Abstract
Fermented vegetable–fruit beverages are a popular fermented food, with many potential health benefits. In this study, two commercial Lactobacillus plantarum strains were selected to ferment a beverage containing apples, pears, and carrots. The metabolites and antioxidant activities were examined during the fermentation process. Results showed that lactic acid and acetic acid accumulated gradually, whereas malic acid decreased. Glucose and fructose increased from 0.48 and 14.8 g/L to 7.7 and 20.8 g/L, respectively, while sucrose decreased slightly. Ascorbic acid also increased continuously during the fermentation to 90.74 mg/100 mL. DPPH and ABTS radical scavenging activity and FRAP reached their maximum value after 4–8 days. The accumulation of TPC, TFC, and SOD reached their maximum value on the 8th day of fermentation. Our study revealed that the L. plantarum-fermented vegetable–fruit beverage showed significant antioxidant activity, which is helpful in evaluating the fermentation end-point and developing a high-quality fermented beverage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fermented beverage, a traditional fermented food, such as yoghurt, fruit vinegar, and fruit wine, is consumed widely throughout the world. With increasing awareness of the relationship between diet and health, non-alcoholic beverages have shown increasing popularity compared to alcoholic beverages, and the demand for fermented beverage products has created a need for new non-dairy substrates for their production. Many common types of available substrates (such as rice, emmer, vegetables, fruits, etc.) could be used for preparation of fermented beverages (Coda et al., 2011; Ghosh et al., 2015; Pereira et al., 2011). For vegetables and fruits, sugars, organics, and minerals are widely distributed and constitute the major form of soluble solids in vegetable–fruit beverages. They can be metabolized to other compounds by bacteria during the fermentation process for quality enrichment, and the final fermented beverage is beneficial for health. Therefore, vegetable–fruit beverages not only are characterized by a pleasant aroma and taste but show many health-promoting benefits, such as free radical scavenging activities and improvement of gastrointestinal health (Marsh et al., 2014; Somanah et al., 2014). These advantages may be due to raw material and metabolites of lactic acid bacteria, such as vitamins, organic acids, and phenolics (Ghosh et al., 2015). Lactobacillus plantarum is frequently used for vegetable and fruit fermentation (Chang and Chang, 2010; Simsek et al., 2014). Some selected L. plantarum strains are also used to develop functional beverages (Connelly, 2008; Salmerón et al., 2015). Previous studies on fermented beverages were mainly focused on optimization of the fermentation process (strains, matrix ratios, and temperature) (Pereira et al., 2011; Prado et al., 2015; Vanajakshi et al., 2015) and analysis of functional activities (Chang and Chang, 2010; Simsek et al., 2014). However, only a few studies have been reported the metabolism of the starter culture or performed physicochemical characterization of the products during fermentation (Đorđević et al., 2010; Filannino et al., 2014; Ghosh et al., 2015). The physicochemical characteristics of vegetable–fruit beverages during the fermentation process remain unclear. Furthermore, analysis of the metabolic characteristics of L. plantarum strains in the long-term fermentation of different fruit and vegetable mixtures has not been studied.
In this study, we decided to use apples, pears, and carrots as they are common fruits and vegetable, which could be obtained easily and have abundant nutrients. Several previous studies showed that they are suitable material for fermented beverages. Apple juice was shown to be the best substrate for lactic acid bacteria growth, considering bacterial viability and overall acceptance by the test (Espirito-Santo et al., 2015). Di Cagno et al. (2008) investigated carrots, French beans and marrows fermented by lactic acid bacteria and found that the fermented material had a significantly higher total concentration of vitamin C.
Therefore, the purpose of this study was to explore the fermentation characteristics of beverages containing a mixture of apples, pears, and carrots fermented by two commercial L. plantarum strains. The metabolites and physicochemical characteristics as well as antioxidant activity were examined during the fermentation process. Altogether, this study improves our knowledge of the fermentation process of vegetable–fruit-fermented beverages from the perspective of the substrates and metabolism.
