Abstract
This study characterized the blends of corn starch with potato amylopectin (PAP) and PAP hydrolysates treated with branching enzyme (BR), pullulanase (PL), and BR-BL cocktail. PAP/PAP hydrolysates were deposited or bound (particularly in intact and PL-treated PAPs) on the surfaces of corn starch granules. Although PAP/PAP hydrolysates rarely affect the X-ray diffraction patterns of the blends, their relative crystallinities decreased. Relative to native starches, the swelling power was higher for all blends. Solubility was higher for normal starch-based blends but lower for waxy starch-based blends. All blends exhibited higher gelatinization temperatures and lower gelatinization enthalpies. Although the pasting viscosities of blends with intact PAP were higher than those of native starches, the opposite trends were found in blends with BR-, PL-, and BR-PL cocktail-treated PAPs. Overall, the PAP structures diversified the characteristics of the corn starch-PAP blends. BR- and BR-PL cocktail-treated PAPs could function as stabilizers for stable paste consistency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starch is a natural polysaccharide that is widely used in the food and non-food industries (Hong et al., 2020). Native starch has some defects, such as cold-water insolubility, high opaqueness, unstable consistency, and rapid retrogradation/gelling tendency of its paste (Lee and Kim, 2020). In addition to starch sweeteners (starch hydrolysates), chemically modified starches are much more prevalent in processed food applications than are their native counterparts (Hong et al., 2020; Lee and Kim, 2020). However, due to the reluctance of consumers to eat chemically synthesized food additives, food manufacturers have shown great interest in clean-label starches as substitutes for chemically modified starches (Park and Kim, 2021; Radeloff and Beck, 2016). The marketing term ‘clean label’ generally applies to foods and ingredients prepared with minimal processing and based on natural sources without synthetic chemical additives (Busken, 2015). Thus, there have been many studies in which native starch has been physically and enzymatically modified without chemicals (i.e., reacting agents and catalysts) or chemical processes (Park and Kim, 2021; Radeloff and Beck, 2016). Even though these do not completely replace the physical and rheological functionalities of chemically modified starches, the utilization of clean-label starch products has increased in the food industry (Busken, 2015; Park and Kim, 2021; Radeloff and Beck, 2016).
In addition to physical and enzymatic starch modifications, starch blending is considered a simple and economical method to prepare clean-label starch products (Waterschoot et al., 2015b). There are many published studies of the impact of blending ratios on the characteristics of blends of native starches with different granule sizes and shapes, amylose content, and physical properties (Fonseca-Florido et al., 2017a; 2017b; Lemmens et al., 2021; Ortega-Ojeda and Eliasson, 2001; Park et al., 2009; Puncha-arnon et al., 2008; Waterschoot et al., 2014, 2015a; 2015b, 2016; Yadav et al., 2016; Zhang et al., 2011). These results demonstrate that either additive or non-additive behavior of individual starch properties in starch blends diversified their swelling, thermal, pasting, and rheological characteristics (Puncha-arnon et al., 2008; Waterschoot et al., 2014). Furthermore, the authors of these studies commonly concluded that starch blending could complement or compensate for the drawbacks of native starch, which are undesirable for processed foods. Nevertheless, the application of starch blends to food systems is case-by-case owing to their unpredictable properties depending on the starch combination and blending ratio. There are too many intrinsic factors (i.e., granule size, relative crystallinity, amylose content, amylose leaching, amylopectin branch-chain length, granule rigidity) as well as extrinsic factors (i.e., starch combination, blending ratio, starch concentration) that affect starch blend properties (Waterschoot et al., 2015b). In addition, each starch in the starch blend independently swells, gelatinizes, and pastes (Ortega-Ojeda and Eliasson, 2001; Puncha-arnon et al., 2008). This makes it difficult to establish mechanisms to explain the interaction between different starches in the starch blend (Waterschoot et al., 2015b).
