Abstract
Objectives
Our aim was to investigate the prevalence value of a high-avidity antinuclear antibody (ANA) of the IgG isotype (HA IgG ANA) compared with that of ANAs of other isotypes in patients with systemic lupus erythematosus (SLE) and to assess the associations of HA IgG ANA with the activity of SLE and lupus nephritis.
Methods
We retrospectively analyzed clinical and laboratory data from subjects. Blood samples were acquired from 101 SLE patients, 67 patients with other autoimmune diseases, and 65 healthy donors. The levels of HA IgG ANA and other isotype ANAs were measured by indirect immunofluorescence (IIF). The prevalence and diagnosis value of HA IgG ANA and other antibodies in SLE patient were tested. The advantage of HA IgG ANA compared with HA anti-dsDNA antibodies IgG (HA dsDNA IgG) was verified by ELISA. We monitored the relative avidity indexes (RAIs) of HA IgG ANA and HA dsDNA IgG at 3 time points after the start of treatment in the same individuals with SLE.
Results
The prevalence of HA IgG ANA was significantly higher in active cases than in inactive cases of SLE and LN, which is consistent with data for IgG ANAs, anti-dsDNA IgG antibodies, low C3 levels, low C4 levels, and anti-C1q antibodies. HA IgG ANA showed moderate sensitivity and specificity (80% and 81.3%) for discriminating active and inactive SLE cases. However, HA IgG ANA showed no significant differences among the different clinical manifestations of SLE. Compared with that of HA dsDNA IgG, the RAI of HA IgG ANA was positively related to SLEDAI scores after treatment at 0, 1, and 3 months (r = 0.6813, p = 0.0026; r = 0.5972, p = 0.0114; r = 0.4817, p = 0.0474).
Conclusions
First, we demonstrated that HA IgG ANA was a reliable diagnostic tool in SLE patients. Furthermore, HA IgG ANA was supposed to be more appropriate for identifying the activity of SLE compared with HA dsDNA IgG. In summary, HA IgG ANA may be a new biomarker for diagnosing SLE and identifying SLE activity.
Key Points • We first introduced the concept of a “high-avidity IgG ANA (HA IgG ANA)” that could distinguish between the early stage of SLE and SLE that had been active for some time. • The relative avidity indexes (RAIs) of HA IgG ANA and HA dsDNA IgG were presented and applied here to evaluate the avidities of antibodies involved in SLE. • In our study, we confirmed the value of HA IgG ANA in diagnosing SLE. In addition, HA IgG ANA was more appropriate for identifying the activity of SLE than was HA dsDNA IgG. • In conclusion, HA IgG ANA could be a potential biomarker for the assessment of the prognosis of SLE activity. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease characterized by the presence of a multitude of autoantibodies. Antinuclear antibody (ANA) positivity is an important criterion for the diagnosis of SLE and is officially recognized as such by its inclusion in the European League Against Rheumatism (EULAR) and American College of Rheumatology criteria [1]. ANA positivity at a serum dilution of > 1:80 is a necessary entry criterion, followed by weighted items including seven clinical (constitutional, mucocutaneous, arthritic, neurologic, serositic, hematological, and renal) and three immunological (anti-phospholipid, complement and highly specific antibody) domains [2]. The indirect immunofluorescence (IIF) ANA test is the “gold standard” for the diagnosis of systemic autoimmune rheumatic disease (SARD) and is used for essentially all patients with SLE. However, both the detection and the titer measurement of anti-double-stranded DNA (anti-dsDNA) antibodies are considered to be key in the diagnosis of SLE, the assessment of the SLE phenotype, especially renal involvement, and the determination of SLE activity [3,4,5]. The levels of anti-dsDNA IgG were detected at least 2 years earlier than clinical diagnosis occurred [6, 7], and the concentration of these antibodies fluctuates with disease activity in SLE [8, 9]. In addition, anti-dsDNA and anti-Sm antibodies are relatively specific for diagnosing SLE. Nonetheless, many SLE patients lack these antibodies, which leads to the possibility of a missed diagnosis [10]. Furthermore, there is a lack of correlation between the titers of anti-dsDNA antibodies and SLE in some patients because of the methodological limitations of ELISA methods and Farr’s assay. Thus, clinicians have been puzzled by the inconsistencies between clinical activity manifestations and the titers found by anti-dsDNA antibody serology tests. Autoantibody involvement in SLE immunopathogenesis still deserves further exploration [11,12,13].
