Abstract
A number of animal species have evolved the cognitive ability to detect when they are being watched by other individuals. Precisely what kind of information they use to make this determination is unknown. There is particular controversy in the case of the great apes because different studies report conflicting results. In experiment 1, we presented chimpanzees, orangutans, and bonobos with a situation in which they had to request food from a human observer who was in one of various attentional states. She either stared at the ape, faced the ape with her eyes closed, sat with her back towards the ape, or left the room. In experiment 2, we systematically crossed the observer’s body and face orientation so that the observer could have her body and/or face oriented either towards or away from the subject. Results indicated that apes produced more behaviors when they were being watched. They did this not only on the basis of whether they could see the experimenter as a whole, but they were sensitive to her body and face orientation separately. These results suggest that body and face orientation encode two different types of information. Whereas face orientation encodes the observer’s perceptual access, body orientation encodes the observer’s disposition to transfer food. In contrast to the results on body and face orientation, only two of the tested subjects responded to the state of the observer’s eyes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies have shown that at least two different species of bird behave differently when caching food items as a function of whether a conspecific is or is not watching the hiding process, and further that this differential hiding has direct benefits for the probability that the hider will be able to retrieve the hidden food later (Bugnyar and Kotrschal 2001; Emery and Clayton 2001). However, in both of these studies and others (e.g. Coussi-Korbel 1994; Leavens et al. 1996), individuals may simply have used the physical presence/absence of a conspecific or a human in the individual’s perceptual field rather than an appreciation of others’ visual access to determine their foraging actions. A recent study with domestic dogs, however, has demonstrated that they are not only sensitive to whether another individual (in this case human) is or is not present as they engage in a forbidden behavior, but they are also sensitive to whether that individual is or is not facing them, and even whether that individual’s eyes are open or closed (Call et al. 2003).
Nonhuman primates, specifically apes, live in complex social groups. It is known from different studies that they use many different gestures to communicate with groupmates and that these gestures are used more or less flexibly (Goodall 1986; Maestripieri 1996, 1997; Tomasello 1997). From an evolutionary perspective it is very important for individuals to show some kind of sensitivity to social context and to be able to adjust these gestural signals to the attentional state of the recipient (Pika et al. 2003). Experiments designed to address the questions of nonhuman primates’ understanding of the attentional state of others have produced mixed results. Povinelli and Eddy (1996) presented juvenile chimpanzees with a choice. They could use a begging gesture to request food from one of two human experimenters who were simultaneously present but oriented to them in different ways. For example, one experimenter might be facing toward the chimpanzee and the other facing away, or one might be wearing a blindfold over the eyes and the other over the mouth (with both bodily oriented towards the subject). In a series of 15 experiments (some replicated with the same subjects at older ages by Reaux et al. 1999), Povinelli and Eddy (1996) determined that in this situation chimpanzees were sensitive not to the orientation of face or eyes but rather to the orientation of the body—so that, for instance, they gestured to a human facing them bodily but with her head turned away in preference to a human facing away from them bodily but with her head turned so that she could see them over her shoulder. Some authors have interpreted these findings as evidence that chimpanzees do not know whether others are or are not looking at them, but they are merely sensitive to front and back body orientation (Povinelli and Eddy 1996).
This interpretation is difficult to reconcile, however, with other recent findings demonstrating that chimpanzees know such things as whether a conspecific competitor’s vision is being blocked by a barrier (Hare et al. 2000, 2001). Moreover, previous research has also found some evidence of sensitivity to the eyes in food-begging situations in one enculturated orangutan and three enculturated chimpanzees (Call and Tomasello 1994; Gómez 1996). It is thus possible that the Povinelli and Eddy’s experimental paradigm is not a good test of chimpanzees’ abilities. Most importantly, choosing which of two individuals to beg from is a very complex task, and indeed chimpanzees needed to be trained over hundreds of trials to participate in this experiment meaningfully.
The aim of the current study was to investigate apes’ sensitivity to the attentional states of humans. We used an experimental paradigm previously used with two orangutans (Call and Tomasello 1994) to administer some of the conditions used by Povinelli and Eddy (1996). There were two crucial differences between our design and that of Povinelli and Eddy. First, there was only one human present from whom individuals could beg. The experimental manipulation thus took place across trials, as the human was either facing the subject, had her back turned, had her eyes closed, and so forth. Second, our procedure did not involve training over hundreds of trials but capitalized on apes’ natural tendency to beg for food that is beyond their reach. Thus, we used the number of communicative behaviors subjects actively directed toward the human to determine whether they distinguished between the various human attention states.