Potato starch, as either a major or minor component in starch blends, plays a role in increasing swelling and peak viscosity, early viscosity development, and rapid gelatinization onset (Ortega-Ojeda and Eliasson, 2001; Park et al., 2009; Puncha-arnon et al., 2008; Waterschoot et al., 2014). The largest granule size of potato starch greatly affects its water holding capacity and swelling power (Puncha-arnon et al., 2008; Waterschoot et al., 2015b, 2016; Zhang et al., 2011). This can severely restrict the utilization of water in the swelling and gelatinization of the counterpart starch in the starch blend sharing available water, ultimately decreasing the overall properties of the starch blend (Park et al., 2009). Moreover, the blends of granular potato starch and other starches depending on blending ratios reported in the literature still reveal dramatic viscosity changes (Puncha-arnon et al., 2008; Waterschoot et al., 2015b, 2016; Zhang et al., 2011) which are undesirable for the food processing process.
The objective of this study was to investigate the effect of potato amylopectin and its enzymatic hydrolysates on the physicochemical properties of corn starch-potato amylopectin blends. This study used waxy potato starch to exclude the impact of amylose of potato starch on the blend properties. Waxy potato starch granules were pre-gelatinized to remove their granular form, after which potato amylopectin was treated with and without the branching enzyme, pullulanase, and the branching enzyme-pullulanase cocktail to modify the potato amylopectin structures. Pullulanase debranches potato amylopectin, generating linear and less-branched α-glucans from short to long lengths (Luo et al., 2021). The branching enzyme cleaves potato amylopectin into amylopectin cluster units (Lee et al., 2013). Building on these results, this study can obtain better insights into the impacts of non-granular starch and amylopectin structure on starch blend properties.
Materials and methods
Materials
Corn starch (25.6% amylose for normal starch, 1.5% amylose for waxy starch), and waxy potato starch (WPS; 1.6% amylose) were obtained from Daesang Co. (Icheon, Korea) and Avebe Food (Veendam, Netherlands), respectively. Food-grade branching enzymes (BR; Branchzyme, from Rhodothermus obamensis, 25,000 BEU/g) and food-grade pullulanase (PL; Promozyme D2, from Bacillus subtilis, 1350 NPUN/g) were purchased from Daejong Co. (Seoul, Korea). The other chemicals and reagents used in this study were of analytical grade.
Preparation of corn starch-potato amylopectin blend
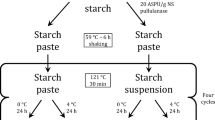
WPS (100 g, d.b) was dispersed in deionized water (DIW; 900 g) and heated for 30 min in a boiling water bath, followed by cooling to 65 °C. The enzyme solution (0.1%, based on dry WPS weight) of the BR, PL, and BR-PL cocktail (50:50, w/w) was added to the clear WPS paste and incubated for 18 h at 65 °C under continuous stirring (100 rpm) using a high-viscosity overhead stirrer (MS-3060, Misung Scientific Co., Ltd., Yangju, Korea) equipped with an anchor-type shaft. Upon completion of the enzyme reaction, the WPS solutions were heated for 30 min in a boiling water bath to inactivate the enzymes, followed by cooling to 25 °C. The corn starch was then mixed with the resultant WPS solution to a mixing ratio of 9:1 (w/w, based on a total solid content) and adjusted to pH 5 with 0.1 M HCl or 0.1 M NaOH as stirred for 30 min using a high-viscosity overhead stirrer (MS-3060, Misung Scientific Co., Ltd., Yangju, Korea). The resultant dispersions were freeze-dried, ground, and passed through an 80-mesh sieve. Native normal and waxy corn starches were treated identically without pre-gelatinized WPS, referred to as NST and WST, respectively. The blend of normal (waxy) corn starch and pre-gelatinized WPS without enzyme treatment was designated as NUT (WUT). The blends of normal (waxy) corn starch and the pre-gelatinized WPS subjected to BR, PL, and BR-PL cocktail treatments were designated as NBR (WBR), NPL (WPL), and NBP (WBP), respectively.
