), t 27 = 2.52, p = 0.018]. In contrast, they failed to do so in the Baseline condition (46.5% of trials), T + = 56.0, N = 12 (17 ties), p = 0.20. There was no evidence of improvement across the two sessions for any of the experimental conditions [Wilcoxon tests: Familiar: T + = 93.0, N = 18 (11 ties), p = 0.769; Novel checking: T + = 59.5, N = 14 (15 ties), p = 0.607; Reversed sequence: T + = 71.0, N = 13 (16 ties), p = 0.081].
Mean percentage of trials in which the subjects chose the “egg with food” in each of the conditions tested in Study 1. Numbers within the bars represent the numbers of subjects that chose the correct egg more than half of the trials : half of the trials : less than half of the trials. See text for statistical details (*result significant at 5% level; **result significant at 1% level)
There were significantly more subjects choosing the correct egg for more than half of the trials (seven or more trials correct) compared to the number of subjects that were correct in less than half of the trials (five or less trials correct) in all experimental conditions (Binomial test: Familiar: 15 vs. 4, p = 0.019; Novel checking: 15 vs. 3, p = 0.008; Reverse sequence: 14 vs. 4, p = 0.031). Analysis at the individual level revealed that one (female chimpanzee) and three subjects (one male orangutan, one female gorilla, and one female chimpanzee) performed above chance (Binomial test: p = 0.039) in the Familiar and Reverse sequence conditions, respectively.
There were no significant species differences in any of the conditions (Familiar behavior: F 3,28 = 0.12, p = 0.95; Novel checking behavior: F 3,27 = 0.96, p = 0.43; Reverse sequence: F 3,27 = 0.85, p = 0.48; Baseline: Kruskal–Wallis H 3,29 = 0.475, p = 0.933). No age effects could be found for any of the conditions (Familiar behavior: r 29 = 0.003, p = 0.99; Novel checking behavior: r 28 = 0.036, p = 0.86; Reverse sequence: r 28 = 0.040, p = 0.84; Baseline: r 29 = 0.008, p = 0.97).
Discussion
Apes preferred eggs that the experimenter had attempted to open. Thus, when the apes observed an experimenter smelling one of the eggs and subsequently attempting to bite it open, they chose this egg over another one that the experimenter had only smelled. Moreover, they also selected eggs that the experimenter had smelled first and then bitten over those that were bitten first and then smelled. Finally, apes also continued to show a preference for the baited eggs even when the experimenter used actions that were not used by these apes but that were still consistent with extractive behavior. Finally, when no cues were given in the Baseline condition, subjects did not show any preference, thus ruling out the use of uncontrolled cues provided by the experimenter or the test materials (e.g., smell cues). Note also that there was no evidence of improvement throughout testing.
There are several possible explanations for the apes’ success in the Familiar behavior condition. One possibility is that the subjects could have simply chosen the correct egg in the Familiar and Novel checking behavior conditions because the baited egg received two different types of actions (as opposed to just one performed on the alternative egg). However, the Reverse sequence condition ruled out this possibility because there was the same number of actions directed toward each egg, albeit in reverse order. We can also rule out the possibility that the subjects were responding to the presence of specific actions such as smell or bite. The Novel checking behavior condition did not include smelling and the subjects still continued to prefer the baited egg consistently. Moreover, the Reverse sequence condition included both smell and bite in both alternatives and subjects still chose correctly. Incidentally, success in this condition demonstrated that subjects were sensitive to the order in which the actions were performed, not just their presence or absence.
The source of the observed preferences remains unclear. One possibility is that subjects may have extrapolated from their own experience in the pretest when all subjects had opened plastic eggs. It is also possible that they may have performed (or witnessed other individuals perform) behaviors similar to the ones presented here. Consequently, experience from everyday life could explain these results. Although this explanation may not apply to the Novel checking behavior condition (recall that we did not observe animals shaking the eggs during pretests), we wanted to see if the subjects could consistently perform successfully with novel behavioral cues, which they did not perform during the pretest. Specifically, we tested whether subjects could also understand a different attempt to open the egg besides biting.
Study 2: uncommon behavioral cues
In Study 1, we showed that great apes used behavioral cues (including their sequence) that were familiar to them (behaviors typically displayed when exposed to plastic eggs). Here, we examined how flexible this ability was by presenting subjects with two conditions in which new actions were performed on the egg containing food. Apes witnessed the experimenter trying to open the egg with her fingers, but failing (Failed Attempt condition, Meltzoff 1995). We compared this condition to an Irrelevant action condition in which the experimenter applied a non-foraging action to the egg.