High-affinity antibodies mediate a variety of biological functions more effectively than low-affinity antibodies, as reported in previous experimental evidence [14]. The maturation of antibody affinity leads to an increase in the avidity of antigen-antibody complexes, which is a basic and universal immunological process [15]. High-avidity anti-dsDNA IgG is considered highly specific for the diagnosis of SLE and evaluation of disease activity [16, 17]. Although anti-dsDNA IgG exhibits a high specificity, its prevalence has been estimated to be approximately 50% [18, 19]. In our study, high-avidity IgG ANA (HA IgG ANA) were first introduced to evaluate the disease activity of SARDs, especially SLE. Until now, the role of HA IgG ANAs in SLE has not been identified. The purpose of our study was to investigate the prevalence of HA IgG ANA, ANAs of other isotypes (IgG, IgM, and IgA), anti-dsDNA IgG antibodies, and C3 and/or C4 levels to evaluate diagnostic value and clarify the association with disease activity of SLE.
Materials and methods
Ethics statement
The study was approved by the Research Ethics Committee of Zhongshan Hospital, the Medical College of Xiamen University, and informed consent was obtained from all the participants. For the patients under 18 years of age participating in the study, written informed consent from their guardians or parents was required.
Study participants
A total of 101 patients with SLE (8 males and 92 females, mean age of 36 years) diagnosed according to the 2012 revised diagnostic criteria of the European League against Rheumatism (EULAR) and American College of Rheumatology criteria were accepted from Zhongshan Hospital, the Medical College of Xiamen University, from April 2018 to August 2019 and enrolled in the present study. The other autoimmune disease group recruited 67 patients (8 males and 59 females, mean age of 36 years old) with other autoimmune diseases. Of these patients, 23 were diagnosed with Sjögren’s syndrome (SS), 19 were diagnosed with rheumatoid arthritis (RA), 13 were diagnosed with mixed connective tissue disease (MCTD), 6 were diagnosed with liver disease (including hepatitis and liver cirrhosis), 4 were diagnosed with systemic sclerosis (SSC), and 2 were diagnosed with anti-phospholipid syndrome (APS).
The healthy control group was composed of age- and sex-matched individuals (7 males and 58 females, mean age of 36 years) without any risk factors or chronic diseases. The disease activity of the patients with SLE was assessed based on the SLE disease activity index 2000 (SLEDAI-2K) [20], and SLEDAI-2K>4 was defined as active SLE [21]. Patients exhibiting a serological cure and those presenting with pregnancy, malignant diseases, heart failure, or hematological disease were excluded from the study. The diagnosis of lupus nephritis (LN) depended on urine analysis, renal function tests, and renal biopsies. An independent pathologist classified the renal biopsies of LN patients according to the International Society of Nephrology-Renal Pathology Society (ISN/RPS) classification. Active LN was defined as urine protein excretion ≥ 500 mg/day or cellular casts [22].
The most common medicine used by patients was prednisone (94/101, 93.06%), and some patients combined prednisone with chloroquine or chloroquine plus azathioprine (64/94, 68.08%). Methotrexate plus prednisone and chloroquine was used in fewer patients (28/94, 29.78%). Cyclophosphamide was rarely used (10/94, 10.63%). These data are not shown.
Detection of ANAs of different isotypes by indirect immunofluorescence
Total ANAs were screened with indirect immunofluorescence on human epithelial type-2 (Hep-2) cells using commercially available ANA Hep-2 (Euroimmun Medizinische Labordiagnostika, Germany) as recommended by the manufacturer. Briefly, serum was diluted to 1:100, 1:10, or 1:40 with PBS for IgG, IgM, and IgA ANAs, respectively, and then applied to substrate glass slides coated with Hep-2 cells. After incubating for 30 min, the slides were washed with PBS, and FITC-labeled anti-human IgG, IgM, and IgA antibodies were added to the corresponding slides. After incubating for another 30 min, the slides were washed with PBS and sealed with coverslips. IgM was extracted by mixing 20-μl serum samples with a 180-μl IgG/RF adsorbent. After incubating at 25 °C for 15 min, the mixture was centrifuged at 2000 rpm for 10 min, and the supernatant of IgM antibody was diluted 10 times.
To detect HA IgG ANAs in the serum of patients, 8-M urea treatment was used, which allowed low-affinity antibodies to dissociate from their cognate antigen, while high-affinity antibodies were unaffected. After continuous exploration, the experimental process was as follows: serum was diluted 1:10 with PBS and incubated with Hep-2 cells for 40 min. After washing with PBS once for 5 min, 8 M urea was applied to denature the low-affinity IgG ANAs and incubated for 30 min. FITC-labeled anti-human IgG (Euroimmun Medizinische Labordiagnostika, Germany) was added after washing once. After incubating for another 30 min and washing with PBS, the slides were sealed with coverslips. All the reactions were performed at room temperature. A single individual, blinded to the clinical data, read all tests with a fluorescence microscope using an LED light source.