Scanning electron microscopy (SEM)
The starch sample was dusted on an aluminum stub equipped with double-sided carbon tape and coated for 3 min with gold using a sputter coater operated at 20 mA under vacuum. The specimens were viewed using a scanning electron microscope (SNE-3000 MB, SEC Co., Ltd., Suwon, Korea) at an accelerating voltage of 30 kV and 1,000 × magnification.
Phosphorus content
The starch sample (2 g, d.b) was dispersed in a 50-mL conical tube with DIW (30 mL) and shaken for 30 min using a wrist action shaker (200 strokes/min; ASA-026–12, Asia Testing Machine Co., Gwangju, Korea). The mixture was centrifuged for 20 min at 2,500 × g, after which the supernatant was discarded. The recovered precipitate was dispersed again with fresh DIW (30 mL). This washing procedure was repeated a total of three times. The precipitate from the final washing was dried at 45 °C and ground using a mortar and pestle. The phosphorus content of starch samples before and after washing was quantified using inductively coupled plasma-atomic emission spectroscopy (ICP-AEC), as outlined by Anderson (1996).
X-ray diffraction (XRD)
The XRD patterns of the starch samples were obtained using an X-ray diffractometer (D8 Advance; Bruker AXS GmbH, Karlsruhe, Germany) operated at 40 mA and 40 kV. The starch samples were scanned from 4° to 40° 2θ at a scan speed of 2°/min. The relative crystallinity was presented as the percentage of the sum of the crystalline peak areas to the total diffractogram area (Hong et al., 2020).
Swelling power and solubility
The swelling power and solubility of the starch samples were analyzed at 70 °C as outlined by Hong et al. (2020). Solubility and swelling power were calculated as follows:
Thermal properties
The gelatinization properties of the starch samples were investigated in the temperature range of 25–150 °C using a differential scanning calorimeter (DSC4000, PerkinElmer, Inc., Waltham, MA, USA), as outlined by Bae et al. (2020). The scanned pan was then stored at 4 °C for 4 weeks, after which it was scanned again under identical DSC conditions. The degree of retrogradation was estimated based on the percentage of melting to gelatinization enthalpy (Bae et al., 2020).
Rapid visco analyzer (RVA)
Pasting viscosity profiles and parameters of the starch samples were investigated with a Rapid Visco Analyzer (RVA-3D, Newport Scientific Pty. Ltd., New South Wales, Australia) according to the programmed temperature profile and RVA operating procedure outlined by Hong et al. (2020).
Statistical analysis
All native starches and blends were prepared three times, and all characteristic measurements were repeated twice for each starch sample. One-way analysis of variance (ANOVA) was conducted for all obtained data, and the significant differences in characteristics among starch samples were investigated using Tukey’s HSD multiple range tests at p < 0.05. All statistical computations and analyses were performed using Minitab 18 (Minitab Inc., State College, PA, USA).
