Abstract
Objectives
To assess the clinical features and functional and psychological status of patients with rheumatoid arthritis (RA) and those with fibromyalgia (FM) in a real-world setting.
Method
Between December 2018 and April 2019, 202 inpatients with RA were enrolled from the Rheumatology and Immunology Department at Peking University People’s Hospital and assessed for the presence of FM using the 1990 American College of Rheumatology’s classification criteria for FM. Disease activity and functional and psychological status were assessed using the Disease Activity Score in 28 joints (DAS-28), Short-Form 36 (SF-36), Health Assessment Questionnaire (HAQ), Hospital Anxiety and Depression Scale, and Visual Analog Scale.
Results
Among the patients with RA, 42 (20.8%) had concurrent FM. Compared with patients without FM, patients with FM had higher DAS-28 (6.0 vs. 4.4, P = 0.011) and notably higher tender joint counts (16.5 vs. 4.5, P < 0.001). Patients with RA and FM had worse HAQ scores (1.24 vs. 0.66, P < 0.001) and lower SF-36 scores (28.6 vs. 58.2, P < 0.001). Patients with RA and FM experienced more fatigue (88.1% vs. 50.6%, P < 0.001) and had higher anxiety (10 vs. 4, P < 0.001) and depression scores (12 vs. 6, P < 0.001). No significant differences in erythrocyte sedimentation rate, C-reactive protein concentration, morning stiffness period, or swollen joint counts were found between the groups.
Conclusions
Patients with RA and FM had higher disease activity, a worse functional and psychological status, and poorer quality of life. The DAS-28 may have been overestimated in these patients. When patients with RA do not reach remission, FM should be considered.
Key Points • Patients with rheumatoid arthritis and fibromyalgia had a worse functional and psychological status compared with those with rheumatoid arthritis alone. • When patients with rheumatoid arthritis do not reach remission, fibromyalgia should be considered. • Physicians should avoid overtreatment and enable these patients to receive the treatment, such as non-drug interventions, that they need. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic, typically progressive, autoimmune disease that causes joint damage and functional impairment. Fibromyalgia (FM)—a chronic disorder characterized by diffuse musculoskeletal pain, fatigue, stiffness, and the presence of multiple tender points [1]—occurs in 2–8% of the general population [2]. Its prevalence is 12–48% in patients with RA [3,4,5,6,7,8]. Most studies concerning patients with RA have reported a relationship between FM and non-inflammatory pain. Chronic unexplained pain may lead to fatigue, sleep disturbances, mental and psychological disorders, and impaired sleep quality [9]. The reduction in overall health in patients with RA with comorbid FM illustrates that FM remains a significant burden in patients with RA [10]. However, the lack of reliable and objective evidence leads to difficulties in accurately diagnosing this disorder. The first diagnostic classification criteria for FM were published in 1990 through the evaluation of 18 anatomical sites. These American College of Rheumatology (ACR) criteria emphasized “pain” as a core symptom, although it did not include the evaluation of other clinical characteristics, such as fatigue, sleep disorders, and psychosocial symptoms. Pollard et al. [11] proposed a simplified standard for fibromyalgic RA, defined as ≥ 7 tender minus swollen joint counts. Furthermore, neither the ACR’s classification criteria nor Pollard’s standard for FM has been entirely or widely used in China to date. This study aimed to assess the utility of the Pollard’s standard and to compare the clinical characteristics as well as the functional and psychological condition of patients with RA and those with FM in a real-world population in China.
Materials and methods
Patients
This study was conducted in the Department of Rheumatology and Immunology of Peking University People’s Hospital. A total of 202 patients who met the 2010 ACR/EULAR classification criteria for RA were enrolled in this study that was conducted over 6 months, from December 2018 to June 2019. All of the patients were evaluated for the presence of FM using the 1990 ACR-FM classification criteria as well as Pollard’s standard for FM [12]. Patients with other rheumatic illnesses, severe somatic or psychiatric diseases, or disabling medical problems were excluded from this study. The study was approved by the Institutional Medical Ethics Review Board of Peking University People’s Hospital and conducted in compliance with the Declaration of Helsinki. Patients were classified into two groups: those with FM and those without FM.
