Abstract
This study investigated the effects of Lactobacillus curvatus MG5246 on periodontitis inflammation. Cell-free supernatants (CFS) prepared from L. curvatus MG5246 decreased prostaglandin E2 production and cyclooxygenase-2 gene expression by 60% and 78% in Porphyromonas gingivalis-lipopolysaccharide stimulated human gingival fibroblasts at 400 μg/mL. Gene expressions of tumor necrosis factor-α, interleukin-6, matrix metalloproteinases, and chemokines were significantly downregulated by CFS treatment (p < 0.05). L. curvatus MG5246 (2 × 108 CFU/day, 8 weeks) administration significantly improved alveolar bone loss in the ligature-induced periodontitis rat model. Elevated mRNA expression of the receptor activator of nuclear factor-κB ligand/osteoprotegerin ratio in the gingival tissue was significantly decreased by L. curvatus MG5246 administration (p < 0.05). Moreover, L. curvatus MG5246 showed sufficient tolerance in simulated gastrointestinal conditions (gastric tolerance: 89.48%, intestinal tolerance: 98.62%) and did not show antibiotic resistance and hemolytic activity. Therefore, L. curvatus MG5246 has the potential as novel oral probiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal disease, one of the most common and frequent chronic diseases, has become a global concern. Periodontitis is highly associated with various systemic diseases, including rheumatoid arthritis and cardiovascular diseases (Bartold et al., 2005; Beck and Offenbacher, 2005). Porphyromonas gingivalis, a Gram-negative anaerobic bacteria, is a major periodontal pathogen, and lipopolysaccharide (LPS) from P. gingivalis stimulates host inflammatory responses (Wang et al., 2000). The binding between LPS and toll-like receptors (TLR) increases the expression of proinflammatory cytokines [interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α)] and promotes the destruction of periodontal tissue (Sun et al., 2010). Human gingival fibroblasts (HGF-1 cells) are dominant resident cells in gingival connective tissue and modulate periodontal homeostasis. HGF produces inflammatory cytokines upon binding of P. gingivalis LPS (Pg-LPS) to TLR4 (Wang et al., 2000).
Allaker and Stephen (2017) reported that probiotics have great potential to be used as an effective interface to recover homeostasis in oral microbial dysbiosis. Lactobacillus curvatus is one of the probiotics isolated from variety of fermented foods, such as vegetables, cereals and cheeses (Terán et al., 2018). L. curvatus SMFM2016-NK effectively decreased osteoclastic cell numbers and improved alveolar bone resorption in the periodontitis rat model (Choi et al., 2021a). In addition, fermented milk prepared with L. curvatus SMFM2016-NK also downregulated inflammatory gene expression in the oral cavity of periodontitis rats and increased the composition and diversity of the gut microbiome (Choi et al., 2021b).
This study was conducted to evaluate the efficacy of L. curvatus MG5246 as an oral probiotic. To achieve this goal, the cell free supernatant (CFS) of L. curvatus MG5246 was prepared, and the anti-inflammatory effects were analyzed using Pg-LPS-stimulated HGF-1 cells. Furthermore, the effects of L. curvatus MG5246 on alveolar bone loss were examined in an experimental periodontitis rat model.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin were purchased from WelGENE Inc. (Daegu, Korea). Prostaglandin E2 (PGE2) enzyme-linked immunosorbent assay kit was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). Taqman® Universal master mix, Taqman® probes (5′-fluorescein based reporter dye; 3′-TAMRA quencher) and high capacity RNA-to-cDNA kit were purchased from Applied Biosystems (Foster City, CA, USA). L. curvatus MG5246 powder was kindly provided from Mediogen (Jecheon, Korea). Pepsin, pancreatin, and other reagents were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA).