Results and discussion
Potato amylopectin as a blending source
In this study, potato amylopectin (PAP) was used to pre-gelatinize WPS granules and PAP hydrolysates treated with BR (branching enzyme), PL (pullulanase), and BR-PL cocktail as blending sources. The molecular weight (Mw) distributions of PAP and PAP hydrolysates are presented in Fig. S1 (supplementary data). WPS exhibited only a single peak eluted at an early retention time, indicating a greater Mw of PAP. The BR-treated PAP showed a peak shape similar to that of intact PAP at the delayed retention time, consistent with the results reported by Lee et al. (2013). This results from the hydrolysis of PAP to amylopectin cluster units by BR, which hydrolyzes the α-1,4 glycosidic linkage of amylopectin B2 and longer branch-chains connecting amylopectin clusters (Lee et al., 2013). The PL-treated PAP exhibited a broad range of chromatograms with four main peaks, consistent with the results reported by Luo et al. (2021). This is due to the varied sizes of amylopectin branch-chains released from PAP by hydrolysis of its α-1,6 glycosidic linkage by the PL action (Luo et al., 2021). The chromatogram of the BR-PL cocktail-treated PAP was very similar in shape and retention time to that of the BR-treated PAP, implying inhibition of the PL debranching action. BR can generate new α-1,6-linked branch-chains by glucosyltransferase action following α-1,4 glycosidic linkage cleavage, often forming highly branched cyclic maltodextrins (Lee et al, 2013). Thus, in this study, BR might mainly form a self-branched cyclic structure in PAP clusters, protecting PAP clusters from PL attacks. Accordingly, the enzymatic treatment of PAP can provide a better understanding of the impact of PAP on corn starch-PAP blend properties. In addition, the characteristics of potato starch are greatly influenced by pH due to the presence of anionic monophosphate groups naturally esterified on its amylopectin (Lim et al., 2003). Thus, the corn starch and corn starch-PAP suspensions were adjusted to pH 5 to exclude the impact of pH on the characteristics of the corn starch-PAP blends.
Morphology
The SEM images of the native starches and blends are shown in Fig. 1. No significant difference in granule morphology was observed between normal and waxy corn starches in any of the treatments. Native corn starch (NST and WST) presented polygonal and round shapes with a smooth surface, consistent with observations by Fonseca-Florido et al. (2017a; 2017b). In addition, irregular granule fragments, not shown in the literature (Fonseca-Florido et al., 2017a; 2017b), were occasionally observed in the NST and WST. These may result from pulverization of starch blocks freeze-dried after pH adjustment of native starch suspensions using a blender. In the corn starch-PAP blends, NUT and WUT had polygonal and irregular granules with rough and uneven surfaces, where PAP appeared to randomly adhere to discrete film forms with varied thicknesses. Irregular granule fragments of varied sizes were more frequently present in NUT and WUT than in their native counterparts. These morphological characteristics did not differ between blends with BR-treated (NBR and WBR) and BR-PL cocktail-treated (NBL and WBL) PAPs. In the blends with PL-treated PAP (NPL and WPL), however, the surfaces of their granules were relatively smoother than those of other blends, contrary to the expectation that corn starch granules were covered by starch microparticles, self-assembled aggregates of PAP branch-chains debranched by PL (Oh et al., 2021). Furthermore, individual starch microparticles or irregular granule fragments were rarely found in the NPL and WPL. This might be because individual PAP branch-chains and less branched PAP (caused by PL debranching action) are evenly laminated on the surface of corn starch granules. Overall, the corn starch granules in the blends were coated with PAP, regardless of the PAP structure.
SEM images of native and waxy potato amylopectin-incorporated corn starches. NST and WST indicate normal and waxy corn starches, respectively. NUT, NBR, NPL and NBP (WUT, WBR, WPL, and WBP) indicate NSTs (WSTs) incorporated with untreated, branchzyme-treated, pullulanase-treated and branchzyme-pullulanase cocktail-treated waxy potato amylopectin, respectively
Phosphorus content
PAP is naturally phosphorylated in the form of phosphate monoester at the C-3 and C-6 positions of anhydroglucose units (McPherson and Jane, 1999; Wikman et al., 2011). Phosphate groups are mainly distributed on amylopectin cluster-interconnected long amylopectin B chains deposited within the amorphous lamellae of amylopectin (McPherson and Jane, 1999; Wikman et al., 2011). These natural phosphate groups affect the swelling, gelatinization, and pasting properties of potato starch (McPherson and Jane, 1999). The net phosphorus content (determined by subtraction of the native starch phosphorus content from the blend phosphorus content) of the blends before and after washing are depicted in Table 1. Before washing, the phosphorus content ranged from 30.1 to 56.5 ppm for the NST-based blends and from 41.3 to 58.4 ppm for WST-based blends. Although only trace amounts of phosphorus were found, significant differences were present among the blends. This might result from the heterogeneity of the phosphorus contents within the WPS granules. After washing, phosphorus was still detected in all the blends, although the phosphorus content decreased. This might indicate that the phosphate groups of PAP are esterified with hydroxyl groups of corn starch molecules (Park et al., 2004), integrating PAP with corn starch granules. Meanwhile, the phosphorus residues of NBR and NBL were much smaller than those of NUT and NPL. This pattern did not differ for the WST-based blends. These results indicate that BR- and BR-PL cocktail-treated PAPs may be much less incorporated with corn starch granules. This may be explained by the BR-derived cyclic PAP cluster structure (Lee et al., 2013), less contact with corn starch granules, and consequently, reduced binding of the granules.
