Abstract
It has been shown that children and non-human animals seem to integrate geometric and featural information to different extents in order to reorient themselves in environments of different spatial scales. We trained fish (redtail splitfins, Xenotoca eiseni) to reorient to find a corner in a rectangular tank with a distinctive featural cue (a blue wall). Then we tested fish after displacement of the feature on another adjacent wall. In the large enclosure, fish chose the two corners with the feature, and also tended to choose among them the one that maintained the correct arrangement of the featural cue with respect to geometric sense (i.e. left-right position). In contrast, in the small enclosure, fish chose both the two corners with the features and the corner, without any feature, that maintained the correct metric arrangement of the walls with respect to geometric sense. Possible reasons for species differences in the use of geometric and non-geometric information are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When an animal is spatially disoriented in such a way that it cannot keep track of its movements (e.g. when it is moved passively in the absence of orienting sensory cues) in order to reorient itself it should make use of the spatial features of the external environment. These spatial features comprise both geometric and non-geometric aspects. For instance, rats (Cheng 1986) and human children (Hermer and Spelke 1994, 1996) who are disoriented in a rectangular room with a single wall of a contrasting colour (the so-called blue-wall task) use the shape of the room to reorient themselves, but they fail to use the colour of the distinctive wall as landmark information to resolve the 180-degree ambiguity imposed by the room's symmetry to reorient correctly (see Fig. 1A).
Top: On the left, sketch of the geometrical information available in a rectangular-shaped environment. The target (filled dot, corner A) stands in the same geometric relations to the shape of the environment as its rotational equivalent (corner C). Metric information (i.e. distinction between a short and a long wall) together with geometric sense (i.e. distinction between left and right) suffices to distinguish between locations A–C and locations B–D, but not to distinguish between A and C (or between B and D). When featural information is added (e.g. a blue-coloured wall, see figure on the right) then disambiguation between geometrically equivalent corners A and C becomes possible. Bottom: Sketch of the information available at corner A. Animals can rely on either an association between metric properties and sense (short wall on the right and long wall on the left) or an association between featural properties and sense (blue on the right and white on the left)
Other species, in contrast, have been proved able to conjoin geometric (the shape of the room) and non-geometric (the blue wall) information to reorient themselves (i.e. fish: redtail splitfins (Xenotoca eiseni): Sovrano et al. 2002, 2003; goldfish (Carassius auratus): Vargas et al. 2004; birds: chicks: Vallortigara et al. 1990, 2004; pigeons: Kelly et al. 1998; mammals: rhesus monkeys: Gouteux et al. 2001; tamarins: Deipolyi et al. 2001; see reviews by Vallortigara 2004, 2006; Cheng and Newcombe 2005).
The results of some recent studies provided a further complication. Learmonth et al. (2001 and 2002) replicated the original finding of Hermer and Spelke (1994) with five-year-old children, concluding that they fail to conjoin geometric and landmark information in a small room (4 ft×6 ft), but demonstrated that the same children succeeded in a large room (8 ft×12 ft).
Work with non-human animals also point out to a role of the spatial scale of the environment on the ability to integrate geometric and non-geometric information. Fish (redtail splitfins, Xenotoca eiseni) tested in the same task used with children proved able to conjoin geometric and non-geometric information to reorient themselves in both the large and the small space used (Sovrano et al. 2005). Moreover, fish proved able to reorient immediately when dislocated from a large to a small experimental space and vice versa. However, they tended to make relatively more errors based on geometric information when transfer occurred from a small to a large space, and to make relatively more errors based on landmark information when transfer occurred from a large to a small space (Sovrano et al. 2005). One-week-old domestic chicks also seemed to be able to conjoin geometric and non-geometric (landmark) information to reorient themselves in both a large and a small space used (Vallortigara et al. 2005). However, when tested with a transformation (affine transformation) that alters the geometric relations between the target and the shape of the environment, chicks tended to make more errors based on geometric information when tested in the small than in the large space (Vallortigara et al. 2005).