Experiment 1
In this study we replicated the procedure that we had used previously with two orangutans (Call and Tomasello 1994). We presented subjects with a choice between two glasses of fruit juice and scored their spontaneous gestures while the experimenter engaged in one of the following four conditions: facing the subject with eyes open, facing the subject with eyes closed, facing away from the subject, and absent (i.e., she left the room).
Methods
Subjects
Seven chimpanzees (Pan troglodytes), three bonobos (Pan paniscus) and six orangutans (Pongo pygmaeus) participated in this experiment (see Table 1). There were nine females and seven males ranging in age from 4 to 30 years. Six apes were nursery reared whereas all other subjects were mother reared. All subjects were housed at the Wolfgang Köhler Primate Research Center in the Leipzig Zoo (Germany) where they lived with conspecifics in social groups of various sizes, with access to indoor and outdoor areas. Subjects were tested in their indoor cages and were fed according to their normal daily routine, that is, four times a day on a diet of fruit, vegetables, cereals and monkey chow. Water was available ad libitum, and subjects were not deprived of food or water during testing. Subjects had previously participated or were concurrently participating in other studies so they were all used to participate in tests (see Table 1 for further details).
Materials
Two identical transparent plastic glasses (7×9 cm) with different amounts of fruit juice (orange or grape juice) were placed on a wooden platform (84 cm×32 cm) that was located outside the subjects’ enclosure behind a Plexiglas panel. At the bottom of the Plexiglas panel were three holes forming a straight line separated by 25 cm from center to center.
Procedure
Subjects received no special training for this experiment since all subjects had learned prior to this experiment to poke through one of the holes of the Plexiglas panel to request the container located in front of the hole (filled with either juice or food depending on the experiment). The experimenter (E) sat behind the platform facing the subject. She placed the filled glasses on the platform behind the two extreme holes of the Plexiglas panel. Two types of trials were presented alternately: filler trials and experimental trials. In filler trials the experimenter placed the glasses on the platform and offered the glass to the subject as soon as she requested it by poking through one of the extreme holes in the Plexiglas panel. In experimental trials the experimenter placed the glasses on the platform and engaged in one of the following four experimental conditions:
-
1.
Eyes open: E sat facing the subject with her eyes fully opened but without reacting to the subject’s behavior.
-
2.
Eyes closed: E sat facing the food with her eyes closed. Her head orientation and posture were identical to those in the eyes open condition.
-
3.
Back turned: E sat facing away from the subject with her back turned to the food
-
4.
Out: E left the room.
After 30 s had elapsed the filler trial started, and the experimenter became responsive again, offered the subject the glass that she requested at that precise moment, and started preparing for the next experimental trial. Each session consisted of a total of eight trials comprising alternating filler and experimental trials. Thus, each experimental condition was presented once per session with conditions being randomized. Every subject participated in a total of 12 sessions with only one session per day.
Data scoring and analysis
All experimental trials were videotaped and later coded by the experimenter. We measured the total behavioral output for each subject. Behavioral output was composed of the following five behavioral categories: poking, knocking, spitting, lip begging, and giving.
-
1.
Poking consisted of inserting the fingers through one of the Plexiglas holes so that parts of the finger were visible on the experimenter’s side.
-
2.
Knocking consisted of hitting the Plexiglas with any body part (usually the hand) so that it made a noise.
-
3.
Spitting consisted of projecting saliva from the mouth to the Plexiglas or executing the spitting behavior (and its associated noise) without successfully ejecting saliva.
-
4.
Lip begging consisted of presenting the lower lip through one of the panel holes.
-
5.
Giving consisted of pushing an item initially located in the enclosure (e.g., orange peel) through the Plexiglas holes.
We combined the frequency of each of these behavioral categories to obtain the total behavioral output for each subject across conditions and used this overall score in our analyses. We ignored other behaviors such as vocalizations, hand clapping and pushing due to their low frequency, which means only one or two subjects of one species showed the behavior.
A second coder who was unaware of the experimental conditions blindly coded 20% of the trials for reliability purposes. Reliability was excellent (Spearman r=0.90, P<0.001, n=212). We used an ANOVA to investigate the effect of various independent variables on the total behavioral output.