Materials and methods
Raw materials
Apples, pears, carrots, and sugar were purchased from a local supermarket in Shanghai, China.
Bacterial cultures
In this study, we selected freeze-dried commercial cultures of L. plantarum 115 (Du Pont, USA) and L. plantarum Vege Start 60 (Chr. Hansen, Denmark) for the fermentation of vegetable–fruit beverages. Strains were activated in MRS (de Man, Rogosa, and Sharpe) broth at 35 °C, 100 rpm for 12 h.
Preparation of the fermented beverage
The raw materials were washed, drained and cut into small pieces, then crushed with 20% (m/v) sugar solution (1:2, v/v). In order to ensure the consistency of the raw materials, we bought the same varieties of vegetable/fruit each time, and prepared the raw materials in strict accordance with the fixed proportion. The compositions of vegetable fruit beverages were shown in Table 1. The mixture was pasteurized at 65 °C for 30 min and then cooled to 35–40 °C for inoculation of the starter cultures. The mixture was fermented at 35 °C for 14 days.
Microbial growth during the fermentation
Colony-counting method was used to determine the growth of the microbial (lactic acid bacteria) during the fermentation process. Briefly, samples of fermented vegetable–fruit beverage were withdrawn at various time intervals, and then immediately for the microbial cultivation. In a series of dilution, samples were inoculated onto MRS ager-plate medium by spread plate method. After inoculation, the plates were inverted and incubated at 37 °C for 72 h in an anaerobic incubator. The colony counts were then calculated and results were presented as log10-transformed data. The colonies with a surrounding clear zone were counted as total lactic acid bacteria, and the average from the three plates recorded for each serial dilution.
pH, total titratable acidity (TTA) and acidification kinetics
pH values were determined using a pH meter (FE20, Mettler Toledo, Switzerland). TTA was determined by homogenizing the sample (5 mL) with 40 mL of distilled water and then titrated with 0.1 M NaOH using phenolphthalein (0.1%, w/v in 95% ethanol) as an indicator. TTA was defined as the amount of lactic acid (g) per 100 mL of fermented sample. The acidification kinetics was modeled according to the Gompertz equation as modified by Zwietering et al. (1990):
where y is the acidification value expressed as dpH/dt (units of pH/h) at time t; k is the initial level of the dependent variable to be modeled (pH units); A is the difference in pH (units) between inoculation and the stationary phase (ΔpH); Vmax is the maximum acidification rate expressed as dpH/h; λ is the length of the lag phase expressed in hours; and t is the time.
Quantification of sugars and organic acids by HPLC
The concentration of sugars (sucrose, glucose, and fructose) and organic acids (lactic, acetic, acetic acid, and malic acid) was determined using high performance liquid chromatography (HPLC, LC-10A, Shimadzu). An ultraviolet (UV) detector and a refractive index detector (RID) were used for determining the levels of organic acids and sugars, respectively. Lactic acid, acetic acid, and malic acid were determined as previously described (Molina et al., 2009). Briefly, organic acids were measured at a UV wavelength of 210 nm, with a C18 column (250 × 4.6 mm, Wondasic, Japan). The column was operated at 25 °C using 0.1% (v/v) phosphoric acid as the mobile phase at a flow of 1.0 mL/min. For sugars, a C-NH2 column (NH2P-50, Shodex, Japan) was equipped with the HPLC system and the mobile phase was acetonitrile and water (70:30). The temperature was kept at 30 °C and a constant flow rate of 1.0 mL/min was maintained (Ghosh et al., 2015). Sample (20 μL) was injected into the chromatograph. Sugars and organic acids were identified and quantified by comparison of their retention times and peak areas to that of the standards.