Characteristics of L. curvatus MG5246
Survival in simulated gastrointestinal conditions
To evaluate the gastrointestinal resistance of L. curvatus MG5246, the strain was harvested by centrifugation (3460 × g, 10 min) after culture in MRS broth at 37 °C for 24 h. The pellets were washed twice with phosphate buffered saline (PBS) adjusted to pH 7 and resuspended (107 CFU/mL) in simulated gastric fluid (SGF; 3 g/L of pepsin in PBS, pH 3 and pH 4) and simulated intestinal fluid (SIF; 1 g/L of pancreatin in PBS, pH 7 and 8). The viable cells were determined on MRS agar after incubated at 37 °C for 3 to 4 h (Maragkoudakis et al., 2006).
Bacterial survival was calculated as follows:
Antibiotic reistance
The antibiotic resistance of L. curvatus MG5246 was assayed using the minimum inhibitory concentration (MIC) test strip method. The L. curvatus MG5246 was harvested by centrifugation (3460 × g, 10 min) after culture in MRS broth at 37 °C for 18 h, washed twice with PBS (pH 7), and resuspended in PBS to obtain 0.5 McFarland turbidity. The suspended solution was incubated in LSM broth (a mixture of 90% Iso-Sensitest (OXOID, Hampshire, UK) and 10% MRS broth) with 1.5% agar using swabs (Klare et al., 2005). The plates were dried for 10 min, and MIC test strips (Liofilchem, Italy) were placed on the agar surface according to the manufacturer’s instructions. Plates were incubated at 37 °C, and the results were taken after 24 h inoculation. Antibiotic susceptibility was determined according to the European Food Safety Authority (EFSA) guidelines.
Hemolytic property
To evaluate the hemolytic activity, L. curvatus MG5246 strain was grown in MRS broth for 18 h at 37 °C and streaked onto tryptic soy agar (Difco, Detroit, MI, USA) with 5% sheep blood (MB cell, Seoul, Korea). After incubation for 48 h (37 °C, 10% CO2), the hemolytic property of L. curvatus MG5246 was evaluated based on the lysis of red blood cells around the colonies (Buxton, 2005).
Preparation of cell free supernatant (CFS)
L. curvatus MG5246 strain was grown in MRS broth at 37 °C for 24 h, aerobically, and CFS was obtained by centrifugation at 3460 × g for 10 min, followed by filtration using 0.2 μm filters (Advantec, Tokyo, Japan). The supernatants were lyophilized and used for the cell culture study.
Cell culture
HGF-1 cells (American Type Culture Collection, Manassas, VA, USA) were incubated and maintained in DMEM containing 10% FBS (v/v) and 1% penicillin–streptomycin (100 units/mL) at standard conditions (37 °C, 5% CO2). The culture medium was changed every 2–3 days, and subcultures were performed when the cells were completely full. Only cells with passage numbers between 4 and 10 were used for the experiments. MTT assay was used to evaluate the cytotoxicity of the sample.
PGE2 production
HGF-1 cells were seeded in 6 cm plate at a density of 4 × 105 cells/well and incubated until 90% confluent. Cells were treated with Pg -LPS (Invivogen, San Diego, CA, USA; 5 μg/mL) in fresh phenol-free DMEM medium to stimulate immune response and coincubated with various CFS concentrations (100, 200, and 400 μg/mL) of CFS for another 24 h. The production of PGE2 was analyzed from the culture supernatant using the enzyme immunoassay kits (Cayman Chemical).