Results
Figure 1 shows the mean number of behaviors that subjects produced across trials for each of the different conditions. An ANOVA with the factors condition, genus (Pan vs Pongo) and trial block (first vs second six trials) revealed a significant effect of trial block, F (1,14)=14.71, P=0.002. Subjects produced significantly fewer behaviors in the second block of trials compared to the first block. However, this decrease affected all conditions equally since there was no interaction effect between trial block and condition, F (3,42)=0.45, P=0.71.
There was also a significant effect of condition, F (3,42)=30.84, P<0.001 (see Fig. 2). A post hoc analysis (paired t-test) across conditions indicated that subjects produced significantly more behaviors in the eyes open (control) condition than in the back turned, t(15)=7.04, P<0.001, and out conditions, t(15)=6.22, P<0.001. In contrast, there were no differences between the eyes open and eyes closed conditions, t(15)=0.16, P=0.87 or any other pairwise comparison.
We restricted this analysis to the first two trials (see Fig. 1) to have the lowest number of trials possible to avoid learning and to obtain data that statistics could be applied to. Restricting this analysis to the first two trials produced identical results. Subjects produced significantly more behaviors in the eyes open (control) condition than in the back turned, t(15)=3.10, P=0.007, and out conditions, t(15)=4.06, P=0.001. In contrast, there were no differences between the eyes open and eyes closed conditions, t(15)=1.10, P=0.29 or any other pairwise comparison.
Although there were no group differences between the eyes open and eyes closed conditions, there were two individuals who provided some evidence of perceiving this distinction for particular behaviors. A juvenile orangutan (Padana) spat significantly more often in the eyes closed than in the eyes open condition (Wilcoxon T=47.5, n=10; P<0.05), and an adult orangutan (Dunja) lip-begged longer in the eyes open than the eyes closed condition (Wilcoxon T=1.5, n=10; P<0.01)—indicating at least some understanding that the eyes in particular are the source for other individual’s visual perception of the world.
Discussion
This study had three major results. First, apes were highly sensitive to front–back body orientation of the experimenter and gestured preferentially when the experimenter was facing them. These findings corroborate previous studies that have shown that chimpanzees and orangutans use visual gestures toward partners who are facing them (Call and Tomasello 1994; Tomasello et al. 1994; Povinelli and Eddy 1996; Hostetter et al. 2001) but use more vocalizations to partners that are not facing them (Hostetter et al. 2001).
Second, subjects significantly reduced their behavioral output when the experimenter left the room. This is important because it shows that the behaviors used by subjects were communicative in nature, not simply an attempt to get the food without the experimenter’s help or a sign of frustration for not receiving the food without delay. Other studies have also found this kind of audience effect (e.g., Call and Tomasello 1994; Leavens et al. 1996; Call et al. 2004).
Third, subjects as a group showed little sensitivity to whether the eyes were open or closed, thus confirming the results of previous studies (Hare et al. unpublished data; Povinelli and Eddy 1996). Nevertheless there were two orangutans that showed sensitivity to the state of the eyes. This finding parallels other studies showing that some enculturated apes also responded differentially when the eyes were open as opposed to closed (Call and Tomasello 1994; Gómez 1996).
In sum, apes responded preferentially to a human facing them regardless of whether her eyes were open or closed and reduced their number of gestures when the experimenter left the room. In the next experiment we investigated in greater detail which aspects of the frontal orientation triggered apes’ gestures.
Experiment 2
In this experiment we systematically manipulated the body orientation and face orientation of the experimenter, who could adopt either a frontal or back body orientation while she could either turn her face toward or away from the subject. This generated four possible conditions that allowed us to investigate the independent effect of the experimenter’s body and face orientation in the apes’ production of gestures.
Methods
Subjects
Subjects included all apes that had participated in experiment 1 except for Kuno.
Materials
The experimental apparatus was the same as that used in experiment 1.
Procedure
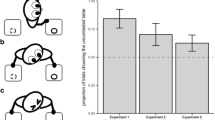
We began this experiment right after completing experiment 1. We followed the general procedure of experiment 1 with some modifications. The main change concerned the experimental conditions since in the current experiment we systematically manipulated the body orientation and face orientation of the experimenter to produce the following four experimental conditions (see Fig. 4):
-
1.