Determination of vitamin levels
Hydro-soluble vitamins were analyzed by HPLC (Agilent, 1200LC, USA) as described (Ghosh et al., 2015). A C18 column (250 × 4.6 mm, Wondasic, Japan) was used, and the mobile phase was 0.02 M KH2PO4 (pH 2.5) and acetonitrile (A). The acetonitrile concentration was 0.6%, and stop time was 20 min. The temperature was kept at 15 °C and a constant flow rate of 1.0 mL/min was maintained. Sample (20 μL) was injected into the chromatograph and measured at a UV wavelength of 204 nm.
Superoxide dismutase activity assay
The determination of superoxide dismutase (SOD) activity was carried out in triplicate during fermentation. The superoxide anion scavenging activity of the fermented beverage was determined by the xanthine oxidase method, using the T-SOD assay kit (Jiancheng, Nanjing, China). This method is based on the xanthine and xanthine oxidase reaction system to produce super oxygen-anion free radicals. The super oxygen-anion free radicals can oxidize hydroxylamine to produce nitrile, resulting in the production of a purple product in the presence of the chromogenic agent, which can be measured at 550 nm using a spectrometer (UV-2000, Unico, China). Antioxidant enzymes such as SOD specifically inhibit the superoxide radical and reduce nitrite production. SOD activity in the fermented vegetable–fruit beverage was calculated using a formula as equivalents of SOD U/mL.
Determination of total phenolics and flavonoids
The Folin–Ciocalteu method was used to determine the total phenolic content (TPC) with slight modifications (Abirami et al., 2014). Sample (0.1 mL) and 0.1 mL distilled water were added to 1.0 mL Folin–Ciocalteu reagent. After 5 min, a 3.0 mL solution of Na2CO3 (7.5%, w/v) was added and the mixture was allowed to incubate for 30 min at 25 °C. Absorbance was measured at 760 nm using a spectrometer. A standard solution of gallic acid (ranging from 0 to 300 mg/L) was used to prepare a calibration curve. TPC was defined as the amount of gallic acid (mg) per L fermented sample.
Aluminum chloride colorimetric method was used for determining the total flavonoid content (TFC) with some modifications (Abirami et al., 2014). Sample (0.5 mL) and 0.5 mL distilled water were combined and then 0.3 mL sodium nitrite (5%, m/v) was added. After 6 min, 0.3 mL of aluminum chloride (7.5%, w/v) solution was added and the mixture was incubated for 6 min at ambient temperature. Next, 2.0 mL sodium hydroxide (4%, w/v) was added to stop the reaction. Absorbance was measured at 510 nm using a spectrometer (UV-2000, Unico, China). A standard solution of rutin (ranging from 0 to 100 mg/L) was used to prepare a calibration curve. TFC was defined as the amount of rutin (mg) per L fermented sample.
1,1-Diphenyl-2-picrylhydrazyl radical (DPPH) scavenging activity
The insoluble debris of fermented beverage sample was removed by centrifugation at 10,000×g for 5 min, and the undiluted supernatant (200 μL each) was added to 1.8 ml of 0.1 mM DPPH and mixed vigorously. The mixture was shaken and then incubated for 30 min in the dark; the absorbance was measured at 515 nm using a spectrophotometer (UV-2000, Unico, China) (Brand-Williams et al., 1995). The absorbance of the control was obtained by replacing the sample with ethanol. The radical scavenging activity was determined using the following equation:
here Asample is the absorbance of the sample and Acontrol is the absorbance of the control containing all of the reaction reagents except the sample.