X-ray diffraction (XRD)
The XRD patterns and relative crystallinities of the native starches and blends are shown in Fig. 2. Native starches and blends exhibited typical A-type crystal packing arrangements, as reported for cereal starches (Bae et al., 2020). In addition, no new peak was found in any of the X-ray diffractograms, indicating that PAP and its hydrolysates blended with native corn starches formed no crystal structures. Furthermore, the relative crystallinity of all the blends was lower than that of their respective native starches. This may be attributed to the dilution of corn starch crystals by PAP and its hydrolysates. Overall, the layers of PAP and its hydrolysates enclosing corn starch granules as well as their irregular aggregates may comprise amorphous structures.
XRD patterns (relative crystallinity) of native and waxy potato amylopectin-incorporated starches from normal (A) and waxy corn (B). NST and WST indicate normal and waxy corn starches, respectively. NUT, NBR, NPL and NBP (WUT, WBR, WPL, and WBP) indicate NSTs (WSTs) incorporated with untreated, branchzyme-treated, pullulanase-treated and branchzyme-pullulanase cocktail-treated waxy potato amylopectin, respectively
Swelling power and solubility
The precipitate and supernatant were incompletely separated by centrifugation from WST heated to over 70 °C. The swelling power and solubility of native starches and blends were measured at 70 °C and are presented in Table 2. The swelling power of the blends was higher than that of their native counterparts, regardless of the starch source. This result may be attributed to the enhanced integrity of swollen starch granules by PAP and its hydrolysates covering starch granules, as shown in Fig. 1 (Kang et al., 2020). In addition, the hydration of amorphous PAP/its hydrolysate covers for starch granules may contribute partially to the increased swelling power of the blends. In a blend of starch with either linear or shortly branched hydrocolloids, the hydrocolloids enhance starch swelling due to their water-holding capacity and protective effect against swollen granule collapse (Gularte and Rosell, 2011; Kang et al., 2020).
The solubility of the NST-based blends was significantly higher than that of the NST. This may be due to the solubilization of PAP and its hydrolysates on the surface of NST granules rather than the leaching of starch molecules from swollen granules. This explanation is further supported by the fact that even though NST and NBR have similar swelling powers, higher solubility is found in NBR. Among the NST-based blends, solubility was highest for NBR and NBL and lowest for NPL. This may result from structural differences between the BR-treated and PL-treated PAPs. As previously proposed (see the “Phosphorus content” section), the BR-derived PAPs in NBR and NBL have the low Mw and highly-branched cyclic form, facilitating their detachment from blend granules (as supported by the lowest phosphorus contents of both blends after washing; Table 1) and solubilization. The linear short- to long-chain α-glucans in the PL-treated PAP form a relatively uniform matrix that tightly attaches to the granules (as partially supported by the higher phosphorus content of NPL after washing; Table 1), hindering the solubilization of the mass of granules. This is further supported by the much smoother NPL surface relative to NBR and NBL (Fig. 1). In contrast to NST-based blends, the highest solubility was found in WST. The lack of amylose in WST weakens swollen granule integrity, resulting in dramatic leaching of starch molecules and easy rupture of swollen granules (Bae et al., 2020). The lower solubility of the WST-based blends compared to WST may be attributed to the fact that based on their higher swelling power, PAP and its hydrolysates on WST granules (Fig. 1) reinforce the integrity of swollen WST granules, restricting starch leaching and preventing swollen granule collapse. Within the WST-based blends, their solubility appears to follow the pattern of solubility of the NST-based blends. This result can also be explained by the properties of the NST-based blends, as described above.