Why should the ability of conjoining geometric and non-geometric information depend on the size of the experimental space? One explanation suggested by various authors is that organisms are prepared to use only distant featural information as landmarks (Wang and Spelke 2002; Spelke 2000, 2003; Spelke and Tsivkin 2001; Hupbach and Nadel 2005; Nadel and Hupbach 2006). However, there is one problem with this view. Several data have provided evidence of a ‘primacy’ of geometric information over non-geometric information (see Vallortigara and Sovrano 2002; Cheng and Newcombe 2005 for reviews). For instance, after training with local landmark information at the corners that would suffice to completely disambiguate the task, a test with complete removal of the local landmarks does not result in random search: animals search on the basis of geometric information (see e.g. Vallortigara et al. 1990; Kelly et al. 1998). This suggests that geometric information is encoded anyway, even when not strictly necessary to solve the task (see Cheng and Newcombe 2005 for a general review of the evidence). Therefore, the basic issue as to the effects of size on spatial reorientation is not to explain why organisms do not use featural information in small spaces (they could do that simply because of the primacy of geometric information), but rather to explain why they do not continue to use geometric information even when tested in large spaces. The latter issue is difficult to understand in terms of the simple hypothesis that animals tend to use as landmarks distal rather than proximal objects. There seems to exist some factors that are specifically associated with a more reduced reliance of geometric information in large spaces.
Sovrano and Vallortigara (2006) have recently put forward a specific hypothesis in this regard. The solution of the blue-wall task actually encompasses the combined use of two sources of information, geometric information provided by the shape of the room (i.e. the arrangements of surfaces as surfaces) and non-geometric landmark information provided by the blue wall. However, geometric information actually comprises two aspects: i.e. metric information and sense. Metric information refers to the ability of the animal to distinguish between a shorter and a longer wall (irrespective of any other non-geometric property associated with the walls’ surfaces, such as colour, brightness, scent and so on). Sense refers in geometry to the ability to distinguish between left and right. The important point to note is that in certain conditions animals might make use of a combination of non-geometric information and sense in order to reorient, without making any use of truly metric properties of the environment.
Consider the situation depicted in Fig. 1B. The correct corner (A) can be distinguished from both its geometric equivalent (C) and its featural equivalent (B) without relying on the use of metric information. It suffices that the animal encodes the information that the correct corner is the corner with a white-blue arrangement (featural information) in which the blue is “on the right” (geometric information). This combination of featural information and sense (without any reference to the metric of the environment) would suffice to disambiguate the problem, because the corner A can now be distinguished easily from both corner C (because corner C lacks any blue colour) and corner B (because in corner B the blue colour, although present, is located in the wrong sense ordering).
Sovrano and Vallortigara (2006) devised a test (Fig. 2) in which such a dissection of sense and metric information was made possible. After training (Fig. 2 left), at test (Fig. 2, right) the blue wall was dislocated from the DA to the AB wall (given that the transformation also implied a change in size of the feature, that was accounted for experimentally by counterbalancing the two types of changes, from a large to a small blue wall and vice versa, see Sovrano and Vallortigara 2006). As a result of the transformation, it would appear impossible for the animal to find a corner that exactly matches featural and geometric information (sense and metric properties) as experienced during initial training (Fig. 2, left). If animals take into consideration both sources of information, geometric and non-geometric, then choices should be expected to be concentrated along corners in the AB wall, because these are the only locations that possess the correct featural information. However, given that geometric information actually comprises two distinct aspects, metric properties and sense, there are two possibilities (or combinations of them). Firstly, if animals rely mainly on metric properties but tend to ignore sense with regard to featural information, then corner A should be preferred. This is because corner A possess the same featural information (the blue colour—even though with the wrong sense because the blue is on the left rather than on the right) and the same metrical arrangement of surfaces as during the initial test (i.e. long wall on the left and short wall on the right). Secondly, if, on the contrary, animals rely mainly on the sense of the feature and tend to ignore metric properties of surfaces, then corner B should be preferred. This is because corner B possess the same featural information (the blue colour) with the same sense properties (i.e. blue on the left) as during the initial test, even though it does not possess the same metrical arrangement of surfaces (i.e. in this case the long wall is on the right and the short one on the left). Results were striking: In the large enclosure chicks chose the corner that maintained the correct arrangement of the featural cue with respect to sense (B in Fig. 2), whereas in the small enclosure they chose the corner that maintained the correct metric arrangement of the walls with respect to sense (A in Fig. 2; see Sovrano and Vallortigara 2006). These findings suggest that there would be a different linkage of sense information with either metric or landmark information depending on the spatial scale of the environment: In small spaces animals link sense with metric properties of surfaces, in large spaces animals link sense with local landmark cues.
