Abstract
We report experiments based on a novel test in domestic chicks (Gallus gallus), designed to examine the encoding of two different geometric features of an enclosed environment: relative lengths of the walls and amplitude of the corners. Chicks were trained to search for a food reward located in one corner of a parallelogram-shaped enclosure. Between trials, chicks were passively disoriented and the enclosure was rotated, making reorientation possible only on the basis of the internal spatial structure of the enclosure. In order to reorient, chicks could rely on two sources of information: the relative lengths of the walls of the enclosure (associated to their left-right sense order) and the angles subtended by walls at corners. Chicks learned the task choosing equally often the reinforced corner and its rotational equivalent. Results of tests carried out in novel enclosures, the shapes of which were chosen ad hoc (1) to induce reorientation based only on the ratio of walls lengths plus sense (rectangular enclosure), or (2) to induce reorientation based only on corner angles (rhombus-shaped enclosure), suggested that chicks encoded both features of the environment. In a third test, in which chicks faced a conflict between these geometric features (mirror parallelogram-shaped enclosure), reorientation seemed to depend on the salience of corner angles. These results shed light on the elements of the environmental geometry which control spatial reorientation, and broaden the knowledge on the geometric representation of space in animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research in comparative spatial cognition has established that finding a place in an enclosed environment after passive disorientation depends critically on cues provided by the geometry of the environment, even when non-geometric perceptual features could be taken into account to determine the position of the goal. The classic paradigm originally developed to study this problem (Cheng 1986; Margules and Gallistel 1988) consisted of a working memory task in which rats were trained to find a reward hidden in one corner of a rectangular enclosure, in the presence of distinctive panels located in the four corners. Disoriented rats made a substantial number of rotational errors during their search, visiting the corner geometrically equivalent to the goal corner even if it was associated with a different panel. What appeared to be an apparently "blind" dependence on the geometric shape of the environment (which was interpreted as depending on a purely "geometric module"; Cheng 1986; Gallistel 1990) seems to be peculiar also to prelinguistic children (Hermer and Spelke 1994), whereas adult humans (Hermer and Spelke 1994), rhesus monkeys (Gouteux et al. 2001), birds (Vallortigara et al. 1990b; Kelly et al. 1998) and even fish (Sovrano et al. 2002, 2003) were shown to rely also on local non-geometric information, such as that provided by distinctive panels in the corners or a differently coloured wall, when they were available. However, it must be remarked that a number of counterexamples have been provided, making this picture less clear-cut. For instance, when rats were trained in a reference memory version of the task, their performance revealed that they could make use of non-geometric information (Cheng 1986, 1987). Also children, when tested in a large rectangular room, were shown to use the information provided by local landmarks (Learmonth et al. 2002). On the other hand, in some cases non-geometric information has been proven to become subordinate to geometric information contingently on task conditions. Adult humans, for instance, reorient themselves neglecting non-geometric information when they are asked to perform a concurrent verbal shadowing task (Hermer-Vasquez et al. 1999). Finally, Rhesus monkeys reorient successfully to the correct corner, provided that non-geometric information is large enough in size: if the angular size of the landmarks is small, the monkeys' choice of the two rotationally equivalent corners falls to chance levels (Gouteux et al. 2001).
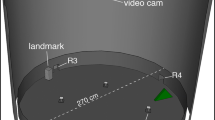
The choice of a rectangular enclosure as the training and testing apparatus, common to all these studies, has been hitherto of great advantage in revealing the existence of spatial representations based on the overall shape of the environment. The sources of information which were usually present in those studies consisted of the geometric shape of the enclosure (relative lengths of walls and sense) and the non-geometric information conveyed by salient objects or panels located in the corners or by the different colour of one of the walls of the enclosure. Here we propose a modified version of the classic paradigm, aimed at assessing the encoding of two different features of the environment: (1) the relative lengths of the walls, together with sense (left and right), and (2) the angle defined by the intersection of walls at corners. Preserving the original idea (Cheng 1986) that rotational errors are indicative of the animals' reliance on the overall geometry, we decided to use a parallelogram-shaped enclosure as the training apparatus (Fig. 1). The corner of a parallelogram has only one geometrically equivalent corner (which is at the opposite end along the diagonal), and this makes a parallelogram as suitable as a rectangle in order to induce animals to make rotational errors.