Thermal property
The gelatinization properties and degree of retrogradation (DR) of the native starches and blends are shown in Table 3. The blends of granular starches from different sources commonly showed two endothermic peaks originating from the respective starches (Ortega-Ojeda and Eliasson, 2001; Waterschoot et al., 2015a). In this study, only a single peak was observed for all the blends. This is due to the removal of the crystalline structure and granular form by pre-gelatinization and enzymatic treatment of WPS. There was no distinct pattern in the gelatinization temperatures of the NST- and WST-based blends depending on the PAP structures. Nevertheless, relative to native starches (NST and WST), the NST- and WST-based blends had higher gelatinization temperatures and lower gelatinization enthalpies. This may be attributed to the restriction of water available for corn starch gelatinization due to the preferred water absorption of the amorphous PAP and its hydrolysates on corn starch granules (Kang et al., 2020). Compared to the gelatinization properties of native starches and blends, the melting properties obtained from their pastes stored at 4 °C for 4 weeks following gelatinization decreased (Table S2 in the supplementary data), similar to those of retrograded starches (Bae et al., 2020; Kang et al., 2020; Ortega-Ojeda and Eliasson, 2001). Whereas the DR of NUT and WUT with intact PAP was higher than that of their respective native counterparts (Ortega-Ojeda and Eliasson, 2001), the opposite trends were observed in other blends. This may be because re-association, re-alignment, and in turn, re-crystallization of amylopectin and amylose from corn starch were restricted by the structured PAP hydrolysates created by BR, PL, and BR-PL cocktail treatments, such as the low Mw, highly branched cyclic amylopectin clusters, and phosphorylated α-glucans.
Pasting viscosity
Pasting viscosity profiles and parameters of native starches and blends are presented in Fig. 3 and Table S3 (supplementary data). Both NUT and WUT with intact PAP (non-granular WPS) had higher pasting viscosities at all points over the temperature profile than their respective native counterparts (NST and WST). These results agree with the increased pasting viscosities of blends of granular corn starch with granular potato starch at blending ratios of 7.5:2.5–9:1 (w/w) (Park et al., 2009; Waterschoot et al., 2014). In previous studies, it was found that the pasting behavior of blends of granular corn and potato starches was non-additive, depending on the starch pasting viscosity at given blending ratios (Park et al., 2009; Waterschoot et al., 2014). However, in the present study, the increased pasting viscosities of NUT and WUT may be explained by the fact that the maximum swelling of corn starch granules can be achieved because of the presence of intact PAP molecules deposited and bound on their surface. In addition, although higher pasting viscosities of blends with PAP hydrolysates were expected, based on their higher swelling powers (Table 2), their pasting viscosities were lower over the temperature profile than those of their respective native starches. Although similar results have not been reported in the literature, this pattern may be explained as follows. The continued shear during RVA operation might tear off the low Mw of PAP hydrolysates deposited on the granule surface. The detached PAP hydrolysates and corn starch granules share available water, restricting the utilization of water in corn starch pasting. Thus, corn starch granules do not fully hydrate and swell, and thus fail to fully develop their pasting viscosities. In addition, the less swollen starch granules may still remain rigid, enhancing their stability on continued shear, as supported by the lower breakdown viscosities of blends with BR-, PL-, and BR-PL cocktail-treated PAPs (Table S3 in the supplementary data). Moreover, the blends with PL-treated PAP exhibited higher pasting viscosities than the blends with BR- and BR-PL cocktail-treated PAPs. This might be due to the coexistence of low Mw PL-treated PAPs deposited and bound on the surface of corn starch granules.