Data collection
Demographic features of all participants, including their age, sex, ethnicity, disease duration, educational level, working status, total family income, and direct medical costs, were assessed. Clinical assessments performed were as follows: (a) the Disease Activity Score, which includes the examination of 28 joints (DAS-28), was used to evaluate the disease activity of the patients with RA; for this purpose, the tender joint count (TJC), swollen joint count (SJC), erythrocyte sedimentation rate (ESR), and global assessment score were calculated. A DAS-28 > 5.1 indicates high disease activity, while a score < 3.2 indicates low disease activity. Besides, data on an inflammatory marker, such as C-reactive protein (CRP), was also collected. (b) Functional status was assessed using the Visual Analog Scale (VAS) for pain severity, where a score of 0 indicates that “there is no pain,” while a score of 10 indicates that “there is very severe pain”; furthermore, the Short-Form 36 survey (SF-36) and Health Assessment Questionnaire (HAQ) were used to assess health-related quality of life. The SF-36 comprises 36 questions in 8 categories: bodily pain, physical functioning, general health perceptions, social functioning, vitality, general mental health, and role limitations due to either physical or social problems. The HAQ score covers 20 questions in 8 dimensions: dressing, grooming, rising, eating, walking, reaching, gripping, and outdoor activities. Each response is scored based on ability, with scores ranging from 0 to 3: no difficulty, some difficulty, much difficulty, and unable to do. (c) Psychological status was assessed using the Hospital Anxiety and Depression Scale. This scale was developed to measure both anxiety and depression among patients in non-psychiatric hospital clinics. Each subscale comprises 7 items, which are rated by the patient on a 4-point (0–3) scale, based on the previous week. A score of 0–7 represents the reference range, 8–10 indicates the presence of a possible case of anxiety or depression, and ≥ 11 suggests the probable case of anxiety or depression. (d) Fatigue was assessed using the Fatigue Assessment Instrument (FAI). The FAI was formulated in 1993 by the American Institute of Psychological and Behavioral Sciences and Laboratory of Neurology. It is a 4-factor model containing 29 entries that reflect different aspects of fatigue: severity (Factor I), sensitivity (Factor II), psychological consequences (Factor III), and response to rest or sleep (Factor IV). Each question is scored on a 7-point (1–7) scale. According to the score of Factor I, the severity of fatigue is divided into four levels: no fatigue (< 4), mild fatigue (= 4), moderate fatigue (= 5), and severe fatigue (≥ 6). Fatigue was also measured using a 10-mm VAS.
Statistical analyses
Numeric data with a normal distribution are presented as means and standard deviations or medians (interquartile ranges [IQRs]). The Shapiro–Wilk test was used to analyze the normal distribution assumption of the quantitative outcomes. Student’s t test and Mann–Whitney test were used for numerical variables, and Chi-square tests were used to compare categorical variables among the groups. P values of < 0.05 were considered statistically significant. Direct medical costs and total family income were missing for 0.48% of all included individuals. Patients with missing data were excluded from the analyses. All analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp.; Armonk, NY).
Results
Sociodemographic characteristics
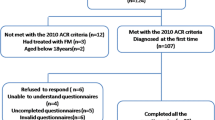
Among the 243 individuals enrolled in this study, 38 were excluded due to the presence of other autoimmune diseases—36 cases of Sjogren’s syndrome, 1 of inflammatory myositis, and 1 of systemic lupus erythematosus. Three other individuals were excluded because of serious deafness. Among the 202 included participants, 42 (20.8%) met the requirements for FM with the 1990 ACR-FM classification criteria, while 76 cases conformed to Pollard’s classification criteria. Furthermore, 40 patients met the requirements for both criteria. The sensitivity of Pollard’s standard was 95.2% in this study, yet the specificity was only 52.6%. The major demographic data of the participants are presented in Table 1. Those with FM were 1.5 years younger and had RA for 2.5 years longer than those without FM. Participants with FM were slightly more likely to be of Han nationality (2.6%), and all of them were married. There were fewer participants with FM who had completed college and were employed. Overall, a comparison of sociodemographic factors revealed that participants with FM had a socioeconomic disadvantage compared with those who did not have FM, with the most significant disadvantages being in educational level and occupational status. Those participants with FM had a slightly higher level of poverty according to total household income than those without FM. The analysis of total household income showed that 59.5% of participants with FM had an income exceeding 50 thousand renminbi (RMB) per year, as compared with 67.5% of participants without FM over the same interval. As for direct medical costs, participants with and without FM with medical costs exceeding 20 thousand RMB per year constituted 26.9% and 31.0%, respectively. Those with higher medical costs used more types of non-steroidal anti-inflammatory drugs. There were no significant differences in demographic features between the two study groups (P < 0.05).