In the present task, domestic chicks (Gallus gallus) were thus trained to search for food in one corner of a parallelogram-shaped enclosure. To further clarify the possible geometric features on which the chicks could rely to reorient, it should be noticed that the goal corner (for example the acute angle A in Fig. 1) is located between one long wall on the left and one short wall on the right: this is usually termed the "metric layout" of the walls of the enclosure, namely the relative lengths of walls associated to sense (left and right). This fact implies that corner C becomes a candidate for rotational errors given that it shares the same metric properties of corner A (it also has a long wall on its left and a short wall on its right). Furthermore, the two walls intersecting at corner A subtend an acute angle, which is the second geometric feature chicks could exploit in their search for the goal. Again, corner A and corner C share this geometric feature, so that corner C can be confused systematically with corner A. Assuming that an animal can be successfully trained to find corner A, this makes us predict that it will search at corner A and corner C with equal likelihood.
In the spirit of a genuine 'transformational approach' (Cheng and Spetch 1998), tests carried out after proper alterations of the geometry of the parallelogram-shaped enclosure could reveal whether the two geometric features are both encoded during learning. In practical terms, to assess this, it would be necessary to carry out (1) a test in an enclosure in which the angles subtended by intersecting walls are made irrelevant whereas the relative lengths of walls together with sense are preserved, and (2) a test in an enclosure in which the ratio of lengths is made irrelevant whereas the corner angles are preserved. The two conditions just described could be satisfied, respectively, by using a rectangular enclosure (same ratio of wall lengths as the parallelogram-shaped enclosure, but all corners having an identical amplitude of 90°) and a rhombus-shaped enclosure (same amplitude of corners as in the parallelogram-shaped enclosure, but all walls having the same length, halfway between the length of the short and the long wall of the parallelogram-shaped enclosure). If the spatial representation formed by chicks during training to localise corner A in the parallelogram-shaped enclosure includes the relative lengths of walls and sense information, then we could predict that in the rectangular enclosure chicks would continue to search more in the two corners having a long wall on the left and a short wall on the right. Moreover, if the spatial representation formed during learning also includes the angle at corner A, then we could predict that in the rhombus-shaped enclosure chicks would continue to search more in the two acute corners.
In addition, a third test was devised to assess whether any of these two geometric features is given a higher priority by chicks in the process of reorientation. To this end, a test in a mirror image of the parallelogram-shaped enclosure (see Fig. 3, bottom panel) fitted the purpose. In this situation, chicks might search mainly in the corners with a long wall on the left and a short wall on the right (although they subtend an obtuse angle) and ignore the acute angles, thus showing them to rely more on the relative lengths of walls and sense. On the other hand, chicks might search mainly in the acute angles (which are associated to the opposite sense order of the surrounding walls, namely a short wall on the left and a long wall on the right) and ignore the corners associated with the correct arrangement of walls, thus showing more reliance on corner angles. A third alternative predicts that chicks would search with equal likelihood in the four corners, thus implying that both features control the search behaviour with the same strength.
In sum, the first aim of the present work was to make available for reorientation two distinct geometric features of an enclosed environment defined by a given arrangement of walls, and to assess whether chicks encoded each of these features during learning. The second aim was to put the features in conflict and to assess which of the two played a major role in spatial search.
Methods
Subjects
The subjects were 13 male chicks of the Hybro strain (a local variety derived from the White Leghorn breed) obtained from a commercial hatchery when they were only a few hours old. Chicks were reared individually, at controlled temperature (30–35°C) in metal cages (35 cm wide × 35 cm deep × 38 cm high) illuminated from above by fluorescent lamps. Food and water were available ad libitum.