ABTS radical scavenging activity
Determination of ABTS radical scavenging activity was carried out as described (Sogi et al., 2013) with some modifications. ABTS stock solution was dissolved in sodium acetate-acetic acid buffer (20 mM, pH 4.5) to make a 7 mM ABTS stock solution. ABTS solution (7 mM) and potassium persulfate (2.45 mM) were mixed 1:1 (v/v) and incubated in the dark for 12–16 h to produce ABTS+ working solution. This solution was further diluted with 80% methanol to reach an absorbance of 0.70 ± 0.02 at 734 nm. The insoluble debris of fermented beverage sample was removed by centrifugation at 10,000×g for 5 min, and the undiluted supernatant (0.1 mL) was added to 3.9 ml ABTS+ working solution and mixed. The absorbance of mixture was measured at 734 nm after a 30 min incubation in the dark. A standard curve was prepared using the Trolox solution. The ABTS radical scavenging activity was defined as the amount of Trolox (mmol) per L fermented sample.
Ferric reducing antioxidant power assays
The ferric reducing antioxidant power (FRAP) assay measures the reduction of ferric iron to the ferrous form in the presence of antioxidant compounds by a previously described method (Suárez et al., 2010) with slight modifications. The working FRAP reagent was freshly prepared by mixing 5.0 mL of TPTZ (10 mM in 40 mM hydrochloric acid), 5.0 mL of ferric chloride (20 mM), and 50 mL of sodium acetate buffer (300 mM, pH 3.6). The insoluble debris of fermented beverage sample was removed by centrifugation at 10,000×g for 5 min, and the undiluted supernatant was used to measure. The FRAP reagent (3.6 mL) was first maintained at 37 °C and then mixed with 0.4 mL of each supernatant sample and mixed vigorously. The reaction mixture was incubated at 37 °C for 4 min and then the absorbance was measured at 593 nm. Trolox was used as the standard solution. The FRAP was defined as the amount of Trolox (mmol) per L fermented sample.
Statistical analysis
All analyses were performed in triplicate. Data are expressed as mean ± standard deviation (SD). Statistical analysis was done using SPSS 22 software (SAS Institute Inc., USA). The significance of difference was tested by one-way ANOVA. Post comparisons were performed by Fisher’s least significant difference (LSD) and Duncan’s multiple range test. Results with p < 0.05 were considered to be statistically significant.
Results and discussion
The growth of the microbial, pH, TTA, and acidification kinetics
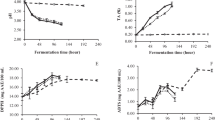
During the fermentation, the growth of the microbial can give important information for the properties of fermented vegetable–fruit beverage. The change of the amount of microbial (lactic acid bacteria) are shown in Fig. 1(A). The amount of lactic acid bacteria in the vegetable–fruit beverage increased rapidly during the first 24 h, which accord with the normal microbial growth curve. The amount of lactic acid bacteria declined slightly after the first 24 h, and then reached stable stage at 2 days to 11 days. The accumulation of substantial lactic acid and other metabolite inhibited the further burst of growth of lactic acid bacteria after the first 24 h.
The amount of microbial (lactic acid bacteria) (A), pH and total titratable acidity (B) during fermentation of the vegetable–fruit beverage. The average from the three plates recorded for each serial dilution. Results represent the means of three independent trials (n = 3). Error bars represent standard deviation values
The pH and TTA dynamics of fermented samples were examined and are shown in Fig. 1(B). The pH values decreased from 6.75 (initial) to 3.56 (14th day). The initial TTA value was 0.05 g/100 mL and increased to 1.03 g/100 mL on the 14th day of fermentation [Fig. 1(B)]. The highest value of ΔpH (3.18) and Vmax (0.08 dpH/h) occurred on the 14th day and 1st day, respectively (Table 2). Similar increases were found when rice-based beverage was fermented (Ghosh et al., 2015). The changes in pH and TTA may be due to the increased concentration of organic acids produced form the lactic acid bacteria fermentation process, especially the production of lactic acid.