Detection of total IgG ANAs, anti-dsDNA antibodies, anti-C1q antibodies, C3 and C4
Total IgG ANA levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (Euroimmun Medizinische Labordiagnostika, Germany). Anti-dsDNA IgG antibody levels were measured using ELISA kits (Shanghai Kexin, China). Anti-C1q antibody levels were measured using ELISA kits (Wuhan Jianglai, China). All assays were analyzed on an automatic ELISA reader (Thermo Scientific, USA) according to the manufacturer’s instructions. Positivity cutoffs were set at ≥ the optical density value of the calibration standard for total IgG ANAs, ≥ 100 IU/ml for anti-dsDNA antibodies and ≥ 638.21 μg/ml for anti-C1q antibodies according to the manufacturer’s instructions. C3 and C4 levels were determined by nephelometry with the Image Immunochemistry System (Roche cobas 8000, Germany).
Detection of the avidities of IgG ANAs and anti-dsDNA IgG antibodies by ELISA
Serum samples were diluted 1:10 or 1:100 for the avidities of IgG ANAs and anti-dsDNA IgG antibodies, respectively. The diluted serum samples were incubated in two parallel sets of microwell plates coated with ANA and dsDNA antigens (Euroimmun Medizinische Labordiagnostika, Germany, and Shanghai Kexin, China). After incubating for 30 min at 25 °C, the plates were washed 5 times with a wash buffer. Then, PBS was added to one plate, and 8 M urea was added to the other plate. After incubating for 6 min, the urea and PBS were washed 3 times with the wash buffer. The plates were then processed for an incubation with a rabbit anti-human antibody as in the standard test. To evaluate the results more objectively, the relative avidity index (RAI) was calculated by the following formula: absorbance value of samples treated with urea/absorbance value of samples not treated with urea×100%, and the cutoff value was 40%.
Statistical analysis
Statistical analyses were carried out using SPSS software (version 20.0; IBM Inc., New York, USA). The associations between qualitative variables were evaluated by the chi-squared test or Fisher’s exact test, as appropriate. Odds ratios (ORs) were estimated with 95% confidence intervals (CIs) from a risk assessment analysis. A t test was performed to analyze the significance of differences between HA IgG ANA and HA dsDNA IgG at the different times evaluated. The Spearman test was used to analyze the correlation between the RAI and disease activity. The significance level was set to p < 0.05.
Results
Participant characteristics and laboratory results in different tests for ANA antibodies
As shown in Table 1, the prevalence of IgG ANAs detected by IIF (χ2 = 117.109, p = 0.000), IgG ANAs detected by ELISA (χ2 = 121.294, p = 0.000), HA IgG ANAs detected by IIF (χ2 = 80.736, p = 0.000), anti-dsDNA IgG detected by ELISA (χ2 = 62.090, p = 0.000), low C3 levels (χ2 = 71.599, p = 0.000), low C4 levels (χ2 = 71.599, p = 0.000), and anti-C1q antibodies (χ2 = 41.845, p = 0.000) were different among the SLE group, the other autoimmune disease group, and the healthy control group. In the SLE group, 93 patients (92.1%) were IgG ANA–positive by IIF, 71 patients (70.3%) were HA IgG ANA–positive by IIF, 35 patients (34.7%) were IgA ANA–positive by IIF, and 26 patients (25.7)% were IgM ANA–positive by IIF. The ELISA results showed positivity for total IgG ANAs, anti-dsDNA IgG antibodies, and anti-C1q antibodies in 43 patients (42.6%), 91 patients (92.1%) and 40 patients (38.5%), respectively. In total, 63 patients (62.4%) and 22 patients (21.8%) were positive for the complement molecules C3 and C4, respectively. In comparisons between the SLE and other autoimmune disease groups, there were statistically significant differences for all antibodies except for the IgA and IgM antibodies. In the other autoimmune disease group, the numbers of positive results for IgG ANA IIF, IgG ANA ELISA, HA IgG ANA IIF, anti-dsDNA IgG ELISA, low C3 level detection, low C4 level detection, and anti-C1q antibody detection were 41 (61.2%), 36 (53.7%), 26 (38.8%), 2 (3.0%), 17 (25.4%), 5 (7.5%), and 4 (5.97%), respectively. In the healthy controls, the numbers of slightly positive results for IgG ANA IIF, IgG ANA ELISA, IgA IIF, IgM IIF, and anti-C1q antibody detection were 5 (8%), 2 (3%), 3 (5%), 2 (3%), and 3 (4.65%), respectively. Moreover, there were no positive cases for HA IgG ANA evaluated by IIF, low C3 expression, or low C4 expression.