Pasting viscosity profiles of native and waxy potato amylopectin-incorporated starches from normal (A) and waxy corn (B). NST and WST indicate normal and waxy corn starches, respectively. NUT, NBR, NPL and NBP (WUT, WBR, WPL, and WBP) indicate NSTs (WSTs) incorporated with untreated, branchzyme-treated, pullulanase-treated and branchzyme-pullulanase cocktail-treated waxy potato amylopectin, respectively
In summary, this study has shown that PAP structures have a number of effects on the physicochemical properties of corn starch-PAP blends. Non-granular WPS (intact PAP) enhanced the solubility, swelling power, and pasting viscosity of the corn starch-potato starch blend at a given blending ratio, similar to those of blends with granular potato starch. Enzymatically hydrolyzed PAPs increased the solubility and swelling power but decreased the pasting viscosities of the blends. The gelatinization temperatures of the blends increased, and their gelatinization enthalpies decreased. These results confirm that the molecular structure of starch is also one of the main factors affecting the characteristics of starch blends. Overall, the results suggest that non-granular or amorphous starch in the starch blends behaves like non-starch hydrocolloids. Granular starches may not be necessary to diversify native starch properties by blending starches. In addition, PAP hydrolysates can delay retrogradation and stabilize the consistency of starch pastes. In particular, the branching enzyme-derived PAP could be used as a stabilizer to modulate paste consistency.
References
Anderson KA. Micro-digestion and ICP–AEC analysis for the determination of macro and micro elements in plant tissues. Atomic Spectroscopy. 17: 30-33 (1996)
Bae JE, Hong JS, Baik MY, Choi HD, Choi HW, Kim HS. Impact of starch granule-associated surface and channel proteins on physicochemical properties of corn and rice starches. Carbohydrate Polymers. 250: 116908 (2020)
Busken DF. Cleaning it up-What is a clean label ingredient? Cereal Foods World. 60: 112-113 (2015)
Fonseca-Florido HA, Hernández-Ávilab J, Rodríguez-Hernández AI, Castro-Rosas J, Acevedo-Sandoval OA, Chavarria-Hernández N, Gomez-Aldapa CA. Thermal, rheological, and mechanical properties of normal corn and potato starch blends. International Journal of Food Properties. 20: 611-622 (2017a)
Fonseca-Florido HA, Castro-Rosas J, Hernández-Hernández E, Mata-Padilla JM, Velazquez G, Ávila-Orta CA, Rodríguez-Hernández AI, Gomez-Aldapa CA. Structural properties of waxy corn and potato starch blends in excess water. International Journal of Food Properties. 20: S353-S365 (2017b)
Gularte MA, Rosell CM. Physicochemical properties and enzymatic hydrolysis of different starches in the presence of hydrocolloids. Carbohydrate Polymers. 85: 237-244 (2011)
Hong JS, Chung HJ, Lee BH, Kim HS. Impact of static and dynamic modes of semi-dry heat reaction on the characteristics of starch citrates. Carbohydrate Polymers. 233: 115853 (2020)
Kang EJ, Bae JE, Hong JS, Choi HD, Choi HW, Lee JK, Kim HS, Park J. Characteristics of wheat starch-pectin hydrolysate complexes by dry heat treatment. Food Science and Biotechnology. 29: 1389-1399 (2020)
Lee H, Kim HS. Pasting and paste properties of waxy rice starch as affected by hydroxypropyl methylcellulose and its viscosity. International Journal of Biological Macromolecules. 153: 1202-1210 (2020)
Lee BH, Yan L, Phillips RJ, Reuhs BL, Jones K, Rose DR, Nichols BL, Quezada-Calvillo R, Yoo SH, Hamaker BR. Enzyme-synthesized highly branched maltodextrins have slow glucose generation at the mucosal α-glucosidase level and are slowly digestible in vivo. PLOS ONE. 8: e59745 (2013)