Examination of organic acid and sugar levels during fermentation
Changes in organic acid levels during fermentation are shown in Fig. 2(A). The concentration of malic acid in the vegetable–fruit beverage changed significantly during the fermentation process. It increased from 0.28 to 0.52 g/L during the initial stage and then almost disappeared by the end of fermentation, which could be attributed to malolactic fermentation given that L. plantarum can transform malic acid to lactic acid (Vegara et al., 2014). The concentration of lactic acid and acetic acid increased along with the fermentation time and the highest values (3.28 and 0.11 g/L, respectively) were found on the 14th day [Fig. 2(A)]. Lactic acid was the major fermentation end product as the malolactic fermentation, which was consistent with a previous report (Vegara et al., 2014). Acetic acid was produced by L. plantarum as a secondary metabolite through the pentose phosphate pathway and showed the same trend as lactic acid.
There were many carbohydrates compositions (glycan, oligosaccharide and monosaccharide) in the vegetable/fruit substrates. They all appropriate carbon source for L. plantarum. However, glycan and oligosaccharide often firstly hydrolyzed by enzymes secreted by L. plantarum and then utilized by L. plantarum. Therefore we only choose sucrose, glucose and fructose for the detection in the fermentation. Changes in the levels of sucrose, fructose and glucose during fermentation were shown in Fig. 2(B). The sucrose content of the fermented beverage was 153 g/L on the 1st day and showed a slight reduction to 142 g/L on the 14th day. A previous study showed that sucrose could be better utilized by lactic acid bacteria than fructose or glucose, consistent with their rapid growth by consuming sucrose during the early stage of fermentation (Cho et al., 2006). Xiong et al. (2014) also reported that sucrose was utilized throughout sauerkraut fermentation. Fructose and glucose increased from 0.48 and 14.8 g/L to 7.7 and 20.8 g/L, respectively. Sucrose was rich in fruits and vegetables material. During the fermentation, sucrose was gradually degraded into glucose and fructose. In the meantime, fructose and glucose can be consumed as the carbon source for L. plantarum (Siragusa et al., 2014). However, the speed of sucrose decomposition was higher than consumption by L. plantarum in the formation. Thus fructose and glucose increased during entire fermentation process of this vegetable–fruit beverage.
Determination of vitamin content
The ascorbic acid and riboflavin content of the fermented samples are shown in Fig. 3(A). The riboflavin and ascorbic acid content of the fermented material were higher than that of the raw material. Riboflavin increased during the entire fermentation process, with a final concentration of 1.18 mg/100 mL. On the other hand, ascorbic acid content increased gradually during fermentation and the final concentration was 90.74 mg/100 mL, whereas little ascorbic acid was detected in the unfermented sample. Ghosh et al. showed that the highest amounts of ascorbic acid occurring in a rice-based fermented beverage was 500.25 μg/100 mL; however, riboflavin levels decreased during the fermentation process (Ghosh et al., 2015). Notably, ascorbic acid levels were very low in the unfermented material after pasteurization [Fig. 3(A)]. The total amount of ascorbic acid in the raw materials (apple, pear, carrot) is about 15 mg/100 g FW (Cotrut and Badulescu, 2016). Thus, ascorbic acid and riboflavin mainly result from the L. plantarum strains during the fermentation process.
Changes in the levels of ascorbic acid and riboflavin during fermentation of the vegetable–fruit beverage (A). Changes in antioxidant activity during fermentation of the vegetable–fruit beverage (B) including DPPH free radical scavenging activity (square), ABTS free radical scavenging activity (circle), and ferric reducing antioxidant power (inverted triangle). Results are the means of three independent trials (n = 3). Error bars represent standard deviation values
DPPH/ABTS free radical scavenging ability and FRAP
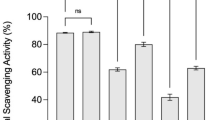
DPPH/ABTS radical scavenging activities and FRAP have been widely used for representing the total antioxidant activities of foods. The antioxidant activities of the vegetable–fruit fermented beverage are shown in Fig. 3(B). The DPPH free radical scavenging activity increased during the first 8 days and then decreased until the 14th day. The maximum value of DPPH free radical scavenging activity (58.7%) was found on the 8th day. The trend in ABTS radical scavenging activity was characterized by an increase for the first 8 days and then a decrease during the fermentation period. It reached its highest value (0.571 mmol Trolox/L) on the 8th day. The FRAP values increased for the first 5 days and then decreased during the remaining fermentation period. The maximum FRAP value (0.481 mmol Trolox/L) was found on the 5th day [Fig. 3(B)]. Therefore, the three antioxidant activities showed a similar trend during the entire fermentation period.