Apparatus
The apparatus used during training consisted of a parallelogram-shaped enclosure (see Fig. 1), with walls made of homogeneously brown wood and the floor made of white opaque plastic. The lengths of the walls were 70 cm (long walls) and 35 cm (short walls), the height of the walls was 35 cm. The angular separation between a 70-cm wall (on the left) and a 35-cm wall (on the right) was 60°. The angular separation between a 35-cm wall (on the left) and a 70-cm wall (on the right) was 120°. Transparent glass containers (diameter 5 cm, height 6 cm), identical to those present in the chicks' home cages, were positioned in the four corners and replenished with food. All containers had a thin plastic net glued over the top opening. The reinforced container also had this covering, but a small hole (diameter 2 cm) was cut on top of it, to allow chicks to access food while making this container identical in appearance to the other three containers. The top of the enclosure was covered by a veil, and two light bulbs (25 W each) hung over the centre of the enclosure illuminated the environment. The enclosure was placed over a turning platform, so that its orientation with respect to the experimental room could be randomised before each trial. This was done to minimise the likelihood that chicks could reorient by using spatial information external to the enclosure.
The testing apparatuses were three enclosures constructed with the same materials and characteristics as the training enclosure but having the following shapes and dimensions: they were a rectangular enclosure (sides 35×70 cm, height 35 cm), a rhombus-shaped enclosure (sides 52 cm, opposite corners 60 ° and 120°, height 35 cm), and a mirror version of the parallelogram-shaped enclosure (sides 70 cm and 35 cm, height 35 cm). All containers in the four corners of the testing enclosures were covered to make food inaccessible during tests.
Procedure
For a group of six chicks ("group 60°") the reinforced container was positioned in one of the acute corners (corner A in Fig. 1); for the remaining seven chicks ("group 120°") the reinforced container was positioned in one of the two obtuse corners (corner B in Fig. 1).
Chicks were deprived of food 8 h before each daily training session. Training started on day 3 of life and consisted of two blocks of 15 trials each, separated by a 15 min interval, for 3 days.
During the intertrial interval, the chick was placed in a closed cardboard box which was slowly rotated for 10 s, in order to exclude the use of compass or inertial information. Meanwhile, the floor of the enclosure was accurately cleaned from any trace of food and other debris, and the enclosure was rotated to a new orientation on the turning platform. At the beginning of each trial, the chick was taken from the restraining box with one hand after being disoriented, taking care to cover its eyes with the other hand for the entire duration of the displacement, and it was placed in the middle of the enclosure with a random orientation of the sagittal axis of the body. One trial lasted until the chick had discovered the accessible container (and eaten some food) or, in the case that the chick failed to find it, for a maximum time of 1 min. The first corner visited by the chick after its release was scored. Approach and close visual scrutiny of the container (usually associated to pecking behaviour) were necessary conditions for a visit to be considered as a score. Given the shape of the enclosure, two pairs of "places" can be identified: the "correct corners", which include the reinforced corner together with its rotational equivalent (Fig. 1: corners A and C for group 60°; corners B and D for group 120°) and the "incorrect corners", which include the other two corners (Fig. 1: corners B and D for group 60°; corners A and C for group 120°). The choice of reinforcing only one of the two correct corners was made in order to check whether they were really indistinguishable (differences unnoticeable to the human eye could help, a priori, chicks in selecting the reinforced container). A criterion of 80% choice of correct corners was established to consider training successfully accomplished.
After that chicks had completed the last training block on day 5, a series of three test blocks (seven trials per block) was carried out in each of the three testing enclosures. The order of the test blocks was randomised for the animals, and a short retraining block (ten trials) in the parallelogram-shaped enclosure (in presence of the baited container) was administered in between test blocks to avoid extinction. Chicks were allowed to search in the novel enclosures and the first corner visited in each trial was scored, after which the chick was removed from the enclosure, it was disoriented, and a new trial began. Both authors independently scored the visits, and the inter-observer agreement was 100%.
Results
On the third day of training, both groups of chicks reached or exceeded the required criterion of 80% choices of the correct corners, and there was an increase in performance over the 3 days (six blocks) of training (Fig. 2). A mixed-design analysis of variance with Training Corner as a between factor (levels: 60° and 120°) and Block as a within factor revealed in fact a main effect of Block (F 5,50=9.02; P<0.0001), no effect of Training Corner (F 1,10=1.62; P=0.22) and no effect of the interaction Training Corner × Block (F 5,50=1.985; P=0.09).