Front–face: E sat with her body and her face oriented toward the subject so that she was able to see the subject (same as eyes open condition in experiment 1).
-
2.
Front–no face: E sat with her body facing the subject and her head turned away from the subject so that she was unable to see the subject.
-
3.
Back–face: E sat with her body turned away from the subject and her face oriented toward the subject so that she was able to see the subject.
-
4.
Back–no face: E sat with her body and face turned away from the subject so that she was unable to see the subject (same as back turned condition in experiment 1).
After 30 s had elapsed the experimenter became responsive again and offered the subject the glass that she requested at that precise moment. To keep subjects motivated the number of filler trials between experimental trials varied between one and two throughout the session. As before there were four experimental trials per session, one for each experimental condition with conditions randomized. Each subject performed a total of ten sessions. Chimpanzees and orangutans performed one session per day, bonobos two sessions per day.
Data scoring and analysis
We coded and analyzed the data in the same way as in experiment 1. A second naive coder unaware of the experimental conditions blindly coded 20% of the trials for reliability purposes. Reliability was excellent (Spearman r=0.81, P<0.001, n=156).
Results
Figure 3 shows the mean number of behaviors that subjects produced across trials for each of the different conditions. An ANOVA with the factors body orientation (front vs back), face orientation (straight vs turned), genus (Pan vs Pongo) and trial block (first vs second five trials) revealed a significant effect of body, F (1,13)=71.10, P<0.001; face, F (1,13)=28.79, P<0.001; and a body × face interaction, F (1,13)=14.01, P=0.002. No other factors including genus and trial block or their interactions had a significant effect.
A post hoc analysis (paired t-test) revealed that when the experimenter’s body was oriented toward the subject, apes produced significantly more behaviors in the straight compared to the turned-face condition, t(14)=5.55, P<0.001 (see Fig. 4). In contrast, when the experimenter had her back turned toward the subject, apes did not significantly produce more behaviors depending on the face orientation, t(14)=1.13, P=0.28. Restricting this analysis to the first two trials (see Fig. 3) produced identical results. Subjects distinguished between the straight and turned-face condition when the experimenter’s body was oriented toward them, t(14)=2.16, P=0.049, but not when the experimenter had her back turned, t(14)=0.34, P=0.74.
Discussion
Results showed that apes were sensitive to the orientation of the body, thus replicating previous findings including those of experiment 1. More importantly, subjects were also sensitive to the orientation of the face. However, this sensitivity was only expressed when the experimenter’s body was oriented toward the subject, in which case subjects responded more when the experimenter had her face oriented toward (as opposed to away from) the subject. When the experimenter had her back turned, subjects responded the same regardless of whether the experimenter had her face turned away or oriented toward them.
Although Povinelli and Eddy (1996, experiment 13) also found that chimpanzees responded differentially to the face, they did so after subjects had extensive experience with their testing paradigm. In fact, Povinelli and Eddy (1996) argued that it was likely that their chimpanzees had simply learned to use the face as a discriminative cue without much understanding of visual perception in others. Initially, the chimpanzees they tested showed no preference toward someone with their face oriented toward them (Povinelli and Eddy 1996, experiment 3). In contrast, apes in the current study responded to the face from the beginning of testing without preparatory training and without differential reinforcement. Recall that all subjects were rewarded regardless of how much they gestured at the end of each trial.
General discussion
Apes distinguished the front–back body orientation and face orientation when begging for food from humans. Unlike other studies, apes showed this sensitivity from the beginning, that is, without an extensive training regime and without differential reinforcement. This may be because our experimental design used one rather than two experimenters.
One of the most interesting findings in this study was the sensitivity to face orientation. However, it is notable that differential responding to face orientation only occurred when the experimenter’s body was oriented toward the subject. If the apes are sensitive to face orientation one must presume that the same sensitivity should apply when the experimenter’s body is oriented away from the subject. One possible interpretation is that apes respond primarily to body orientation and secondarily to face orientation—with little sensitivity for the state of the eyes. This interpretation is univariate and hierarchical. It is univariate because body and face orientation carry information about a single dimension—in this case perceptual access. Moreover, it is hierarchical because body orientation carries more weight (it is richer) than face orientation. Thus, the main difference between body and face is that the former carries more weight than the latter. However, the results of experiment 2 do not fully support this interpretation. In particular, if the univariate interpretation was correct, the back–face and back–no face conditions should be different, but they were not.