However, before any generalization can be drawn it should be considered that the evidence currently available suggests that the relative role of geometric and non-geometric (landmark) information can vary in different species (likely because of differences in ecology and sense organs properties). Fish and birds can provide an interesting case in point. Although in species of both classes it has been demonstrated the capacity to integrate geometric and non-geometric information in the blue-wall task (e.g. chicks: Vallortigara et al. 2004; redtail splitfins: Sovrano et al. 2002), the effects of tests in which geometric and non-geometric cues provided contradictory information produced very different results: chicks, for instance, seemed to be little affected by geometric cues and tended to rely mainly on local landmark information (Vallortigara et al. 1990), whereas redtail splitfins tended to be severely affected by the metric properties of the surfaces of the environment (Sovrano et al. 2003).
Thus, it appeared to be interesting to test fish with the task developed by Sovrano and Vallortigara (2006), with the displacement of the blue wall, in order to verify whether the association between sense and metric versus landmark information would follow different rules in different species.
Method
Subjects
Twenty-six mature male fish (ranging 3–5 cm in length) of the species Xenotoca eiseni were used. Fish came from a stock maintained in our laboratory within vegetation rich (Ceratophillum sp.) large tanks (55–120 l) provided with artificial illumination 16 h per day. Water temperature was maintained between 22 and 25°C and fish were fed dry food twice a day.
Apparatus
The apparatus was the same as described in Sovrano et al. (2005). The large-size apparatus consisted of a rectangular tank (31 cm long, 14 cm wide and 16 cm high) covered with a one-way screen to eliminate extra-tank cues and lit centrally with a 75 W light bulb. Three of the walls of the testing tank were white, whereas one of the smaller walls was blue. The testing tank was inserted in a larger tank (60 cm×36 cm×25 cm) so as to create an annular region (see Fig. 3) with vegetation and food where the test fish was located together with other four female conspecifics (not tested) that provided motivation for social reinstatement (see for evidence Sovrano et al. 1999, 2001). The small-size apparatus was identical in all respect, apart from the size (15 cm×7 cm×16 cm) and was inserted in the same larger tank used for the large-size apparatus. The size used for the large tank was the same used in previously published work (Sovrano et al. 2002, 2003, 2005), in which fish had proved able to conjoin geometric and non-geometric information.
In each of the two tanks, two conditions were devised (see Fig. 5): one in which the blue wall was located along the shorter wall and one in which the blue wall was located along the longer wall. This was done to check for any possible different effect of changing the size of the blue wall from large to small or vice versa from training to test (see below Procedure).
In each corner of the two apparatus was inserted a small tunnel (2.5 cm in length, 2 cm in size, 3 cm in height, located 4.5 cm from the floor of the tank), of white plastic material (Poliplak®), allowing the fish to pass through it to rejoin conspecifics in the annular region in the outer tank (see Fig. 4). At the end of each tunnel was a door (2.5 cm×3 cm), made of a sheet of an opaque plastic material on top and of a transparent very flexible plastic material on the bottom (1 cm×2 cm) that could be easily pushed and bended by the fish with the snout. Only one door could be opened, the others being blocked (hereafter the correct door will be conventionally indicated with A in figures. For the blocked doors the flexible plastic material was completely glued to the outer walls of the tunnel, so that it could not be opened. For the correct door, in contrast, the flexible plastic material was glued to the wall of the tunnel only on the top side so that the plastic material could be easily bent by the animal. Fish thus could open the correct door to rejoin conspecifics by pressing on it with the snout; choices for each door were clearly visible from videorecording, because of characteristic movements of the tail and the most caudal part of the body of the fish that remained visible outside the tunnel (observations reliability among different observers was 100%).