Induction of experimental periodontitis
Male Sprague–Dawley rats (6 weeks old; 400 ~ 430 g; Orient Bio, Gapyeong, Korea) were maintained in a controlled temperature (23 ± 3 °C) and relative humidity (55 ± 15%) with a 12 h light–dark cycle. Rats had free access to purified water and a standard chow diet (Cargill Inc., Seongnam, Korea). Animal experiments protocols were approved by the Institutional Animal Care and Use Committee (KNOTUS 21-KE-032). After 1 week adaptation period, the rats were randomly divided into three groups: (1) no ligation (n = 10), (2) ligation control (n = 10), and (3) ligation + L. curvatus MG5246 (2 × 108 CFU/day) (n = 10). Based on the result of toxicity assessment that none of the Lactobacillus strains caused any sign of toxicity at a concentration as high as 1 × 1010 CFU/kg/day (Shokryazdan et al., 2016) and the suggested human dose of most probiotics is 108 to 109 CFU, a dosage of 2 × 108 CFU/kg/day was decided. Ligatures were placed around right mandibular molar teeth as described previously (Kim et al., 2019). After 8 weeks of ligation induction and L. curvatus MG5246 administration, rats were sacrificed and its gingival tissue were taken for further analysis.
Micro-computerized tomography (Micro-CT) analysis
Morphometric analysis of ligated molar tooth and the alveolar bone of rats was done using Viva CT 80 (SCANCO Medical, Switzerland) after CFS administration (8 weeks). The distance from the cemento enamel junction (CEJ) to the alveolar bone crest (ABC) was used as an index of alveolar bone loss.
RNA extraction and quantitative real time polymerase chain reaction (qRT-PCR)
qRT-PCR analysis was performed using HGF-1 cell lysates and rat gingival tissues as described previously (Kim et al., 2019). Target gene expressions [cyclooxygenase-2 (COX-2), TNF-α, IL-6, MMP-2, MMP-8, CXCL12, CXCR4, RANKL, and OPG)] were analyzed by StepOne Plus RT-PCR system (Applied Biosystems; Foster City, CA, USA). The relative changes in target gene expression levels were normalized to the value of β-actin.
Statistical analysis
All cell culture experiments and analytical measurements were conducted in triplicate. Data were presented as the mean ± SD. One-way analysis of variance and Duncan’s multiple comparison test (SPSS 25; SPSS, Inc., Chicago, IL, USA) were performed to examine significant differences among treatment means (p < 0.05).
Results and discussion
Simulated gastrointestinal survivability of L. curvatus MG5246
The viability of probiotics in the gastrointestinal tract is one of the key functional requirements to provide health benefits to the body (Tokatlı et al., 2015). The viability of L. curvatus MG5246 in simulated gastrointestinal conditions is shown in Table 1. Under the simulated gastric conditions adjusted pH 3, L. curvatus MG5246 had an 82% survival rate compared to the initial. In addition, L. curvatus MG5246 exhibited a viable cell count of > 6 log CFU/mL, and the survival rate was > 96% after exposure to pH 4–8. Generally, the viability of probiotic strains was in the range of 70% ~ 103% in SGF (Kim et al., 2020). Thus, L. curvatus MG5246 has acceptable viability to be used as a probiotic strain.
Antibiotic resistance and hemolytic activity of L. curvatus MG5246
The possible antibiotic resistance gene transfer to the pathogen is one of the safety issues in probiotic strains. In addition, evaluation of hemolytic activity of probiotics is strongly recommended to use in food products, even if they have GRAS (Generally Recognized as Safe) or QPS (Quality Presumption of Safety) status (FAO/WHO, 2002). Thus, the MICs of eight antibiotics and hemolytic activity were determined to ensure the safety of L. curvatus MG5246.
In Table 2, the MICs of all eight antibiotics against L. curvatus MG5246 showed lower values than the cut-off MIC values of the European Food Safety Authority guidelines. Also, MG5246 showed neither alpha nor beta hemolytic activity on the blood agar plates (data not shown). Based on these results, MG5246 does not pose safety concerns regarding antibiotic resistance and hemolytic activity.