Associations of the presence of ANAs of specific isotypes, anti-dsDNA IgG antibodies, low C3 levels, low C4 levels, and anti-C1q antibodies with active/inactive SLE patients, LN/non-LN groups, and active/inactive LN cases
In our cohort of 101 SLE patients, the mean value of the SLEDAI scoring system used for the assessment of disease activity was 12. The prevalences of IgG ANAs detected by IIF (97.6% vs. 62.5%, p = 0.000), IgG ANAs detected by ELISA (95% vs. 62.5%, p = 0.000), HA IgG ANA detected by IIF (80% vs. 18.75%, p = 0.000), IgM ANAs detected by IIF (30.6% vs. 0, p = 0.010), anti-dsDNA IgG detected by ELISA (50.6% vs. 0, p = 0.000), low C3 levels (71.8% vs. 12.5%, p = 0.000), and low C4 levels (25.9% vs. 0, p = 0.020) were significantly higher in the active SLE group than in the inactive group. However, there were no significant differences in the prevalences of IgA ANAs detected by IIF and anti-C1q antibodies between the active and inactive SLE patients (Table 2).
In the SLE with renal involvement group, 35 patients (34.7%) were diagnosed with lupus nephritis (LN). We found that the presence of anti-C1q antibodies was correlated with renal involvement (74.29% vs. 13.64%, p = 0.000). However, there were no significant differences in the ANA isotypes, anti-dsDNA IgG antibodies, and low complement molecule levels between the LN and non-LN patients.
We compared the positivity of study ANA isotypes between the group with active LN and the group with inactive LN. The results showed that the prevalences of IgG ANAs detected by IIF (100% vs. 75%, p = 0.009), and HA IgG ANA detected by IIF (65.6% vs. 5.56%, p = 0.000) in the active LN group were significantly higher than those in the inactive group. Associations of IgG ANAs detected by ELISA, IgA ANAs, IgM ANAs, anti-dsDNA antibodies, low C3 levels, and the presence of anti-C1q antibodies with active or inactive LN were not found, as displayed in Table 2.
Diagnostic value of ANA isotypes, anti-dsDNA IgG antibodies, and low C3 and/or C4 levels in determining the disease activity of SLE
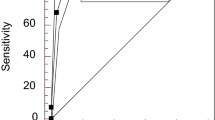
There were no significant differences in the sensitivity or specificity of ANA IgG-class IIF and ELISA for evaluating the disease activity of SLE (97.6% vs. 95.3% and 37.5% vs. 37.5%, respectively, p > 0.05), as shown in Table 2. However, because the IIF ANA test is considered the “gold standard” for the diagnosis of SARD, the IgG ANA ELISA was excluded in the diagnosis of SLE activity. The analysis findings for the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and odds ratio (OR) of ANA isotypes and anti-dsDNA IgG antibodies in predicting the disease activity of SLE are shown in Table 3 and Fig. 1. ANA IgG-class IIF was shown to have the highest sensitivity and lowest specificity (97.6% and 37.5%, respectively), whereas ANA IgM showed the lowest sensitivity and highest specificity (30.6% and 100%, respectively) when assessed alone. HA IgG ANA displayed moderate sensitivity and specificity (80% and 81.3%, respectively). Therefore, we combined HA IgG ANA-class IIF with other antibodies in various combinations to identify active and inactive SLE. The best combination of both high sensitivity (78.9%) and specificity (81.3%) was found for the HA IgG ANA-class IIF and ANA IgG-class IIF groups. However, combination with ANA IgM-class IIF and/or dsDNA IgG ELISA was also positive, with a specificity as high as 100%, despite exhibiting low sensitivity (16.45–45.9%).
However, a moderate PPV, NPV, and OR for the diagnosis of SLE disease activity were found for ANA IgG-class IIF (89.2%, 75%, and 25). The PPV, NPV, and OR for HA IgG ANA-class IIF were 95.8%, 43.3%, and 17.24, respectively, and the values for the combination of the HA IgG ANA-class IIF and ANA IgG-class IIF groups were 95.7%, 40.6%, and 15.385, respectively. The PPV of ANA IgM-class IIF and/or dsDNA IgG ELISA alone or in combination with ANA IgG-class IIF and HA IgG ANA-class IIF were as high as 100%, despite occurrence of a low NPV (18.8–27.6%) and low OR (1.204–2.024). In addition, low C3 and/or C4 levels displayed the second highest sensitivity and specificity (72.9% and 81.3%, respectively). Moreover, the PPV, NPV, and OR were 95.4%, 36.1%, and 36.1%, respectively (Table 3).