Procedure
Before testing, fish underwent a shaping procedure in their home-tank (30 cm×40 cm×20 cm) for 10 days, using a partition that divided their home-tank in two halves, one (‘enriched’) with food and vegetation and the other (‘unenriched’) without any food and vegetation. Two moveable doors identical to those subsequently used at test were positioned on the partition, allowing the fish to move between the two compartments. In this way fish were accustomed to the use of the moveable doors before testing.
The experiment comprised two parts: training and test. Twelve animals were trained in the large-size apparatus and 14 in the small-size apparatus. To account for any effect of a change from small to large or from large to small blue-wall during test, some animals were trained with the blue wall located along the short wall (large tank: N=6; small tank: N=8) and some with the blue wall located along the long wall (large tank: N=6; small tank: N=6). During training, fish were given three daily sessions of 10 trials. In each trial the fish was brought to the inner tank by gently inserting it into an opaque plastic cylinder (6 cm in diameter; without top and bottom) placed in the centre of the inner tank. After 10 s, the cylinder was removed by lifting it gently, thus leaving the fish in the middle of the test tank. In each trial the number of choices for the four doors was videorecorded, until the fish was able to exit and rejoin conspecifics in the annular region (in each trial, the maximum time allowed to escape was 20 min). Inter-trial interval was 10 min, during which the fish was allowed to remain in the annular region (reinforcement time). After that, the fish was placed in a closed, opaque container and slowly passively rotated on a rotating chair in order to eliminate use of compass and inertial information before being tested again. Every two trials the entire apparatus was rotated 90°.
Number of choices for the four corners, i.e. total number of choices per fish summed over the session of 10 trials were used as individual data, entering analyses of variance (ANOVAs) with size of the apparatus (large versus small) as a between-subject factor, and daily sessions and corners as within-subjects factors (see also Sovrano et al. 2003). (Note that multiple choices for the correct corner A could occur, either because fish explored the door without actually exiting or because not enough strength was exerted; see Sovrano et al. 2003.) A rejection criterion of alpha < 0.05 was used throughout.
In the morning of the day after the last session of training fish were given three further trials to reinstate motivation and then the test started. During the test, the animals were located in the tanks with the same size as those used during training, but with the blue wall displaced (see Fig. 5). During test, fish were given a single session of 10 trials with all four doors blocked. Number of choices during the test session entered an analysis of variance with size and position of the blue wall (from large to small versus from small to large) as a between-subject factor, and corners as a within-subjects factor. A rejection criterion of alpha < 0.05 was used throughout.
Results
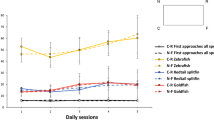
Results for training are shown in Fig. 4a and b. The data have been analyzed by Anova with size (large versus small) and position of the blue wall (along a short versus along a long wall) as between-subjects factors, and corners (A, B, C, D) and sessions (1st, 2nd and 3rd session) as within-subjects factors. Since the Anova (below) did not reveal any significant effects associated with the location of the blue wall along a short or a long wall, the data relative to these two conditions were lumped together in the graphs. The Anova revealed significant main effects of corners (F(3,66)=112.283; p<0.0001), sessions (F(2,44)=7.301; p=0.002) and size (F(1,22)=10.613; p=0.004). There were also significant corners × size (F(3,66)=3.600; p=0.018), sessions × size (F(2,44)=6.695; p=0.003), corners × sessions (F(6,132)=2.252; p=0.042) and corners × sessions × size (F(6,132)=2.378; p=0.033) interactions. There were no other statistically significant effects. As can be seen from the graphs (Fig. 4a and b), fish learned to choose the correct corner (A) very rapidly. In general, fish showed more attempts to exit in the small than in the large tank – likely due to shorter distances among different doors. During the initial sessions, however, there were more errors in fish tested in the small than in the large tank. An analysis limited to errors (i.e. choices for the corners B, C and D) showed that the main effect of corners was not significant (F(2,44)=1.198; p=0.311), whereas the main effect of size was significant (F(1,22)=8.187; p=0.009). An analysis limited to the last session, however, did not reveal any significant effects associated with corners and size (corners F(2,48)=0.470; p=0.628; size F(1,24)=0.510; p=0.482) and the interaction was not significant (F(2,48)=2.994; p=0.060). Overall, these data indicate that errors tended to be higher in the smaller-sized tank, but there was no evidence of any specificity in the types of errors shown by fish (e.g. more geometric than non-geometric errors).