Effect of CFS on PGE2 production and inflammatory gene expression in Pg-LPS-stimulated HGF-1 cells
The effects of CFS on PGE2 production and inflammatory gene expression were analyzed in Pg-LPS-stimulated HGF-1 cells. As shown in Fig. 1a, PGE2 production was significantly decreased by CFS treatment at 200 and 400 μg/mL. No cytotoxicity was observed at all tested concentration ranges (Fig. 1b). COX-2 gene expression was significantly downregulated at 200 and 400 μg/mL of CFS (p < 0.05) (Fig. 1c), whereas TNF-α was substantially decreased by 60% with 200 μg/mL CFS (p < 0.05) (Fig. 1d). The expression of IL-6, one of the major proinflammatory cytokines, was significantly suppressed at 200 and 400 μg/mL CFS (p < 0.05) (Fig. 1e). Gene expression of both MMP-2 and MMP-8 was also effectively downregulated even with 100 μg/mL CFS (p < 0.05; Fig. 1f and g).
Effects of CFS on a PGE2 production, b cell viability, c COX-2, d TNF-α, e IL-6, f MMP-2, and g MMP-8 mRNA expression in Pg-LPS-stimulated human gingival fibroblasts. Cells were treated for 24 h in the presence of Pg-LPS (5 μg/mL) and samples. a PGE2 production was determined by enzyme immunoassay kit. b Cell viability was measured by MTT assay. The level of target gene expressions was analyzed after normalization to that of β-actin using qPCR. Data are expressed as the mean ± SD. Bars with different letters (a–c) indicate significant differences at p < 0.05. Pg-LPS: Porpyromonas gingivalis lipopolysaccharide; PGE2: prostaglandin E2; COX-2: cyclooxygenase-2, TNF- α: tumor necrosis factor- α; IL-6: interleukin-6; MMP: matrix metalloproteinases
PGE2 plays an important role in the progression of inflammatory response. Proinflammatory immune responses such as cytokine secretion, extracellular matrix degradation, and alveolar bone loss are caused by the action of PGE2 (Offenbacher et al., 1993). TNF- α is a primary inflammatory mediator and promotes induction of secondary mediators such as chemokines and COX-2. The COX-2 expression and PGE2 production are significantly upregulated in inflamed gingival tissue, and IL-1β and periodontal bacterial components accounted for the elevated PGE2 production (Morton and Dongari-Bagtzoglou, 2001).
There have been several reports that orally administered probiotics resulted in beneficial effects in periodontitis. Lactobacillus gasseri SBT2055 positively prevented Pg-mediated periodontal disease in mice by decreasing levels of IL-1β and TNF- α levels. Theses reports also demonstrated that the antiperiodontitis effect caused by LG2055 administration was related to β-defensin expression in the oral cavity (Kobayashi et al., 2017). The application of probiotic oral tablet containing Lactobacillus reuteri significantly suppressed inflammatory cytokines in chronic periodontitis patients and principal clinical parameters, including bleeding index, periodontal probing depth, and clinical attachment level (Szkaradkiewicz et al., 2014).
Progression of periodontitis leads to increased collagen degradation via MMP secreted from gingival fibroblasts. Fibroblasts express multiple MMPs and increased MMP-2 level was observed in gingival tissue of chronic adult periodontitis (Makela et al., 1994). MMP-8 is an important periodontitis-associated biomarker, as it is a major collagen degrading protease and is closely related to the severity of periodontal inflammation (Sorsa et al., 2011). Based on these reports, inhibition of MMP-2 and MMP-8 is an important target to delay the progression or severity of the periodontal disease (Fig. 2).