An alternative explanation for our results is a bivariate and hierarchical interpretation in which body orientation and face orientation convey two different types of information. Whereas the experimenter’s body orientation may indicate her disposition and/or ability to give food at all, her face orientation may indicate the ability to perceive a communicative signal. This would explain why subjects only distinguished between face and no face when the experimenter was facing them, because it was only then that she had a disposition to transfer food. When she had no such disposition (i.e., her back was turned), subjects did not distinguish between the condition with the face oriented towards or away from them. Like the univariate interpretation, this bivariate account is also hierarchical because the effect of face orientation is subordinate to body orientation. However, unlike the previous interpretation, body and face orientation carry information about two different dimensions.
Previous studies involving begging from a human have failed to distinguish these two different dimensions (i.e., ability and perceivability), but have mostly concentrated on the perceivability aspect—a common feature for many social cognition studies as well. Thus, sensitivity to the body’s frontal orientation (but not the eyes) has been interpreted as a lack of sophistication in understanding visual perspective. However, apes may have simply perceived a human with a frontal orientation as more predisposed to give food than someone with her back turned to them. Moreover, some of the studies that have reported sensitivity to body orientation were not designed to tease apart the separate contribution of the body and the face (e.g., Call and Tomasello 1994; Tomasello et al. 1994; Hostetter et al. 2001). It is therefore conceivable that subjects were spontaneously responding not just to body orientation but also to the orientation of the face, as the current study has shown.
Another finding was the apes’ minimal sensitivity to the state of the eyes. From a human point of view, such a lack of sensitivity to the eyes in communicative situations is puzzling, especially given the fact that apes use others’ face/head and body orientation very effectively. However, perceiving the eyes may not be so important for apes, perhaps because apes may not be predisposed to focus primarily on their conspecifics’ eyes. Unlike human eyes, ape eyes do not have a white sclera that makes them highly visible (Kobayashi and Koshima 2001). Therefore, apes may focus on other features that are more easily perceivable such as the presence of the face or head direction rather the state of the eyes or their direction because they are difficult to see, especially from a distance. If detecting the eyes reliably represents a large effort, subjects may opt for focusing on stimuli that are easier to detect (e.g., face/head) even though they may be less reliable. Focusing on those more gross features such as the face (as opposed to the eyes) represents a conservative and safe strategy for subordinates who, for instance, will not attempt to obtain food or mate when they can perceive the dominants’ face, regardless of the state of the eyes.
Povinelli and Eddy (1996, see also Theall and Povinelli 1999) and other interpreters of their work claim that apes understand basically nothing about the visual perception of others; they simply learn discriminative cues. Recent work in competitive experimental paradigms (Hare et al. 2000, 2001) calls this conclusion into question, and the current study does as well from within the context of a communicative paradigm very similar—but with a few crucial differences—to that of Povinelli and Eddy (1996). Here, in a communicative context not requiring training or a complex choice of communicative partner, we found that apes without special training know that another individual can see them when her face—and for a minority of subjects, her eyes—is oriented toward them. Much has been made of the implications of this reduced sensitivity to the eyes and the link between understanding the eyes as the organ of vision and the development of perceptual mental states (see Baron-Cohen 1995; Tomasello 1996). Although it is true that humans attribute special importance to the eyes, there is no reason why other organisms could not develop perceptual mental states based on the face as opposed to the eyes. Although someone could argue that using the face as a whole may lead to the development of ‘faulty’ perceptual mental states because there are some parts of the face that do not have any visual perception function, the same reasoning could be extended to the eyes. One could argue that some parts of the eye (e.g., retina) but not others (e.g., sclera) are responsible for vision and therefore focusing on the eyes as a whole may produce an unsatisfactory attribution of perceptual mental states. Therefore, we do not think that lacking sensitivity to the eyes necessarily implies that an organism cannot develop perceptual mental states such as seeing.
Another interesting case is dogs that show an extreme sensitivity to the eyes and gestural communication in general of humans (Miklósi et al. 1998; Mckinley and Sambrook 2000; Call et al. 2003). In addition to their specific ontogenetic history, dogs also have a phylogenetic history of domestication in close contact with humans that may have selected them to respond to certain human configurations. Research on animal cognition in many different species is revealing, on a daily basis, the individuals’ sensitivity to the presence of their social partners (Bugnyar and Kotrschal 2001; Emery and Clayton 2001; Call et al. 2003). The current study helps to elucidate what is the basis of social sensitivity by showing that certain body and face orientations play an important role in social interactions.