The results for the test are shown in Fig. 5a and b. Data were analysed by Anova with size and position of the blue wall as between-subjects factors, and corners as a within-subjects factor. The Anova revealed a significant main effect of corners (F(3,66)=16.756; p=0.0001) and of corners × size (F(3,66)=3.799; p=0.017) and corners × position × size (F(3,66)=3.631; p=0.017) interactions. There were no other statistically significant effects.
Separate analyses of choices associated with use of featural and/or geometric information (i.e., choices in the A, B and C corners) for the large and the small tank revealed a significant heterogeneity between corners in the large (F(2,20)=13.468; p=0.0001), but not in the small (F(2,24)=3.623; p=0.081) tank. This seems to be largely due to more geometric errors in the small tank. As can be seen from the Fig. 5, fish tested in the large tank concentrated they choices along the two corners marked with the blue wall. In particular, there was no evidence of rotational (i.e. geometric) errors (blue wall along the long wall: A versus D t(5)=0.334; p=0.752; blue wall along the shorter wall: C versus D: t(5)=1.074; p=0.332). Fish tested in the large enclosure chose, however, more the corner that maintained the same sense arrangement of the feature as learned during training (i.e. corner (B)), rather than the corner in which the blue feature did not maintain the same left-right arrangement as learned during training (i.e. corner (C) for fish tested with the blue feature located along the longer wall and corner (A) for fish tested with the blue feature located along the shorter wall: t(11)=2.790; p=0.018). Fish tested in the smaller tank, in contrast, showed clear evidence of rotational errors, though the effect was slightly more pronounced when the blue feature was located along the shorter wall (blue wall along the long wall: A versus D t(7)=2.945; p=0.022; blue wall along the shorter wall: C versus D: t(5)=2.423; p=0.054). On the other hand, there was no evidence that fish tested in the small enclosure chose more the corner that maintained the same sense arrangement of the feature as learned during training (t(13)=1.714; p=0.110) as occurred in fish tested in the large enclosure (above).
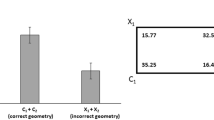
To summarize, fish tested in the large tank chose the corner that maintained the correct arrangement of the featural cue with respect to geometric sense. In contrast, fish tested in the small tank chose almost identically between the two corners with the blue feature, but also chose quite clearly the corner in the geometric position lacking any featural cue.
Discussion
The results of the experiments with fish revealed both basic similarities and some differences with respect to the results obtained with chicks and human infants. As to training, the results confirmed that, similarly to chicks but differently than human infants, there was no evidence of more geometric errors (or of any neglect of featural information) in the small than in the large tank. Fish showed indeed more errors in the smaller tank, but these errors were equally distributed among the corners and there was no evidence of higher level of geometric errors. Why fish should make more errors in the small tank is unclear. The most likely explanation is that exploratory behaviour towards wrong corners is more likely to occur (or more difficult to inhibit) when corners are located at a short distance rather than further away. Note, in fact, that in the small tank there was higher levels of responding in general (i.e. more attempts were done even to the correct corner-door), and this might have also contributed to higher level of errors.