Methods
Subjects
All subjects included in Study 1 participated in this study.
Materials
We used the same materials as in Study 1.
Procedure
We used the same procedure as in Study 1. All subjects experienced the following two conditions in a counterbalanced order across subjects.
Uncommon relevant behavior (smell vs. smell and pull apart)
Baited egg
After the demonstrator had taken the egg, he moved it to his nose and smelled it (three sniffs, duration: 3 s). He then moved the egg in front of his body and grasped it with both hands on opposite sides. He “tried” to pull both parts of the egg apart but failed because his fingers on his left hand slid off the egg. The experimenter, who looked at the object while acting with it, performed this action for 7 s while making the same effortful facial expressions described in the Familiar Behavior condition from Study 1. Afterwards, he placed the egg back in the container.
Distractor egg
The demonstrator took the egg, moved to his nose and smelled it (three sniffs, duration: 3 s). He repeated this exploratory behavior twice before he placed the egg back in the container.
Irrelevant behavior (smell vs. smell and egg on the head)
Baited egg
The demonstrator examined the egg as in the New Behavior condition (sniffing for 3 s). Next, he took the object to his head and twice moved the egg around the crown of his scalp in a circular fashion for a total duration of 7 s. While the person was performing this action, his head was positioned as it was in the previous condition; the only difference was that in this condition he looked straight at the table. Once this action was completed, he placed the egg back in the container.
Distractor egg
The demonstrator used the same actions as in the New Behavior (distractor egg) condition.
Data analysis
We coded and analyzed the data in the same way as in Study 1.
Results
Figure 2 presents the percentage of correct trials for each condition. Subjects selected the baited eggs above chance in the Uncommon relevant behavior condition (57.4% of trials), t 27 = 4.29, p < 0.001. In contrast, the apes significantly preferred the unbaited egg in the Irrelevant behavior condition by choosing the baited egg in 44.7% of trials, T + = 108.0, N = 15 (13 ties), p = 0.004. The subjects’ performance in both conditions differed significantly, Wilcoxon, T + = 225.5, N = 21 (seven ties), p < 0.001. There was no evidence of improvement across the two sessions for the Uncommon, Wilcoxon, T + = 110.0, N = 18 (ten ties), p = 0.296, or the Irrelevant behavior condition, Wilcoxon, T + = 79.0, N = 16 (12 ties), p = 0.605.
Mean percentage of trials in which the subjects chose the “egg with food” in each of the conditions tested in Study 2. Numbers within the bars represent the numbers of subjects that chose the correct egg more than half of the trials : half of the trials : less than half of the trials (**result significant at 1% level; ***result significant at 0.1% level)
There were significantly more subjects choosing the correct egg in more than half of the trials compared to the number of subjects that were correct in less than half of the trials in the Uncommon behavior condition (Binominal test, 16 vs. 1, p < 0.001). The reverse was true for the Irrelevant behavior condition in which most subjects selected the baited egg in less than six trials (Binominal test, 3 vs. 12, p = 0.035). Individual analyses revealed that one female chimpanzee performed above chance in the Uncommon behavior condition (Binomial test: p = 0.039).
There were no significant species differences in the Uncommon behavior, F 3,27 = 0.76, p = 0.53, or the Irrelevant behavior conditions, Kruskal–Wallis H 3,28 = 1.074, p = 0.80. No effect of age could be found in any of the conditions (Uncommon behavior: r 28 = −0.077, p = 0.70, Irrelevant behavior: r 28 = 0.349, p = 0.07).
Discussion
Apes successfully selected the baited egg after witnessing the experimenter trying to open it with a set of actions that they themselves had not used or seen the experimenter use prior to the test, but failed to do so when the experimenter used an irrelevant action on the egg. In fact, they avoided the egg upon which the experimenter had applied an irrelevant action. This means that apes paid close attention to the experimenter’s specific actions (ruling out stimulus enhancement in the process) and that they use them to assess which egg was baited with food.
Taken together, Studies 1 and 2 provide a very consistent picture. Most apes preferred one of the eggs above chance levels, and their preferences appear to have been driven by the experimenters’ actions. Specifically, when the actions were consistent with foraging behavior, the apes preferred those eggs. However, one problem with these results is that the subjects’ preference for the baited egg did not exceed 60% in any of the conditions. In the next study we examined the previous results more in depth, specifically to determine why the apes’ performance, though significant, was not at higher levels.