Chicks in both groups confused the reinforced corner with its rotational equivalent. This was observed throughout the 3 days of training, but analysis will be here limited to the visits scored during the last training block. As it is evident from Fig. 3 and Fig. 4 (top panels), chicks visited with equal likelihood the reinforced corner and its rotational equivalent. A test on the frequency of visits during the last block, based on an univariate loglinear model, with Corner as factor, confirmed this impression (see Table 1 for group 60° and Table 2 for group 120°), excluding that chicks could distinguish the two geometrically correct corners on the basis of uncontrolled non-geometric cues.
Top panel Proportion of visits to the four corners in the last training block for chicks in group 60°. Second panel from top Proportion of visits to the four corners during test in the rectangular enclosure. Third panel from top Proportion of visits to the four corners during test in the rhombus-shaped enclosure. Bottom panel Proportion of visits to the four corners during test in the mirror parallelogram-shaped enclosure
Top panel Proportion of visits to the four corners in the last training block for chicks in group 120°. Second panel from top Proportion of visits to the four corners during test in the rectangular enclosure. Third panel from top Proportion of visits to the four corners during test in the rhombus-shaped enclosure. Bottom panel Proportion of visits to the four corners during test in the mirror parallelogram-shaped enclosure
Results of test in the rectangular enclosure (Figs. 3, 4, second panel from top) clearly indicate that chicks in both groups visited the corners that preserved the correct lengths and sense properties of the surrounding surfaces. Namely, chicks in group 60° visited mainly the two corners that lay at the intersection of a long wall on the left and a short wall on the right [Wilcoxon two-tailed test (A–C vs B–D): n=6; T=0; P<0.05], whereas chicks in group 120° visited mainly the two corners with a short wall on the left and a long wall on the right (Wilcoxon two-tailed test (B–D vs A–C); n=7; T=1; P<0.02).
In the rhombus-shaped enclosure (Figs. 3, 4, third panel from top), chicks in both groups visited the corners that preserved the appropriate corner angle: visits of chicks in group 60° were directed mainly to corners A and C [Wilcoxon two-tailed test (A–C vs B–D): n=6; T=0; P<0.05], whereas visits of chicks in group 120° were directed mainly to corners B and D [Wilcoxon two-tailed test (B–D vs A–C): n=7; T=1; P<0.02].
Results of tests in the mirror parallelogram-shaped enclosure (Figs. 3, 4, bottom panel) showed that chicks in group 60° chose the corners with an amplitude of 60°, even though they were surrounded by walls with a sense order opposite to that experienced during training (Wilcoxon two-tailed test (B–D vs A–C): n=6; T=0; P<0.05). Unexpectedly, chicks in group 120° chose the corners surrounded by walls with the relative lengths and sense order they had learned, even though they subtended an angle of 60° (Wilcoxon two-tailed test (A–C vs B–D): n=7; T=1; P<0.02). At first sight this result was puzzling, and we could hardly find an immediate explanation to account for it (but see Discussion for a possible explanation). One first possibility that had to be discarded, however, could be that chicks have a spontaneous preference for approaching acute corners, which would explain why animals in group 120° chose the 60° corner instead of the 120° corner on which they were trained. It should be remarked, however, that if this hypothesis holds true, then the same bias should also have emerged during the test in the rhombus-shaped enclosure, which instead was not the case.
To further investigate this possibility we devised a simple control experiment aimed at testing the presence of a spontaneous preference in the domain of corner angles. A new group of four chicks (same provenience and rearing conditions as the other chicks) took part in this control experiment, which consisted in a single test session, without any training. Chicks were deprived of food 8 h before the test, which was carried out on day 4 of life. The apparatus was the same rhombus-shaped enclosure described above, with the exception that all food containers positioned in the four corners were freely accessible to the chicks. All other methodological details concerning the apparatus and the procedure were the same as described above. Each animal was introduced in the enclosure and it was allowed to eat some food from the containers for 20 consecutive trials (1 min each). The first corner visited in each trial was scored. All chicks chose the obtuse angles more often than the acute angles (average number of first visit to an obtuse corner: 13.8, SE=1.3). This result suggests that even whether the choice of corners might be random and not significantly biased towards the choice of obtuse angles, there was no evidence of preference or spontaneous choice of acute angles.