As to the results of test, the most striking effect seems to be that fish tested in the small tank made more geometric errors than fish tested in the large tank. There were interesting differences with respect to data obtained in chicks. In the large enclosure, chicks chose the corner that maintained the correct arrangement of the featural cue with respect to sense, whereas in the small enclosure they chose the corner that maintained the correct metric arrangement of the walls with respect to sense (Sovrano and Vallortigara 2006). Fish tested in the large tank also chose the corner that maintained the correct arrangement of the featural cue with respect to sense. However, in the small enclosure fish did not limit themselves to choosing the corner that maintained the correct metric arrangement of the walls with respect to sense between the two corners with the blue feature, but also chose quite clearly the corner in the geometric position lacking any featural cue.
All this seems to suggest that, although the general hypothesis put forward by Sovrano and Vallortigara (2006) appears to be correct, i.e. that in small spaces animals tend to link sense with metric properties of surfaces and in large spaces animals tend to link sense with local landmark cues, there seem to be species differences in the reliance of using prevalently geometric or landmark information. Basically, it seems that for redtail splitfins geometric information is relatively more important than featural information than it is for chicks. (A result that confirms previous findings based on tests in which geometric and landmark cues provided contrasting information, e.g. Vallortigara et al. 1990 for chicks and Sovrano et al. 2003 for redtail splitfins; see also Introduction.) Perhaps such a difference could be expected considering that birds are highly visual animals with considerable spatial resolution capabilities; fish, in contrast, because of adaptation to an aquatic environment, show comparatively reduced spatial resolution.
Recent evidence has revealed similar species differences in birds. Differently than domestic chicks and pigeons, wild-caught mountain chickadees (Poecile gambeli) do not spontaneously encode the geometry of an enclosure when salient features are present near the goal; moreover, when trained without salient features they encode geometric information but this encoding is overshadowed by features (Gray et al. 2005). It is unclear at present why these differences may occur. One explanation could be that wild-caught birds have little experience with small enclosures, thus leading to reliance on featural over geometric information. Somewhat the reverse could be true for small fish that live in shallow, transparent water with pebbles and rich vegetation such as redtail splitfins (see Meyer et al. 1985; and see also Burt de Perera 2004 for evidence of use of geometric information in a species of blind fish that obviously cannot make any use of visual featural information).
It cannot be excluded that differences in cognitive abilities such as those involved in spatial tasks can be observed even in individuals of the same species, as a function of ecological variables such as predatory pressures (e.g. Brown and Braithwaite 2005).
References
Brown C, Braithwaite VA (2005) Effects of predation pressure on the cognitive ability of the poeciliid Brachyraphis episcope. Behav Ecol 16:482–487
Burt de Perera T (2004) Spatial parameters encoded in the spatial map of the blind Mexican cave fish, Astyanax fasciatus. Anim Behav 68:291–295
Cheng K (1986) A purely geometric module in the rat's spatial representation. Cognition 23:149–178
Cheng K, Newcombe NS (2005) Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon Bull Rev 12:1–23
Deipolyi A, Santos L, Hauser MD (2001) The role of landmarks in cotton-top tamarin spatial foraging: evidence for geometric and non-geometric features. Anim Cogn 4:99–108
Gouteux S, Thinus-Blanc C, Vauclair J (2001) Rhesus monkeys use geometric and nongeometric information during a reorientation task. J Exp Psychol: Gen 130:505–519
Gray ER, Bloomfield LL, Ferrey A, Spetch ML, Sturdy CB (2005) Spatial encoding in mountain chickadees: features overshadow geometry. Biol Lett 1:314–317
Hermer L, Spelke ES (1994) A geometric process for spatial reorientation in young children. Nature 370:57–59