Study 3: additional controls
The following two experiments were designed to check whether the subjects’ overall performance might increase if the demonstrator did not check the empty egg and then perform an irrelevant action on it. In addition, in the Empty Eggs condition we wanted to investigate whether the apes really understood that the experimenter’s actions were directed to the content of the eggs, not the eggs themselves.
Methods
Subjects
All subjects from Study 2 participated in the current study except for a male orangutan (“Walter”), a male gorilla (“N’Kwango”), and a female gorilla (“Viringika”) because they were not available when testing took place.
Materials
We used the same materials as in Study 1.
Procedure
We used the same procedure as in Study 1 including counterbalancing between and within trials. All subjects experienced the two conditions in the following order.
Empty eggs
This condition tested whether the apes simply reacted to the exploratory action combined with the attempt to open the object, or if they actually understood that there was food inside this particular egg and that the demonstrator had the goal of obtaining this food. This condition was identical to the Familiar behavior condition from Experiment 1 except that the experimenter showed the apes that both eggs were empty before he gave his behavioral cues. To do so he took one egg, opened it with his hands (by pushing it with his thumbs) and showed it to the subject for 2 s. After that he showed the subject the second egg in the same way. After the demonstration was over, the subject could select one of the eggs. If she chose the egg that the experimenter had bitten, the subject was given the chosen egg and rewarded with a grape (within the next second). If the subject chose the other egg, she was simply handed the chosen egg.
Distractor action
This condition addressed issues concerning the frequency with which the subjects chose the Baited egg. Although they preferred the egg that the demonstrator had attempted to open significantly more than the alternative without food, they still chose the distractor in about 40% of the trials. Did they do so because the treatment of the alternative was ambiguous, implying that it could possibly contain food? In this condition we wanted to decrease the food-relevant information the demonstrator gave about the distractor egg. The experimenter smelled and bit the Baited egg as in the Familiar behavior condition of Experiment 1, but performed a new, neutral action on the Distractor egg: he took the egg, moved it to the middle of his chest and, while looking at the platform, twisted it for 9 s. Afterwards he placed it back on the platform.
Data analysis
We coded and analyzed the data in the same way as in Study 1.
Results
Figure 3 presents the percentage of correct trials for each condition. Subjects selected the baited egg above chance in the Distractor condition (57.3% of trials), t 24 = 4.18, p < 0.001, but not in the Empty eggs condition (49.7% of trials), T + = 30.0, N = 10 (16 ties), p = 1.00. There was no evidence of improvement across the two sessions for the Distractor, Wilcoxon, T + = 52.0, N = 13 (13 ties), p = 0.709, or the Empty eggs condition, Wilcoxon, T + = 66.0, N = 14 (12 ties), p = 0.438.
Mean percentage of trials in which the subjects chose the “egg with food” in each of the conditions tested in Study 3. Numbers within the bars represent the numbers of subjects that chose the correct egg more than half of the trials : half of the trials : less than half of the trials (***result significant at 0.1% level)
There were significantly more subjects choosing the correct egg in more than half of the trials compared to the number of subjects that were correct in less than half of the trials in the Distractor condition (Binominal test, 14 vs. 1, p = 0.001) but not in the Empty eggs condition (Binomial test: 4 vs. 6, p = 0.75). There were no significant species differences in the Empty eggs condition, Kruskal–Wallis H 3,26 = 0.480, p = 0.917. In contrast, there were significant differences between species in the neutral (Distractor) behavior condition, F 3,25 = 3.17, p = 0.045. Post hoc analyses (Tukey HSD) indicated that bonobos outperformed orangutans (p = 0.039). No effect could be found for the subjects’ age on the results for any condition (Empty eggs: r 26 = 0.043, p = 0.83; Distractor action: r 26 = −0.178, p = 0.40).
Discussion
When the apes were shown that both options were empty before the experimenter performed any cues, they choose randomly between the two eggs (despite being rewarded for selecting the egg associated with foraging actions). In contrast, they continued to use those cues when one of the eggs was baited and the experimenter performed non-foraging actions on the empty egg. This implies that in previous studies the subjects did in fact infer from the experimenter’s behavior whether there was food inside the eggs, rather than simply preferring one of the cues. As in previous experiments, there was no evidence that subjects learned to respond to the correct alternative during the course of the experiment.