Hur et al. (2014) reported that increased antioxidant activity in fermented foods was due to the accumulation of antioxidant compounds, including phenolic compounds, flavonoids, and SOD. Torres et al. (2015) also found that the high phenolic and flavonoid content in the fruit extract were directly related to the antioxidant activity. In addition, some lactic acid bacteria have the ability to produce SOD, which increases the antioxidant activity in the fermented system. The two Lactobacillus fermentum strains provided significant antioxidant activity in the intestinal microbial ecosystem via the production of SOD (Kullisaar et al., 2002). Therefore, the fermented beverage showed significant antioxidant activities, which indicates its potential as health-promoting drink for daily use and suggests that it should be developed.
Antioxidants (SOD, TPC and TFC)
SOD is one of the most important antioxidant enzymes, which can remove reactive oxygen effectively in catalyst systems. Some lactic acid bacteria have the ability to produce SOD to increase antioxidant activity in fermented systems. However, to date, there are few studies examining SOD production by L. plantarum during the preparation of fermented beverages. The SOD activity in the vegetable–fruit fermented beverage is shown in Fig. 4. The unfermented materials showed nearly no SOD activity after pasteurization. SOD activity then increased significantly along with the fermentation period up to the 8th day where the maximum activity (357 U/mL) was obtained and decreased after 8 days of fermentation and remained constant thereafter. The increase in SOD activity is consistent with the reported trend in apple juice fermentation using commercial lactic acid bacteria strains (Espirito-Santo et al., 2015). The increasing SOD activity during fermentation may be due to the growth of the L. plantarum strains. SOD is an intracellular enzyme and can be released by cell lysis of microorganisms. The SOD activity was found to be strongly correlated with the amount of bacteria (r = 0.624, p < 0.001) in apple juice fermentation (Espirito-Santo et al., 2015). The reduction of SOD activity may be due to the instability of SOD at the temperatures used for fermentation and inactivation by chemical compounds present in the beverage and/or produced by the lactobacilli as a reaction to the acidic, stressful environment (Welman and Maddox, 2003). The general trend observed for SOD is consistent with the trend of DPPH and ABTS radical scavenging ability.
Changes in phenolics level, flavonoid content, and SOD activity during fermentation of the vegetable–fruit beverage. Phenolics (circle), flavonoids (inverted triangle), and SOD activity (square). Phenolics results expressed in mg gallic acid equivalents (GAE) per L of beverage and flavonoid results expressed in mg rutin equivalents per L of beverage. Results are the means of three independent trials (n = 3). Error bars represent standard deviation values
Changes in the levels of TPC and TFC are shown in Fig. 4. The total phenolic concentration in the vegetable–fruit beverage was 96.8 mg/L prior to fermentation. It increased during the first 8 days of fermentation and then decreased in conjunction with the fermentation period. The maximum content (121 mg/L) was found on the 8th day, which was 1.25-fold higher than that of the unfermented material. A previous study also showed that plant parts show an increase in the total phenolic content after fermentation, and it may explain the observed increase in antioxidant activity (Ng et al., 2011). It was also suggested that microbial transformation and depolymerization of compounds might explain the increase of total phenolic content that occurs during fermentation (Chu and Chen, 2006). During fermentation, a certain concentration of phenols can result in bacteriostasis, and lactic acid bacteria will degrade phenols to keep growing. This action may result in a decrease in the total phenolic content (Rodriguez et al., 2009).