Effects of CFS on a CXCL12, b CXCR4 and c ICAM-1 mRNA expression in Pg-LPS stimulated human gingival fibroblasts. Cells were treated for 24 h in the presence of Pg-LPS (5 μg/mL) and samples. The level of target gene expressions was analyzed after normalization to that of β-actin using qPCR. Data are expressed as the mean ± SD. Bars with different letters (a-c) indicate significant differences at p < 0.05. Pg-LPS: Porpyromonas gingivalis lipopolysaccharide; ICAM-1: intercellular adhesion molecule-1
Effects of CFS on chemokine gene expression in Pg-LPS stimulated HGF-1 cells
Chemokine is a type of cytokine that promotes the migration of immune cells through specific receptors. Based on the result of qRT-PCR, L. curvatus MG5246 treatment resulted in significant differences in chemokine gene expression of Pg LPS-stimulated HGF-1 cells. The gene expression of CXCL12 and its receptor CXCR4 was decreased by 42% and 70%, respectively, with 400 μg/mL of L. curvatus MG4265 (Fig. 3a and b). The gene expression of ICAM-1 was downregulated in a dose-dependent manner by the addition of L. curvatus MG5246 (Fig. 3c).
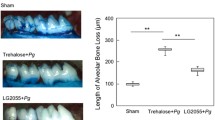
Effects of L. curvatus MG5246 administration on CEJ-ABC distance in ligature-induced periodontitis rats. The micro-CT images were used to measure the distance between the cemento-enamel junction (CEJ) and the crest of the alveolar bone (ABC). Data are expressed as the mean ± SD. Bars with different letters indicate significant differences at p < 0.05
Grassi et al., (2004) reported that CXCL12 expression significantly increased in synovial and bone tissue of rheumatoid arthritis and osteoarthritis patients. Elevated CXCL12 was closely linked with osteoclast-induced bone resorption. Pg-LPS-stimulated the expression of CXCL12 and its receptor CXCR4 in HGF-1 cells and amplified NF-κB signaling. LPS-mediated elevated CXCL12 expression was counteracted by the phosphatidylinositol 3-kinase inhibitor, LY2940002 (Sun et al., 2017). Nagashima et al. (2017) demonstrated that administration of a CXCR4 neutralizing antibody significantly reduced alveolar bone loss in the periodontitis mice model. This suggested that suppression of CXCR4 signaling can be a potential target for periodontitis treatment.
The expression of ICAM-1 was augmented by typical proinflammatory cytokines such as TNF- α and IL-1β in HGF, but suppressed by NF-κB inhibitor (MG-132) (Hosokawa et al., 2006). Chu et al. (2010) reported that Lactobacillus plantarum treatment for 4 weeks improved chronic inflammation by lowering proinflammatory cytokine and ICAM-1 expressions in the IL-10 knockout clotis mice model. Based on this speculation, suppression of CXCL12, CXCR4, and ICAM-1 expression in Pg- LPS-stimulated HGF might play an important role in alleviating periodontal inflammation.
Although the exact active components in the CFS of L. curvatus MG5246 are unclear, culture supernatants may contain various compounds that originated from microbial action, such as bacterial metabolites and fragments (Aguilar-Toalá et al., 2018). Postbiotics derived from Lactobacillus species include various surface proteins, bacteriocins, neurotransmitters, and short-chain fatty acids. They were actively involved in immune modulation and antimicrobial activity (Teame et al., 2020).
Effects of L. curvatus MG5246 on alveolar bone loss in experimental periodontitis in rats
As periodontitis progresses, alveolar bone loss increases. The effects of L. curvatus MG5246 on alveolar bone loss were evaluated after 8 weeks of the feeding trial, and the changes in periodontal bone height were calculated by the distance between cemento-enamel junction (CEJ) and crest of the alveolar bone (ABC). The distance of CEJ-ABC increased in the ligation control group, and L. curvatus MG5246 administration significantly decreased the distance of CEJ-ABC distance (Fig. 4).
Effects of L. curvatus MG5246 administration on a RANKL, b OPG mRNA expression, and c RANKL/OPG ratio in gingival tissue of ligature-induced periodontitis rats. The level of target gene expressions was analyzed after normalization to that of β-actin using qPCR. Data are expressed as the mean ± SD. Bars with different letters indicate significant differences at p < 0.05. RANKL: receptor activator of nuclear factor kappa-B ligand; OPG: osteoprotegerin
Similar to human periodontitis, the ligation of rat molar teeth leads to periodontal tissue destruction and alveolar bone loss. Plaque accumulation was the main factor for bone resorption and the bone loss was decreased by antibiotic treatment (Abe and Hajishengallis, 2013). The reduction in CEJ-ABC distance indicated that L. curvatus MG5246 administration improved alveolar bone loss associated with severe periodontitis.