Contrary to our expectations, in this study subjects did not increase their preference for the baited egg compared to previous conditions in Studies 1 and 2, even though only the baited egg received actions such as smelling and biting that are consistent with foraging. A possible explanation for this result is that the apes thought the novel cue was not irrelevant. Such an explanation, though speculative, could also account for the species differences we found in this condition. For example, orangutans may find this particular action as being more indicative of the presence of food than bonobos do, which could be supported by the fact that we have observed orangutans play with food in many situations.
General discussion
Great apes were able to use systematically varied behavioral experimenter-given cues associated with foraging actions to locate hidden food. Study 1 established that subjects’ success was independent from the kind of inspection behavior shown by the human experimenter (smelling and shaking). Furthermore, that study also indicated that subjects were sensitive to the specific sequence of first inspecting the egg’s contents, and then trying to open it: when given a choice between this sequence and a reversed sequence, they preferred the egg that the experimenter first inspected and then tried to bite open. Study 2 extended this ability to opening actions that subjects had not performed themselves in the pretest or any of the later experiments (representing a total of 3,052 trials), thus ruling out the possibility that observing a biting action was necessary to succeed. Indeed, if the experimenter tried to pull the egg apart with both hands (e.g., Meltzoff 1995), apes could also use this cue to infer the food location. Study 3 showed that subjects only used the information provided by the actions when there was a possibility that one of the eggs contained food; when both eggs were known to be empty, they ignored the cues. Thus, actions were meaningful insofar they informed subjects about the location of food. Two conditions, one in Study 1 (Baseline) and one in Study 2 (Egg on the head) ruled out the possibility of inadvertent cuing either by the experimenter or the test materials (e.g., food smell) because subjects failed to select the baited egg above chance. Likewise, we found no evidence that subjects learned to use those cues during the course of any of the experiments. Thus, subjects were able to use a variety of experimenter-given cues associated with foraging to select one of two alternatives.
Subjects may have solved the task by mapping their own experience to the information they observed and then judging what alternative was more likely to contain food. Note, however, that this assessment was not based on the presence or absence of a particular cue. Subjects solved the task after using various actions both for inspection (e.g., smelling or shaking) or access to the egg’s contents (i.e., biting or pulling apart). More importantly, they also succeeded when the same actions were performed on both eggs, albeit in different orders, as well as when they never had performed the behaviors themselves during any of the experiments. Finally, the very same actions that had been effectively used in some conditions were ignored when subjects knew that the eggs were empty.
In addition to mapping the observed actions with their own experience, subjects may have learned to identify those actions displayed by other individuals that were consistently associated with positive outcomes. Again, one problem with this possibility as a primary explanation for our results is that the apes still selected the baited egg over the alternative when presented with novel or uncommon foraging actions that appear to be absent from the apes’ behavioral repertoire when exposed to plastic eggs. Another possibility is that the apes simply preferred novelty. However, this does not explain why both apes and rhesus monkeys (Santos et al. 2001) do not prefer novel actions in a distractor condition (see Study 3). Therefore, subjects must have made some sort of inference regarding the food location when presented with novel or uncommon foraging actions.
The extent to which the apes made such inferences in the current task is therefore an important and outstanding question. Did subjects make their choices based on purely observable behavioral indicators only, no matter how complex? Or did they go beyond such observable information and interpreted the meaning of the cues? For instance, subjects may have inferred the content of the baited egg from the demonstrator’s actions (‘there is food inside that egg’), or they even may have understood that the experimenter was attempting to open the object to get the food inside it (“the experimenter is trying to open that egg because there is food inside that egg”). This mentalistic account, although more interpretive, is supported by the findings of Call et al. (2004) in which chimpanzees attended to a human experimenter’s goals. That is, chimpanzees waited more patiently in front of a human experimenter when he was unable to give them food compared to when he was behaving in a very similar way but was unwilling to give the chimpanzee the food. Second, some of the actions presented to subjects in the current study were novel or uncommon actions, so it is unlikely that the apes had a simple behavioral understanding of the cues. Nevertheless, current research has not determined exactly what great apes understand about the goals of agents.