TFC showed a similar trend as TPC. It increased up to the 8th day and then decreased along with the fermentation period. The maximum value of TFC was 75.2 mg/L, whereas the TFC level of the unfermented sample was 65.7 mg/L. Xiao et al. (2015) reported that L. plantarum could significantly increase the total flavonoid content during soybean whey fermentation. Previous studies reported that lactic acid bacteria could produce enzymes and acids that can facilitate the release of flavonoids from their complex form (Katina et al., 2007). Therefore, fermentation can improve the content of flavonoids from vegetable–fruit fermented beverages. These findings may help to improve development of a high-quality fermented beverage.
In this study, we explored the substrate utilization and metabolic products of two L. plantarum strains as well as the antioxidant activity of a fermented vegetable–fruit beverage during the fermentation process. Which indicated that the selected L. plantarum strains can be successfully used for the formulation of vegetable–fruit beverages. Furthermore, the fermented beverage showed its potential as health-promoting drink for daily use and suggests that it should be developed.
References
Abirami A, Nagarani G, Siddhuraju P. In vitro antioxidant, anti-diabetic, cholinesterase andtyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci. Hum. Wellness 3: 16–25 (2014)
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28: 25–30 (1995)
Chang JY, Chang HC. Improvements in the quality and shelf life of kimchi by fermentation with the induced bacterioc in-producing strain, Leuconostoc citreum GJ7 as a starter. J. Food Sci. 75: 103–110 (2010)
Cho J, Lee D, Yang C, Jeon J, Kim J, Han H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol. Lett. 257: 262–267 (2006)
Chu SC, Chen C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 98: 502–507 (2006)
Coda R, Rizzello CG, Trani A, Gobbetti M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 28: 526–536 (2011)
Connelly P. Lactobacillus plantarum—a literature review of therapeutic benefits. J. Aust. Tradit-Med. Soc. 14: 79–82 (2008)
Cotruţ R, Bădulescu L. UPLC rapid quantification of ascorbic acid in several fruits and vegetables extracted using different solvents. AASRI Procedia 10: 160–166 (2016)
Di Cagno R, Surico RF, Siragusa S, Angelis DM, Paradiso A, Minervini F, Gara DL, Gobbetti M. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows. Int. J. Food Microbiol. 127: 220–228 (2008)
Đorđević TM, Šiler-Marinković SS, Dimitrijević-Branković SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 119: 957–963 (2010)
Espirito-Santo AP, Carlin F, Renard CMGC. Apple, grape or orange juice: Which one offers the best substrate for lactobacilli growth? A screening study on bacteria viability, superoxide dismutase activity, folates production and hedonic characteristics. Food Res. Int. 78: 352–360 (2015)
Filannino P, Cardinali G, Rizzello CG, Buchin S, Angelis MD, Gobbetti M, Cagnoa RD. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 80: 2206–2215 (2014)
Ghosh K, Ray M, Adak A, Halder SK, Das A, Jana A, ParuaMondal S, Vagvolgyi C, Das Mohapatra PK, Pati BR, Mondal KC. Role of probiotic Lactobacillus fermentum KKL1 in the preparation of a rice based fermented beverage. Biores. Technol. 188: 161–168 (2015)
Hur SJ, Lee SY, Kim YC, Choi I, Kim GB. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 160: 346–356 (2014)
Katina K, Laitila A, Juvonen R, Liukkonen KH, Kariluoto S, Piironen V, Landberg R, Aman P, Poutanen K. Bran fermentation as a means to enhance technologicalproperties and bioactivity of rye. Food Microbiol. 24: 175–186 (2007)
Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, Kilk A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 72: 215–224 (2002)