Clinical measures
The clinical characteristics of the two study groups are compared in Table 2. In comparison to participants with RA without FM, those with FM had a higher DAS-28 (6.0 vs. 4.4, P = 0.011) and a markedly higher TJC (16.5 vs. 4.5, P < 0.001). The trend for differences in the duration of morning stiffness and SJC was not significant. The CRP concentration was 27.9 mg/dL and 14.9 mg/dL, and the ESR was 50.5 mm/h and 43.5 mm/h, for the participants with and without FM, respectively. However, no differences in inflammatory index values were found.
Functional status
A significant difference was found between the HAQ and SF-36 scores of the two different groups (Table 3). The median HAQ score was 0.66 (IQR: 0.2–0.9) and 1.24 (IQR: 0.26–1.5) in participants without and those with FM, respectively. The median VAS score for pain was 5.0 for participants with FM compared with 3.0 for participants without FM. The SF-36 scores revealed that the quality of life of participants with RA and FM was unsatisfactory, with median values of 58.22 (IQR: 47.26–73.17) and 28.63 (IQR: 19.80–39.97) in those without and those with FM, respectively.
Psychological status
Participants with FM reported considerably greater anxiety and depression ratings, and a higher level of fatigue, than those without FM (Table 4). They also had higher scores with respect to the severity (Factor I), sensitivity (Factor II), and psychological consequences (Factor III) of fatigue. As for the response to rest or sleep (Factor IV), there were no significant differences between the two groups. According to the VAS-fatigue data, participants with FM reported a higher level of fatigue than participants without FM.
Discussion
There are a large number (several millions) of patients with RA in China; however, the combination of FM in RA is often underestimated. Many primary care physicians are not aware of this problem; they do not realize the coexistence of FM with RA, so they tend to prescribe more disease-modifying anti-rheumatic drugs once the symptoms cannot be controlled, which in turn leads to the occurrence of adverse drug reactions. We conducted this study based on the fact that there is a lack of clinical studies on RA combined with FM in the Chinese population. Our findings verified the utility of the Pollard’s standard in the Chinese population and suggested that physicians should be vigilant regarding possible FM complications with RA, especially when no laboratory or imaging evidence exists, which is consistent with previous findings [13, 14]. These patients tended to undergo excessive examination and treatment for their disease, and their disease activities might also have been overestimated. Physicians must avoid overestimation and ensure that patients receive appropriate interventions that they really need.
It is challenging to distinguish FM from RA given that both present as joint pain. Therefore, the Pollard’s standard uses ≥ 11 tender points as a key diagnostic criterion to differentiate FM from RA based on the ACR criteria. Pollard et al. proposed that tender minus swollen joint counts of ≥ 7 predicted the identification of fibromyalgic RA with 83% sensitivity and 80% specificity. This standard increases the sensitivity and specificity for identifying RA with concomitant FM because it eliminates the effects of active synovitis by subtracting the number of swollen joints, which is applicable clinically and can be considered a screening criterion for RA combined with FM [9]. In our study, the diagnostic sensitivity of Pollard’s criteria was 95.2%, and the specificity was only 52.6%, which indicated that the criteria may result in overdiagnosis. Besides, the evaluation of tender joints may be susceptible to other factors. Therefore, the use of Pollard’s criteria needs to be combined with a comprehensive assessment by a rheumatologist in the Chinese population. Previous studies have shown that the prevalence of FM in patients with RA is approximately 20% [4, 5]. In our study, 20.8% (42/202) of patients with RA met the 1990 ACR classification criteria for FM, and 37.6% (76/202) met Pollard’s standard for FM. The probability of “fibromyalgic RA” was higher than we had predicted.
Our study shows that patients with RA and FM had a poor functional condition with higher SF-36 and HAQ scores, which is consistent with the results of previous studies [7, 15]. Studies have shown that the concomitant presence of FM and severity of FM symptoms are independent predictors of physical deterioration in patients with RA [16]. We should be cautious that patients with RA may have concomitant FM if their disease activity ratings are rather high, while their objective inflammatory markers, such as CRP and ESR, are not elevated, especially when complaining of tender joints that are not related to synovitis, fatigue, or quality of life disability due to high HAQ scores.
Meanwhile, we emphasized the significance of a nurse-assisted strategy for specialty care for patients with RA. Nurses usually spend longer time with the patients and have more opportunities to perceive mental or psychological abnormality. Therefore, the role of the rheumatological specialist nurse should not be neglected. Instead, regular and effective training of nurses aimed at improving their specialty assessment skills would help doctors make an early and accurate diagnosis and offer appropriate interventions to patients with RA and FM.