Lactobacillus rhamnosus GG (LGG) Administration reduced gingival inflammation and alveolar bone loss in Pg inoculation-induced periodontitis mice. LGG treatment resulted in positive effects on periodontitis regardless of the way of administration (oral gavage vs. oral inoculation) (Gatej et al., 2018). Topical administration of Bifidobacterium animalis subsp. lactis inhibited bone resorption and detachment of connective tissue in ligature-induced periodontitis rats. The probiotic treatment group also showed a greater proportion of healthy oral commensal bacteria than the control group (Oliveira et al., 2017).
Effects of L. curvatus MG5246 on alveolar bone loss-related gene expression in gingival tissue
The effects of L. curvatus MG5246 administration on the bone resorption and tissue damage- related gene expression in the gingival tissue were analyzed using qRT-PCR. The L. curvatus MG5246 group significantly decreased RANKL expression, whereas OPG expression was increased by 2.2-fold compared to the ligation control group (Fig. 4a and b). Thus, the significantly elevated RANKL/OPG ratio by ligation was effectively lowered by L. curvatus MG5246 administration (Fig. 4c).
RANKL is a cell membrane-bound ligand and stimulates the differentiation and maturation of osteoclasts, followed by bone resorption. The binding of RANKL to its receptor RANK is modulated by OPG, a decoy receptor of RANKL (Belibasakis and Bostanci, 2012). Decreased RANKL but increased OPG expression inhibited bone loss by reducing the number of osteoclasts in periodontitis (Teng et al., 2000). Thus, RANKL/RANK/OPG modulation is a therapeutic target for periodontitis associated bone lysis (Belibasakis and Bostanci, 2012). Bostanci et al. (2007) reported that the relative mRNA expression of RANKL/OPG was significantly different between healthy and periodontitis groups. In addition, they found that OPG expression was reduced in chronic periodontitis, whereas acute periodontitis displayed increased RANKL expression. These results indicated that the decrease in alveolar bone resorption by L. curvatus MG5246 administration is closely related to the lowering relative expression of mRNA expression of RANK/OPG in gingival tissue of periodontitis rats.
The mode of action of probiotics in oral inflammation is not fully elucidated. Some indirect antiinflammatory effects through the modulation of the gut immune system have been proposed, in addition to direct local inhibition of pathogenic bacteria in the oral cavity (Kitamoto et al., 2020). Oral administration of L. gasseri SBT2055 improved the disintegration of the periodontal ligament and alveolar bone loss by modulation of the gastrointestinal immune system. In gingival tissue, the level of proinflammatory cytokines, such as TNF-α and IL-6, was also significantly decreased in P. gingivalis-infected mice (Kobayashi et al., 2017).
In this study, L. curvatus MG5246 CFS showed anti-inflammatory effects on Pg-LPS-stimulated HGF-1 cells. Expressions of inflammatory mediators (PGE2), proinflammatory enzymes (COX-2 and MMPs), cytokines (TNF-α and IL-6), and chemokines (CXCL12 and CXCR4) and adhesion molecule (ICAM-1) were significantly downregulated. Administration of L. curvatus MG5246 effectively improved alveolar bone loss in an experimental periodontitis rat model, and the decreased bone resorption was associated with the suppression of mRNA expression RANKL/OPG in gingival tissue. Therefore, L. curvatus MG5246 has great potential to be developed as novel oral probiotic.