Discussion
This is the first experiment attempting to isolate the representation of two distinct geometric features of an environment defined by a given arrangement of surfaces: relative lengths of walls and their associated sense order, and corner angle. One first conclusion which is suggested by the results here reported is that chicks can learn to localise a place which is defined by the association of these two cues. This is not surprising, since it was already known that chicks can be trained to localise a rewarded corner in a rectangular-shaped enclosure (Vallortigara et al. 1990b), thus showing that they can encode the relative lengths of surfaces plus sense. The results of the chicks' training in the present study suggest that the presence of the angle cue provided an additional source of information, useful in the process of reorientation, and this can be appreciated by the very fast rate at which chicks learned the task. However, the fact that chicks learned the task would not necessarily mean that they also relied on the geometric feature provided by corner angles during training. One might even think that neither of the two cues was encoded, and that chicks based their search strategy on other sources of information. One such possible alternative could be that chicks encoded the goal as a region in space defined by the two principal axes and sense. For instance, chicks in group 60° might have encoded the goal corner as the leftward region at the end of the major axis. According to this alternative interpretation, tests in the rectangular enclosure would predict the observed results. Tests in the rhombus-shaped enclosure would also predict the observed results, provided that the chicks had also encoded the relative orientation of the axes, since in the rhombus-shaped enclosure the two axes had the same length. In sum, this alternative explanation would assume that chicks encoded the relative lengths of the axes as well as their relative orientation, and that a matching mechanism based on the extraction of principal axes (along the lines of that proposed by Gallistel, 1990) was carried out by chicks during searching in the novel enclosures. This possibility must however be discarded when taking into consideration the results obtained in the mirror version of the parallelogram-shaped enclosure. Clearly, if the heuristic based on the principal axes and sense had also been exploited in that test, chicks should have searched with a similar pattern in both experimental groups, which instead was not the case. Proceeding by elimination, and accepting prima facie the results obtained in the first two tests, it appears that chicks actually encoded both features. In the rectangular enclosure they recognised the walls with the correct lengths ratio and sense order, choosing the corners which lay at the intersection of the short and the long wall in the same left/right arrangement they had learned during training. In the rhombus-shaped enclosure they recognised the angular amplitude of corners, choosing those corners which had the same amplitude they had learned during training. We can thus conclude that chicks encoded both the relative lengths of walls (together with sense) and the amplitude of angles subtended by walls at corners.
This conclusion could in principle be explained by the fact that corners are perceived and encoded by chicks as local cues, in the same manner as distinct panels or objects were found to be encoded in previous similar studies on geometric modules in avian species (Vallortigara et al. 1990b; Kelly et al. 1998). It is clear that encoding the angle subtended by two walls requires a visual estimation over a narrow portion of the environment, as in the case where a salient object located in a corner is encoded. Corners could thus be assigned at least some of the attributes of objects, in terms of a relatively small extension of the field of view, but also in terms of their appearance and distinctiveness. Undoubtedly they can be regarded as more local than global in nature, when compared to the information conveyed by the relative lengths of walls. We suggest that angles are processed by chicks as local geometric information, in contrast to the local non-geometric information which usually refers to discrete objects and landmarks. One complication arises, however, when considering the results obtained during the tests in the mirror parallelogram-shaped enclosure. Assuming that corners were encoded by chicks as local features, we know from previous results that when global and local information are put into conflict, chicks make greater use of local information (Vallortigara et al. 1990b; see also Tommasi et al. 2000 for similar results), and we should expect that in the mirror-enclosure test chicks would visit mainly those corners subtending the same angle as during training. This prediction was confirmed only for the chicks trained on the 60° angle, whereas chicks trained on the 120° angle did not confirm the prediction.