Our studies also hint that apes perceive sequential series of actions and attribute greater importance to specific sequences. In Study 1, subjects preferred the “inspect then try to open” sequence compared to the “try to open then inspect” sequence. To human subjects, the first sequence indicates the presence of food whereas the second one indicates its absence because this sequence can be interpreted as a rejection. Apes also made this distinction. However, it is currently unclear whether they did so based on their past experience with such sequences (A + B = baited; B + A = empty) without analyzing the causal structure of such sequences, or if they actually made their decision based on such causal information. Some data does suggest that chimpanzees may engage in certain kinds of causal reasoning (e.g., Premack and Premack 1983; Call 2004, 2006), which lends support to the second interpretation. Additionally, subjects did not use similar causal information when it was given about empty eggs, suggesting that they linked the experimenter’s behavior with the food located inside one of the eggs.
These findings highlight an important psychological distinction between attributing meaning to actions, and attributing meaning to communicative actions (e.g., Tomasello 1999), and a closer inspection of the Empty eggs condition is very revealing. In that condition, subjects were shown that both eggs were empty and then the experimenter smelled and bit one egg and only smelled the other egg. If subjects selected the egg bitten by the experimenter, they were rewarded. Subjects failed to select the correct alternative above chance, despite the fact that those same subjects had consistently used identical cues to identify the baited egg in other conditions. Thus, when the behavior became an irrelevant cue, subjects completely disregarded it. However, had they understood the cue to be communicative (“Look, choose this egg and you’ll get food!”), as humans might have, they would have been successful. This converges with other data showing that chimpanzees are more proficient at using pure behavioral cues indicating an intentional act than using communicative cues. In particular, chimpanzees located hidden food if they saw an experimenter reaching for a container in a competitive setting, but did not when the experimenter communicated the location of the food by pointing to the correct container in a cooperative manner (Hare and Tomasello 2004). It is therefore possible that the subjects in the current task also viewed the experimenter as a competitor who was trying to extract the (subjects’) food from one of the two eggs (which then would have resulted in the subjects receiving nothing).
One caveat of our results is that although the great majority of subjects preferred the egg on which the human performed foraging behaviors (but did not show this preference in control conditions without such behavioral cues) their overall levels of preference were relatively low. We tried to boost the subjects’ performance by contrasting actions with and without foraging components. Although the data again revealed consistent differences in the apes’ preference between the two options, general levels of preference remained the same. This makes us cautious regarding the robustness of apes’ knowledge and their explicit understanding about the foraging actions as indicators for the location of food. In particular, certain procedural aspects of the experiment may have affected subjects’ performance. For instance, we may speculate that subjects may have assumed that both eggs were baited (despite never experiencing that situation during the test), or the apes may have had position biases or made alternating choice that contributed to a noisier data set. Indeed, the subjects’ low performance may have been influenced by the fact that the experimenter placed the objects on the table and then proceeded to inspect and attempt to open them. That is, if a second experimenter brought in the eggs and placed them on the table for the first experimenter to inspect, this may have increased the subject’s performance.
In conclusion, great apes were able to use a variety of experimenter-given cues associated with foraging actions to locate hidden food. Since apes successfully understood a variety of cues, including some that they do not use, this suggests that the subjects were partially sensitive to the general purpose underlying these actions. Future research needs to determine what exactly the great apes understand about behavioral experimenter-given cues, specifically what type of inferences they use and to what extent their use of these cues reflects an understanding of others’ goals.
References
Anderson JR, Montant M, Schmitt D (1996) Rhesus monkeys fail to use gaze direction as an experimenter-given cue in an object-choice task. Behav Process 37:47–55
Anderson JR, Sallaberry P, Barbier H (1995) Use of experimenter-given cues during object-choice tasks by capuchin monkeys. Anim Behav 49:201–208