Hermer L, Spelke ES (1996) Modularity and development: the case of spatial reorientation. Cognition 61:195–232
Hupbach A, Nadel L (2005) Reorientation in a rhombic environment: no evidence for an encapsulated geometric module. Cogn Dev 20:279–302
Kelly DM, Spetch ML, Heth CD (1998) Pigeons (Columba livia) encoding of geometric and featural properties of a spatial environment. J Comp Psychol 112:259–269
Learmonth AE, Nadel L, Newcombe NS (2002) Children's use of landmarks: implication for modularity theory. Psychol Sci 13:337–341
Learmonth AE, Newcombe NS, Huttenlocher J (2001) Toddlers’ use of metric information and landmarks to reorient. J Exp Child Psychol 80:225–244
Meyer MK, Wischnath L, Foerster W (1985) Lebendgeba¨rende Zierfishe: Arten der Welt. Mergus Verlag, Melle
Nadel L, Hupbach A (2006) Cross-species comparisons in development: the case of the spatial “module”. In: Johnson MH, Munakata Y (eds) Attention and Performance XXI. Oxford University Press, Oxford, pp 499–511
Sovrano VA, Vallortigara G (2006) Dissecting the geometric module: a sense-linkage for metric and landmark information in animals’ spatial reorientation. Psych Sci 17
Sovrano VA, Bisazza A, Vallortigara G (2001) Lateralization of response to social stimuli in fishes: a comparison between different methods and species. Physiol Behav 74:237–244
Sovrano VA, Bisazza A, Vallortigara G (2002) Modularity and spatial reorientation in a simple mind: encoding of geometric and nongeometric properties of a spatial environment by fish. Cognition 85:B51–B59
Sovrano VA, Bisazza A, Vallortigara G (2003) Modularity as a fish views it: conjoining geometric and nongeometric information for spatial reorientation. J Exp Psychol: Anim Behav Process 29:199–210
Sovrano VA, Bisazza A, Vallortigara G (2005) Animals’ use of landmarks and metric information to reorient: effects of the size of the experimental space. Cognition 97:121–133
Sovrano VA, Rainoldi C, Bisazza A, Vallortigara G (1999) Roots of brain specializations: preferential left-eye use during mirror-image inspection in six species of teleost fish. Behav Brain Res 106:175–180
Spelke ES (2000) Core knowledge. Am Psychol 55:1233–1243
Spelke ES (2003) What makes us smart. Core knowledge and natural language. In: Gentner D, Goldin-Meadow S (eds) Language in mind. Advances in the study of language and thought. MIT Press, Cambridge, MA, pp 277–311
Spelke ES, Tsivkin S (2001) Initial knowledge and conceptual change: space and number. In: Bowerman M, Levinson S (eds) Language acquisition and conceptual development. Cambridge University Press, Cambridge, UK
Vallortigara G (2004) Visual cognition and representation in birds and primates. In: Rogers LJ, Kaplan G (eds) Vertebrate comparative cognition: are primates superior to non-primates? Kluwer Academic/Plenum Publishers, Boston, New York, pp 57–94
Vallortigara G (2006) The cognitive chicken: visual and spatial cognition in a non-mammalian brain. In: Wasserman EA, Zentall TR (eds) comparative cognition: experimental explorations of animal intelligence. Oxford University Press, Oxford, UK, pp 41–58
Vallortigara G, Sovrano VA (2002) Conjoining information from different modules: a comparative perspective. Behav Brain Sci 25:701–702
Vallortigara G, Feruglio M, Sovrano VA (2005) Reorientation by geometric and landmark information in environments of different size. Dev Sci 8:393–401
Vallortigara G, Pagni P, Sovrano VA (2004) Separate geometric and non-geometric modules for spatial reorientation: evidence from a lopsided animal brain. J Cogn Neurosci 16:390–400
Vallortigara G, Zanforlin M, Pasti G (1990) Geometric modules in animal's spatial representation: a test with chicks. J Comp Psychol 104:248–254
Vargas JP, Lopez JC, Salas C, Thinus-Blanc C (2004) Encoding of geometric and featural spatial information by Goldfish (Carassius auratus). J Comp Psychol 118:206–216
Wang RF, Spelke ES (2002) Human spatial representation: insights from animals. Trends Cogn Sci 6:376–382
Acknowledgements
We thank Rosa Damiani for help with training of the animals. G.V was supported by grants MIUR Cofin 2004, 2004070353_002 “Intel-lat” and MIPAF “Ben-o-lat”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sovrano, V.A., Bisazza, A. & Vallortigara, G. How fish do geometry in large and in small spaces. Anim Cogn 10, 47–54 (2007). https://doi.org/10.1007/s10071-006-0029-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-006-0029-4