References
Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. Journal of Immunological Methods. 294: 49-54 (2013)
Aguilar-Toalá J. E, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A. Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology. 75: 105-114 (2018)
Allaker RP, Stephen AS. Use of probiotics and oral health. Current Oral Health Reports. 4: 309–318 (2017)
Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. Journal of Periodontology. 76: 2066-2074 (2005)
Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. Journal of Periodontology. 76: 2089-2100 (2005)
Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. Journal of Clinical Periodontology. 39: 239-248 (2012)
Bostanci N, Ilgenli T, Emingli G, Afacan B, Han B, Toz H, Berdeli A, Atilla G, McKay IJ, Hughes FJ, Belibasakis GN. Differential expression of receptor activator of nuclear factor-κB ligand and osteoprotegerin mRNA in periodontal diseases. Journal of Periodontal Research. 42: 287-293 (2007)
Buxton R. Blood agar plates and hemolysis protocols. American Society for Microbiology. 1–9 (2005)
Choi Y, Park E, Kim S, Ha J, Oh H, Kim Y, Choi KH. Alleviation of periodontal disease using Lactobacillus curvatus SMFM2016-NK. Journal of Functional Foods. 83: 104531 (2021a)
Choi Y, Park E, Kim S, Ha J, Oh H, Kim Y, Choi KH. Fermented milk with Lactobacillus curvatus SMFM2016-NK alleviates periodontal and gut inflammation, and alters oral and gut microbiota. Journal of Dairy Science. 104: 5197-5207 (2021b)
Chu ZX, Chen HQ, Ma YL, Zhou YK, Zhang M, Zhang P, Qin HL. Lactobacillus plantarum prevents the upregulation of adhesion molecule expression in an experimental colitis model. Digestive Diseases and Sciences. 55: 2505-2513 (2010)
FAO, WHO. Guidelines for the evaluation of probiotics in food, report of a joint FAO/WHO working group on drafting guideline for the evaluation of probiotic in food. World Health Organization, Geneva. (2002).
Gatej SM, Marino V, Bright R, Fitzsimmons TR, Gully N, Zilm P, Bartold PM. Probiotic Lactobacillus rhamnosus GG prevents alveolar bone loss in a mouse model of experimental periodontitis. Journal of Clinical Periodontology. 45: 204-212 (2018)
Grassi F, Cristino S, Toneguzzi S, Piacentini A, Facchini A, Lisignoli G. CXCL12 chemokine up‐regulates bone resorption and MMP‐9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. Journal of Cellular Physiology. 199: 244-251 (2004)
Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Cytokines differentially regulate ICAM‐1 and VCAM‐1 expression on human gingival fibroblasts. Clinical & Experimental Immunology. 144: 494-502 (2006)
Kitamoto S, Nagoa-Kitamoto H, Hein R, Schmidt TM, Kamada N. (2020). The bacterial connection between the oral cavity and the gut diseases. Journal of Dental Research. 99: 1021–1029 (2020)
Kim H., Kim J-S, Kim Y, Jeong Y, Kim J-E, Paek N-S, Kang C-H. Antioxidant and probiotic properties of Lactobacilli and Bifidobacteria of human origins. Biotechnology and Bioprocess Engineering. 25: 421-430 (2020)
Kim S, Choi S-I, Kim G-H, Imm J-Y. Anti-inflammatory effect of Ecklonia cava extract on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages and a periodontitis rat model. Nutrients 11: 1143 (2019)
Klare I, Konstabel C, Müller-Bertling S, Reissbrodt R, Huys G, Vancanneyt M, Swings J, Goossens H, Witte W. Evaluation of new broth media for microdilution antibiotic susceptibility testing of lactobacilli, pediococci, lactococci, and bifidobacteria. Applied and Environmental Microbiology. 71: 8982-8986 (2005)
Kobayashi R, Kobayashi T, Sakai F, Hosoya T, Yamamoto M, Kurita-Ochiai T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Scientific Reports. 7: 1-10 (2017)