Consideration should be given to non-drug interventions, such as exercise and psychotherapy, in managing patients with RA. In 2016, the European League Against Rheumatism suggested that aerobic exercise and strength training should be strongly recommended in the management of FM [17]. If the addition of new disease-modifying anti-rheumatic drugs or adjustments to biological therapies still do not improve the disease condition of patients with RA, we should consider the possibility of concurrent FM. However, further research is required to assess whether these activities could enhance the physical and mental health of patients with RA.
Our study has some limitations. First, the sample size was relatively small, and selection bias exists. This is a single-center study that is unable to represent the whole picture of “fibromyalgic RA” in China. Second, the FAI is a self-reported measure of fatigue that, according to some research, does not correspond with objective assessments. In contrast, the four-variables evaluation approach can be used to evaluate different components of fatigue.
In conclusion, if patients with RA do not experience relief from symptoms for an extended period, physicians should consider the possibility of the concurrent presence of FM. In this study, patients with RA and FM had a worse functional and psychological status compared with those with RA alone, while their DAS-28 may have been overestimated. It is of vital importance that physicians avoid overtreatment in this sub-phenotype and enable these patients to receive the treatment, such as non-drug interventions, that they need. Meanwhile, enhancing educational programs for patients may help them better understand the possible coexistence of FM with RA, which may lead to better treatment cooperation. Furthermore, it may greatly reduce the burden on society and can improve the prognosis of the patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbasi L, Haidri FR (2014) Fibromyalgia complicating disease management in rheumatoid arthritis. J Coll Physicians Surg Pak 24:424–427
Clauw DJ (2014) Fibromyalgia: a clinical review. JAMA 311:1547–1555
Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L (1995) The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 38:19–28
Wolfe F, Clauw DJ, Fitzcharles MA et al (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 38:1113–1122
Wolfe F, Häuser W, Hassett AL, Katz RS, Walitt BT (2011) The development of fibromyalgia: examination of rates and predictors in patients with rheumatoid arthritis (RA). Pain 152:291–299
Vincent A, Lahr BD, Wolfe F et al (2013) Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken) 65:786–792
Joharatnam N, Mcwilliams DF, Wilson D, Wheeler M, Pande I, Walsh DA (2015) A cross-sectional study of pain sensitivity, disease-activity assessment, mental health, and fibromyalgia status in rheumatoid arthritis. Arthritis Res Ther 17:11
Kiliçarslan A, Yurdakul FG, Bodur H (2018) Diagnosing fibromyalgia in rheumatoid arthritis: the importance of assessing disease activity. Turk J Phys Med Rehab 64:133–139
Ulus Y, Akyol Y, Tander B, Durmus D, Bilgici A, Kuru O (2011) Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol 29(6 Suppl 69):S92–S96
Wolfe F, Michaud K (2004) Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 31:695–700
Pollard LC, Kingsley GH, Choy EH, Scott DL (2010) Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford) 49:924–928
Wolfe F, Smythe HA, Yunus MB et al (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33:160–172
Mu R, Li C, Zhu JX et al (2013) National survey of knowledge, attitude and practice of fibromyalgia among rheumatologists in China. Int J Rheum Dis 16:258–263
Queiroz LP (2013) Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 17:356
Gist AC, Guymer EK, Eades LE, Leech M, Littlejohn GO (2018) Fibromyalgia remains a significant burden in rheumatoid arthritis in Australia. Int J Rheum Dis 21:639–646
Kim H, Cui J, Frits M et al (2017) Fibromyalgia predicts two-year changes in functional status in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 69:1871–1877
Macfarlane GJ, Kronisch C, Dean LE et al (2017) EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 76:318–328
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author information
Authors and Affiliations
Contributions
CG conducted most of the experiments. LC and TL conceived the study and design it. HZ drafted the manuscript and participated in data analysis. LW, HY, XH, and YJ helped to collect data. TL and CL participated in interpretation of results and reviewed the manuscript. All authors approved the final version to be submitted for publication.
This study was supported by funds from the National Natural Science Foundation of China (81671609), the Beijing Science and Technology Planning Project (Z191100006619111), the Beijing Municipal Natural Science Foundation (7192211), and the Peking University People’s Hospital Research and Development Found (RDN2019-02).
Corresponding authors
Ethics declarations
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors’ equal contribution has been added.
Chao Gao and Hua Zhong are shared first authors.
Rights and permissions
About this article
Cite this article
Gao, C., Zhong, H., Chen, L. et al. Clinical and psychological assessment of patients with rheumatoid arthritis and fibromyalgia: a real-world study. Clin Rheumatol 41, 1235–1240 (2022). https://doi.org/10.1007/s10067-021-06026-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-06026-6