Having excluded the possibility that chicks' choices in this test were due to a spontaneous preference for approaching acute corners, as confirmed by the control experiment, our suggested explanation for the pattern of results obtained could be that angles are perceived by chicks with a variable degree of distinctiveness, and that they acquire different values of predictive strength as a function of their distinctiveness. To conclude, the data obtained in the mirror-parallelogram test could be reinterpreted assuming that acute corners are encoded by chicks as more distinctive than obtuse angles, thus acquiring a higher level of stimulus control than the information conveyed by the ratio of surface lengths. Obtuse angles, on the other hand, would not be distinctive enough (even though they could be easily discriminated from acute angles in the rhombus-shaped enclosure) to gain control, and the residual information extracted from the metric layout of surfaces would predominate. Data reported here would thus suggest a conclusion based on the perceptual salience of corners (see also Vallortigara et al. 1990a, for analogous considerations on the nature of perceptual objects in chicks). However, an alternative explanation of the results obtained in the mirror transformation can be considered. One possibility would be that chicks actually encoded the amplitude of the corner angle during training but responded to change according to a threshold generalisation gradient. Chicks trained on the 60° corner faced an incremental change equal to 100% of the training angle, whereas chicks trained on the 120° corner faced a decremental change equal to 50% of the training angle. The former group might have responded to the more noticeable change (angle doubled) moving away from the region of space defined by the relative lengths of walls, whereas the latter group might have accepted the less noticeable change (angle halved) and stayed in the corresponding region. However, this alternative explanation is made less tenable when considering the derived prediction that chicks trained on the 120° corner would search randomly in the rhombus-shaped enclosure, since the results show that instead they searched mostly in the 120° corners. We believe that an explanation based on the encoding and the perceived salience of corner angles should be regarded as the most likely, although it would be interesting to check whether the use of other values of corner angles than those used in this research would produce results compatible to such an explanation.
Experiments carried out in our laboratory showed that chicks can learn to localise the centre of a geometric-shaped enclosure and that they can also transfer their search behaviour to novel enclosures, differing in size and/or in geometric shape (Tommasi et al. 1997; Tommasi and Vallortigara 2000). Those studies suggested that, in order to evaluate distances, the shape of the enclosure must be encoded by making use of some stable features, and possible candidate features were supposed to be the discontinuities between surfaces, in other words edges and corners. The present study extends that observation and offers further details on the structure of spatial representation in chicks: not only do these animals rely on surface discontinuities in order to reorient, but they also seem capable of parsing the world in meaningful spatial elements. Corners seem to constitute one such category. It should be remarked, however, that in the studies on central place learning just mentioned, chicks could search at any location inside the enclosure, to which end the ground-scratching response on a sawdust substrate was chosen as the measured variable. The same response was adopted in the only research published so far on the geometric module in chicks using the classic rectangular enclosure (Vallortigara et al. 1990b). Our results confirm the finding of that study, namely that chicks encode the ratio of lengths of surfaces. In the present study, however, the transfer test in the rectangular enclosure showed that chicks could still make use of the relative lengths of walls despite the overall geometry of the novel environment being distorted in terms of absolute orientation of the walls. In our opinion, this result bears some resemblance to the result of a recent study by Kelly and Spetch (2001), who found that pigeons trained to find a corner in a rectangular enclosure transferred their search behaviour to the corresponding corners in enclosures of similar shape, but differing in absolute size, showing that they encoded the relative geometry of the enclosure. Absolute length (as shown by Kelly and Spetch 2001), but also absolute orientation of walls (as suggested by our results), seems to be discounted in the process of reorientation, provided that the overall geometry of the enclosure is somehow preserved.
In the present case, however, reorientation based on the relative lengths of surfaces was not observed when the information conveyed by one corner angle was salient enough to act as a sort of local landmark (test in the mirror parallelogram-shaped enclosure, group 60°). From this viewpoint, acute corners seem to have the same role, in terms of stimulus control over the environment, as do panels or objects. On the other hand, obtuse corners seem to be too little distinctive or salient, and in the case where they are simultaneously present, together with the information conveyed by the relative lengths of surfaces, the latter gains control.