Marsh AJ, Hill C, Ross RP, Cotter PD. Fermented beverages with health-promoting potential: past and future perspectives. Trends Food Sci. Technol. 38: 113–124 (2014)
Molina AM, Guadalupe V, Varela C, Swiegers JH, Pretorius IS, Agosin E. Differential synthesis of fermentative aroma compounds of two related commercial wine yeast strains. Food Chem. 117: 189–195 (2009)
Ng CC, Wang CY, Wang YP, Tzeng WS, Shyu YT. Lactic acid bacterial fermentation on the production of functional antioxidant herbal Anoectochilus formosanus Hayata. J. Biosci. Bioeng. 111: 289–293 (2011)
Pereira ALF, Maciel TC, Rodrigues S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res. Int. 44: 1276–1283 (2011)
Prado FC, Lindner DJ, Inaba J, Thomaz-Soccol V, Brar KS, Soccol CR. Development and evaluation of a fermented coconut water beverage with potential health benefits. J. Funct. Foods 12: 489–497 (2015)
Rodriguez H, Curiel JA, Landete JM, Rivas DB, Lopez FF, Gomez-Cordoves C, Mancheno, JM, Munoz R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 132: 79–90 (2009)
Salmerón I, Thomas K, Pandiella SS. Effect of potentially probiotic lactic acid bacteria on the physicochemical composition and acceptance of fermented cereal beverages. J. Funct. Foods 15: 106–115 (2015)
Simsek S, El SN, Kilinc KA, Karakaya S. Vegetable and fermented vegetable juices containing germinated seeds and sprouts of lentil and cowpea. Food Chem. 156: 289–295 (2014)
Siragusa S, Angelis DM, Calasso M, Campanella D, Minervini F, Di Cagno R, Gobbetti M. Fermentation and proteome profiles of Lactobacillus plantarum strains during growth under food-like conditions. J. Proteomics 96: 366–380 (2014)
Sogi DS, Siddiq M, Greiby I, Dolan KD. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 141: 2649–2655 (2013)
Somanah J, Bourdon E, Rondeau P, Bahorun T, Aruoma OI. Relationship between fermented papaya preparation supplementation, erythrocyte integrity and antioxidant status in pre-diabetics. Food Chem. Toxicol. 65: 12–17 (2014)
Suárez B, Álvarez ÁL, García YD, Barrio G, Lobo AP, Parra F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 120: 339–342 (2010)
Torres CA, Romero LA, Diaz RI. Quality and sensory attributes of apple and quince leathers made without preservatives and with enhanced antioxidant activity. LWT Food Sci. Technol. 62: 996–1003 (2015)
Vanajakshi V, Vijayendra SVN, Varadaraj MC, Venkateswaran G, Agrawal R. Optimization of a probiotic beverage based on Moringa leaves and beetroot. LWT Food Sci. Technol. 63: 1268–1273 (2015)
Vegara S, Marti N, Lorente J, Coll L, Streitenberger S, Valero M, Saura D. Chemical guide parameters for Punica granatum cv. ‘Mollar’ fruit juices processed at industrial scale. Food Chem. 147: 203–208 (2014)
Welman AD, Maddox IS. Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol. 21: 269–274 (2003)
Xiao Y, Wang L, Rui X, Li W, Chen X, Jiang M, Dong M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. J. Funct. Foods 12: 33–44 (2015)
Xiong T, Li X, Guan Q, Peng F, Xie M. Starter culture fermentation of Chinese sauerkraut: growth, acidification and metabolic analyses. Food Control 41: 122–127 (2014)
Zwietering MH, Jongenburger I, Rombouts FM, Van K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56: 1875–1881 (1990)
Acknowledgements
Financial support from the Fundamental Research Funds for the Central Universities (No. 222201717026) and the “Shu Guang” project supported by Shanghai Municipal Education Commission Development (No. 15SG28) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Zhou, J., Fan, L. et al. Antioxidant properties of a vegetable–fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci Biotechnol 27, 1719–1726 (2018). https://doi.org/10.1007/s10068-018-0411-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0411-4