Makela M, Salo T, Uitto V-J, Larjava H. Matrix metalloproteinases (MMP-2 andMMP-9) of the oral cavity: cellular originand relationship to periodontal status. Journal of Dental Research.73: 1397–1406 (1994)
Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E. Probiotic potential of Lactobacillus strains isolated from dairy products. International Dairy Journal 16: 189-199 (2006)
Morton RS, Dongari‐Bagtzoglou AI. Cyclooxygenase‐2 is upregulated in inflamed gingival tissues. Journal of Periodontology. 72: 461-469 (2001)
Nagashima H, Shinoda M, Honda K, Kamio N, Hasuike A, Sugano N, Iwata K. CXCR4 signaling contributes to alveolar bone resorption in Porphyromonas gingivalis-induced periodontitis in mice. Journal of Oral Science. 59: 571-577 (2017)
Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. Journal of Periodontology. 64: 432-444 (1993)
Oliveira LF, Salvador SL, Silva PH, Furlaneto FA, Figueiredo L, Casarin R, Messora MR. Benefits of Bifidobacterium animalis subsp. lactis probiotic in experimental periodontitis. Journal of Periodontology. 88: 197-208 (2017)
Shokryazdan P, Faseleh JM, Liang JB, Kalavathy R, Sieo CC, Ho YW. Safety assessment of two new Lactobacillus strains as probiotic for human using a rat model. PLoS ONE, 11: e0159851 (2016)
Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, Lauhio A, Pussinen PJ, Mäntylä, P. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacological Research. 63: 108-113 (2011)
Sun J, Nemoto E, Hong G, Sasaki K. Modulation of stromal cell-derived factor 1 alpha (SDF-1α) and its receptor CXCR4 in Porphyromonas gingivalis-induced periodontal inflammation. BMC Oral Health. 17: 1-8 (2017)
Sun Y, Shu R, Li CL, Zhang MZ. Gram‐negative periodontal bacteria induce the activation of Toll‐like receptors 2 and 4, and cytokine production in human periodontal ligament cells. Journal of Periodontology. 81: 1488-1496 (2010)
Szkaradkiewicz AK, Stopa J, Karpiński TM. Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on pro-inflammatory cytokine response in patients with chronic periodontitis. Archivum Immunologiae et Therapiae Experimentalis. 62: 495-500 (2014)
Teame T, Wang A, Xie M, Zhang Z, Yang Y, Ding Q, Zhou Z. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Frontiers in nutrition. 7. (2020)
Teng YTA, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. The Journal of Clinical Investigation. 106: R59-R67 (2000).
Terán LC, Coeuret G, Raya R, Zagorec M, Champomier-Vergès MC, Chaillou S. Phylogenomic analysis of Lactobacillus curvatus reveals two lineages distinguished by genes for fermenting plant-derived carbohydrates. Genome Biology and Evolution 10: 1516-1525 (2018)
Tokatlı M, Gülgör G, Bağder Elmacı S, Arslankoz İşleyen N, Özçelik F. In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. BioMed Research International. Article ID 315819 (2015)
Wang PL, Azuma Y, Shinohara M, Ohura K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochemical and Biophysical Research Communications. 273: 1161–1167 (2000)
Acknowledgements
This research was financially supported by the Ministry of Small and Medium Sized Enterprises (SEMs) and Startups (MSS), Korea, under the “Regional Star-Enterprise Development Program (R&D, S2908142)” supervised by the Korea Institute for Advancement of Technology (KIAT)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
YongGyeong Kim and Chang-Ho Kang are employees of Mediogen. The authors declare that they had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jung, JI., Kim, Y.G., Kang, CH. et al. Effects of Lactobacillus curvatus MG5246 on inflammatory markers in Porphyromonas gingivalis lipopolysaccharide-sensitized human gingival fibroblasts and periodontitis rat model. Food Sci Biotechnol 31, 111–120 (2022). https://doi.org/10.1007/s10068-021-01009-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-01009-4