One parallel can be traced between these results and those obtained in the recent study by Learmonth et al. (2002) using the classic paradigm of the rectangular space in children. In the study by these authors, confirming the result previously obtained by Hermer and Spelke (1994), young children systematically confused the two equivalent corners of a rectangular room even when one of the short walls was marked by a different colour, provided that the room was small enough in absolute size. However, when the experimental room was larger in size, children were able to conjoin geometric and non-geometric information, paying attention to the disambiguating identity of the differently coloured wall: the salience of the local information (coloured wall) was apparently modulated by the salience of the global information (room shape). It is likely that a similar explanation also holds for our results, but in the reverse order: the salience of the geometric information (enclosure shape) seemed in fact to be modulated by the salience of the local information (angle amplitude). A similar comparison can be made when considering the aforementioned study carried out on rhesus monkeys (Gouteux et al. 2001), which showed that the conjoint encoding of relative lengths of walls and local landmarks depends on the angular size of landmarks; another result attributed to a salience effect. It should be noticed that the absolute size of the enclosure used in the present study was smaller than the size of the enclosure used in the study by Vallortigara et al. (1990b), and this difference might also be relevant in the case of chicks, thus suggesting the need for further research on effects of the size of the environment in this species.
A conclusion common to the present study and those by Learmonth et al. (2002) and Gouteux et al. (2001), although they might appear distant from each other both in the details of the task and the species under study, is that the interaction between global and local information is a delicate matter when dealing with the representation of geometric information, deserving an accurate analysis of the elements which constitute the attended structure of space in animals and humans.
References
Cheng K (1986) A purely geometric module in the rat's spatial representation. Cognition 23:149–178
Cheng K (1987) Rats use the geometry of surfaces for navigation. In: Ellen P, Thinus-Blanc C (eds) Cognitive processes and spatial orientation in animals and man. Martinus Nijhoff, Dordrecht, pp 153–159
Cheng K, Spetch ML (1998) Mechanisms of landmark use in mammals and birds. In: Healy S (ed) Spatial representation in animals. Oxford University Press, Oxford, pp 1–17
Gallistel CR (1990) The organization of learning. MIT Press, Cambridge, Mass.
Gouteux S, Thinus-Blanc C, Vauclair J (2001) Rhesus monkeys use geometric and nongeometric information during a reorientation task. J Exp Psychol Gen 130:505–519
Hermer L, Spelke ES (1994) A geometric process for spatial reorientation in young children. Nature 370:57–59
Hermer-Vasquez L, Spelke E, Katsnelson A (1999) Source of flexibility in human cognition: dual-task studies of space and language. Cogn Psychol 39:3–36
Kelly DM, Spetch ML (2001) Pigeons encode relative geometry. J Exp Psychol Anim Behav Process 27:417–422
Kelly DM, Spetch ML, Heth DC (1998) Pigeons' (Columbia livia) encoding of geometric and featural properties of the environment. J Comp Psychol 112:259–269
Learmonth AE, Nadel L, Newcombe NS (2002) Children's use of landmarks: implications for modularity theory. Psychol Sci 13:337–341
Margules J, Gallistel CR (1988) Heading in the rat: determination by environmental shape. Anim Learn Behav 16:404–410
Sovrano AV, Bisazza A, Vallortigara G (2002) Modularity and spatial reorientation in a simple mind: encoding of geometric and nongeometric properties of a spatial environment by fish. Cognition 85:B51–B59
Sovrano AV, Bisazza A, Vallortigara G (2003) Modularity as a fish views it: conjoining geometric and non-geometric information for spatial reorientation. J Exp Psychol Anim Behav Process 29:199–210
Tommasi L, Vallortigara G (2000) Searching for the centre: Spatial cognition in the domestic chick (Gallus gallus). J Exp Psychol Anim Behav Process 26:477–486
Tommasi L, Vallortigara G, Zanforlin M (1997) Young chickens learn to localize the centre of a spatial environment. J Comp Physiol A 180:567–572
Tommasi L, Andrew RJ, Vallortigara G (2000) Eye use in search is determined by the nature of task in the domestic chick (Gallus gallus). Behav Brain Res 112:119–126
Vallortigara G, Zanforlin M, Compostella S (1990a) Perceptual organization in animal learning: cues or objects? Ethology 85:89–102
Vallortigara G, Zanforlin M, Pasti G (1990b) Geometric modules in animals' spatial representations: a test with chicks (Gallus gallus domesticus). J Comp Psychol 104:248–254
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tommasi, L., Polli, C. Representation of two geometric features of the environment in the domestic chick (Gallus gallus). Anim Cogn 7, 53–59 (2004). https://doi.org/10.1007/s10071-003-0182-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-003-0182-y