References
Baron-Cohen S (1995) Mindblindness. MIT Press, Cambridge, Mass.
Bugnyar T, Kotrschal K (2001) Deception in ravens. Nature 414:445–446
Call J, Tomasello M (1994) The production and comprehension of referential pointing by orangutans, Pongo pygmaeus. J Comp Psychol 108:307–317
Call J, Bräuer J, Kaminski J, Tomasello M (2003) Domestic dogs (Canis familiaris) take food differentially as a function of being watched. J Comp Psychol 117:257–263
Call J, Hare BH, Carpenter M, Tomasello M (2004) Unwilling or unable: chimpanzees’ understanding of human intentional action. Dev Sci (in press)
Coussi-Korbel S (1994) Learning to outwit a competitor in mangabeys (Cercocebus torquatus torquatus). J Comp Psychol 108:164–171
Emery NJ, Clayton NS (2001) Effects of experience and social context on prospective caching strategies by scrub jays. Nature 414:443–446
Gómez JC (1996) Non-human primate theories of (non-human primate) minds: some issues concerning the origins of mind-reading. In: Carruthers P, Smith PK (ed) Theories of theories of mind. Cambridge University Press, Cambridge, pp 330–343
Goodall J (1986) The chimpanzees of Gombe, patterns of behaviour. Belknap, Cambridge, UK
Hare B, Call J, Agnetta B, Tomasello M (2000) Chimpanzees know what conspecifics do and do not see. Anim Behav 59:771–785
Hare B, Call J, Tomasello M (2001) Do chimpanzees know what conspecifics know? Anim Behav 61:139–151
Hostetter A, Cantero M, Hopkins W (2001) Differential use of vocal and gestural communication by chimpanzees (Pan troglodytes) in response to attentional status of a human (Homo sapiens). J Comp Psychol 115:337–343
Kobayashi H, Koshima S (2001) Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol 40:419–435
Leavens DA, Hopkins WD, Bard KA (1996) Indexical and referential pointing in chimpanzees (Pan troglodytes). J Comp Psychol 110:346–353
Maestripieri D (1996) Gestural communication and its cognitive implications in pigtail macaques (Macaca nemestrina). Behaviour 133:997–1022
Maestripieri D (1997) Gestural communication in macaques. Evol Commun 1:193–222
McKinley J, Sambrook TD (2000) Use of human-given cues by domestic dogs (Canis familiaris) and horses (Equus caballus). Anim Cogn 3:13–22
Miklósi A, Polgárdi R, Topál J, Csányi V (1998) Use of experimenter-given cues in dogs. Anim Cogn 1:113–121
Pika S, Liebal K, Tomasello M (2003) Gestural communication in young gorillas (Gorilla gorilla) gestural repertoire and use. Am J Primatol 60:95–111
Povinelli DJ, Eddy TJ (1996) What young chimpanzees know about seeing. Monogr Soc Res Child Dev 61:152
Reaux JE, Theall LA, Povinelli DJ (1999) A longitudinal investigation of chimpanzees’ understanding of visual perception. Child Dev 70:275–290
Theall LA, Povinelli DJ (1999) Do chimpanzees tailor their gestural signals to fit the attentional states of others? Anim Cogn 2:207–214
Tomasello M (1996) Chimpanzee social cognition. Monogr Soc Res Child Dev 61:161–173
Tomasello M (1997) The ontogeny of chimpanzee gestural signals. Evol Commun 1:224–259
Tomasello M, Call J, Nagell K, Olguin R, Carpenter M (1994) The learning and use of gestural signals by young chimpanzees: a trans-generational study. Primates 35:137–154
Acknowledgements
We thank the caretakers of the Wolfgang Köhler Primate Research Center in the Leipzig Zoo, Leipzig, Germany, for their help in collecting the data and several anonymous reviewers for their comments on an earlier version of this manuscript. The reported experiments comply with all laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaminski, J., Call, J. & Tomasello, M. Body orientation and face orientation: two factors controlling apes’ begging behavior from humans. Anim Cogn 7, 216–223 (2004). https://doi.org/10.1007/s10071-004-0214-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-004-0214-2