Lemmens E, Waterschoot J, Smolders E, Delcour JA. Impact of mineral ions and their concentrations on pasting and gelation of potato, rice, and maize starches and blends thereof. Starch-Stärke. 73: 2000110 (2021)
Lim HS, BeMiller JN, Lim ST. Effect of dry heating with ionic gums at controlled pH on starch paste viscosity. Cereal Chemistry. 80: 198-202 (2003)
Luo K, Ryu J, Jeong KB, Kim HS, Kim YR. Colorimetric assay for the determination of molecular weight distribution and branching characteristics of starch hydrolysates. Carbohydrate Polymers. 251: 117046 (2021)
McPherson AE, Jane J. Comparison of waxy potato with other root and tuber starches. Carbohydrate Polymers. 40: 57-70 (1999)
Oh SM, Park CS, Kim YR, Baik MY. Preparation and characterization of self-assembled short-chain glucan aggregates (SCGAs) derived from various starches. Food Hydrocolloids. 114: 106517 (2021)
Ortega-Ojeda FE, Eliasson AC. Gelatinization and retrogradation behavior of some starch mixtures. Starch-Stärke. 53: 520-529 (2001)
Park S, Kim YR. Clean label starch: production, physicochemical characteristics, and industrial applications. Food Science and Biotechnology. 30: 1-17 (2021)
Park EY, Kim HN, Kim JY, Lim ST. Pasting properties of potato starch and waxy mize starch mixtures. Starch-Stärke. 61: 352-357 (2009)
Park DP, Sung JH, Kim CA, Choi HJ, Jhon MS. Synthesis and electrorheology of potato starch phosphate. Journal of Applied Polymer Science. 91: 1770-1773 (2004)
Puncha-arnon S, Pathipanawat W, Puttanlek C, Rungsardthong V, Uttapap D. Effects of relative granule size and gelatinization temperature on paste and gel properties of starch blends. Food Research International. 41: 552-561 (2008)
Radeloff MA, Beck RHF. "Clean label"-Starches and their functional diversity. Sugar Industry/Zuckerindustrie. 141: 209-215 (2016)
Waterschoot J, Gomand SV, Delcour JA. Impact of swelling power and granule size on pasting of blends of potato, waxy rice and mize starches. Food Hydrocolloids. 52: 69-77 (2016)
Waterschoot J, Gomand SV, Delcour JA, Goderis B. Direct evidence for the non-additive gelatinization in binary starch blends: A case study on potato starch mixed with rice or maize starches. Food Hydrocolloids. 50: 137-144 (2015a)
Waterschoot J, Gomand SV, Fierens E, Delcour JA. Starch blends and their physicochemical properties. Starch-Stärke. 67: 1-13 (2015b)
Waterschoot J, Gomand SV, Willebrords JK, Fierens E, Delcour JA. Pasting properties of blends of potato, rice and maize starches. Food Hydrocolloids. 41: 298-308 (2014)
Wikman J, Larsen FH, Motawia MS, Blennow A, Bertoft E. Phosphate esters in amylopectin clusters of potato tuber starch. International Journal of Biological Macromolecules. 48: 636-649 (2011).
Yadav RB, Kumar N, Yadav BS. Characterization of banana, potato, and rice starch blends for their physicochemical and pasting properties. Cogent Food & Agriculture. 2: 1127873 (2016)
Zhang Y, Gu Z, Hong Y, Li Z, Cheng Li. Pasting and rheological properties of potato starch and maize starch mixtures. Starch-Stärke. 63: 11-16 (2011)
Acknowledgements
This work was supported by Kyonggi University Research Grant 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, R.J., Kim, HS. Development and characterization of potato amylopectin-substituted starch materials. Food Sci Biotechnol 30, 833–842 (2021). https://doi.org/10.1007/s10068-021-00919-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-00919-7