Baillargeon R (1995) A model of physical reasoning in infancy. In: Rovee-Collier C, Lipsitt LP (eds) Adv infancy res, vol 9. Ablex, Norwood, NJ, pp 305–371
Barth J, Reaux JE, Povinelli DJ (2005) Chimpanzees (Pan troglodytes) use of gaze cues in object-choice tasks: different methods yield different results. Anim Cogn 8:84–92
Behne T, Carpenter M, Tomasello M (2005) One-year-olds comprehend the communicative intentions behind gestures in a hiding game. Dev Sci 8:492–499
Bräuer J, Kaminski J, Riedel J, Call J, Tomasello M (2006) Making inferences about the location of hidden food: social dog—causal ape. J Comp Psychol 120:38–47
Byrnit J (2004) Nonenculturated orangutans’ (Pongo pygmaeus) use of experimenter-given manual and facial cues in an object-choice task. J Comp Psychol 118:309–315
Call J (2004) Inferences about the location of food in the great apes (Pan paniscus, Pan troglodytes, Gorilla gorilla, Pongo pygmaeus/). J Comp Psychol 118:232–241
Call J (2006) Descartes’ two errors: reasoning and reflection from a comparative perspective. In: Hurley S, Nudds M (eds) Rational animals. Oxford University Press, Oxford, pp 219–234
Call J (2006) Apes know that hidden objects can affect the orientation of other objects. Cognition. doi:10.1016/j.cognition.2006.08.004
Call J, Agnetta B, Tomasello M (2000) Cues that chimpanzees do and do not use to find hidden objects. Anim Cogn 3:23–34
Call J, Hare BA, Tomasello M (1998) Chimpanzee gaze following in an object-choice task. Anim Cogn 1:89–99
Call J, Hare B, Carpenter M, Tomasello M (2004) ‘Unwilling’ versus ‘unable’: chimpanzees’ understanding of human intentional action. Dev Sci 7:488–498
Call J, Tomasello M (1994) The social learning of tool use by orangutans (P.p.). Hum Evol 9:297–313
Hare B, Brown M, Williamson C, Tomasello M (2002) The domestication of social cognition in dogs. Science 298:1636–1639
Hare BA, Tomasello M (2004) Chimpanzees are more skillful in competitive than in cooperative cognitive tasks. Anim Behav 68:571–581
Inoue Y, Inoue E, Itakura S (2004) Use of experimenter-given directional cues by a young white-handed gibbon (Hylobates lar). Jpn Psychol Res 46:262–267
Itakura S, Agnetta B, Hare B, Tomasello M (1999) Chimpanzee use of human and conspecific social cues to locate hidden food. Dev Sci 2:448–456
Itakura S, Anderson JR (1996) Learning to use experimenter-given cues during an object-choice task by a capuchin monkey. Cah Psychol Cogn 15:103–112
Itakura S, Tanaka M (1998) Use of experimenter-given cues during object-choice tasks by chimpanzees (Pan troglodytes), an orangutan (Pongo pygmaeus), and human infants (Homo sapiens). J Comp Psychol 112:119–126
King BJ (1994) The information continuum: social information transfer in monkeys, apes, and hominids. School of American Research Press, Santa Fe
Meltzoff AN (1995) Understanding the intentions of others: re-enactment of intended acts by 18-month-old children. Dev Psychol 31:1–16
Miklósi A, Soproni K (2006) A comparative analysis of animals’ understanding of the human pointing gesture. Anim Cogn 9:81–93
Peignot P, Anderson J (1999) Use of experimenter-given manual and facial cues by gorillas (Gorilla gorilla) in an object-choice task. J Comp Psychol 113:253–260
Povinelli DJ, Bierschwale DT, Cech CG (1999) Comprehension of seeing as a referential act in young children, but not juvenile chimpanzees. Br J Dev Psychol 17:37–70
Premack D, Premack AJ (1983) The mind of an ape. Norton, New York
Rijksen HD (1978) A field study on Sumatran orang utans (Pongo pygmaeus abelii Lesson 1827): ecology, behaviour and conservation. H.Veenman and Zonen, Wageningen
Santos LR, Hauser MD, Spelke ES (2001) Recognition and categorization of biologically significant objects by rhesus monkeys (Macaca mulatta): the domain of food. Cognition 82:127–155
Tomasello M (1999) Emulation learning and cultural learning. Behav Brain Sci 21:703–704
Tomasello M, Call J, Gluckman A (1997) Comprehension of novel communicative signs by apes and human children. Child Dev 68:1067–1080
Vick S, Anderson JR (2000) Learning and limits of use of eye gaze by capuchin monkeys (Cebus apella) in an object-choice task. J Comp Psychol 114(2):200–207
Vick SJ, Anderson JR (2003) Use of human visual attention cues by olive baboons (Papio anubis) in a competitive task. J Comp Psychol 117:209–216
Whitehead JM (1986) Development of feeding selectivity in mantled howling monkeys, Alouatta palliata. In: Else JG, Lee PC (eds) Primate ontogeny, cognition and social behaviour. Cambridge University Press, Cambridge, pp 105–117
Acknowledgments
We thank three anonymous reviewers for helpful comments. The experiments comply with the current German laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buttelmann, D., Call, J. & Tomasello, M. Behavioral cues that great apes use to forage for hidden food. Anim Cogn 11, 117–128 (2008). https://doi.org/10.1007/s10071-007-0095-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-007-0095-2