Associations of clinical manifestations with ANA isotypes, anti-dsDNA IgG antibodies, low C3 levels, low C4 levels, and anti-C1q antibodies in SLE patients
To evaluate the possible associations of clinical manifestations with each antibody and complement molecule in SLE patients, 94 of the SLE patients who were enrolled in our study were evaluated, but seven patients who had no clinical data were excluded. Since one patient might display one or more clinical manifestations, the number of patients participating in the evaluation of clinical manifestations might include double counts. The clinical profiles of the study participants were categorized into cutaneous (75.53%, 71/94), renal (38.3%, 36/94), arthritic (25.53%, 24/94), neurologic (12.77%, 12/94), hematologic (7.45%, 7/94), gastrointestinal (2.13%, 2/94), and pulmonary (2.13%, 2/94). A chi-square test, a continuous correction chi-square test, and Fisher’s exact test were used to identify associations between the positive results for each antibody and clinical manifestations. As shown in Table 4, the presence of anti-C1q antibodies was positively correlated with renal involvement (65.00% vs. 16.67%, p = 0.000). In contrast, anti-C1q antibodies were negatively correlated with arthritis (12.5% vs. 35.1%, p = 0.013), and low C3 levels were negatively correlated with the hematologic classification (p = 0.012). There were no correlations between ANA isotypes or ds-DNA IgG and clinical manifestations (Table 4).
Associations of HA IgG ANA and HA dsDNA IgG in SLE patients
In our study, 17 patients with SLE were followed as study subjects. HA IgG ANA -class ELISA and HA dsDNA IgG ELISA were performed at 0 months, 1 month, and 3 months. We observed that the HA IgG ANA relative avidity index (RAI) decreased at the same rate as the HA dsDNA IgG RAI after treatment. However, in the comparison between the HA IgG ANA RAI and HA dsDNA IgG RAI, there were significant differences at 1 month (X2 = 30.040, p = 0.046) but not at 0 or 3 months (p > 0.05) (Fig. 2a).
a Comparisons of HA ANA IgG and HA dsDNA IgG before and after treatment of SLE. 0 months indicates the first study visit before treatment, while 1 month and 3 months indicate the visits that occurred at 1 month and 3 months after treatment. Declines in the RAIs of HA ANA IgG and HA dsDNA IgG were observed after treatment. There were significant differences between the HA ANA IgG RAI and HA dsDNA IgG RAI at the 1-month time point (X2 = 30.040, p = 0.046) but not at the first or 3-month time point (p > 0.05). b–d compare the association of the RAI and disease activity between HA ANA IgG and HA dsDNA IgG at different times after treatment. b–d show that the HA ANA IgG RAI was positively related to the SLEDAI at three time points (r = 0.6813, p = 0.0026; r = .5972, p = 0.0114; r = 0.4817, p = 0.0474). There was only one significant correlation between the HA dsDNA IgG RAI and the SLEDAI at the first time points (dsDNA RAI 0) (r = 0.7177, p = 0.0012)
To assess the association between the RAI and disease activity, the Spearman rank correlation test was used. The results showed that the HA IgG ANA RAI was positively related to the SLEDAI after treatment (r = 0.6813, p = 0.0026; r = .5972, p = 0.0114; r = 0.4817, p = 0.0474). There was only one significant correlation between the HA dsDNA IgG RAI and the SLEDAI at the first time point (r = 0.7177, p = 0.0012). However, there were no correlations between the HA dsDNA RAI and disease activity after treatment (r = 0.1115, p = 0.6700; r = 0.3446, p = 0.1755) (Fig. 2b–d).
Discussion
ANAs are a specific class of autoantibodies that have the capability of binding to and destroying certain structures within the nucleus of cells [23], which leads to autoimmune disorders. However, it is assumed that ANA testing should usually be performed only once, and positive results do not need to be repeated [24]. Interestingly, we notice that there are some patients who show decreases in their ANA titers after receiving recommended therapy. In addition, some patients exhibit a continually positive serological reaction that can be disconcerting. Therefore, we introduced high-affinity ANAs to compare the diagnostic value and disease correlation of ANA affinity with other SLE indicators in SLE patients.
In our study, we investigated the diagnostic value of HA IgG ANA, IgG ANAs, IgA ANAs, IgM ANAs, anti-dsDNA IgG, and C3/C4 in SLE patients. Consistent with previous studies, this study showed that IgG ANAs, anti-dsDNA IgG, low C3/C4 levels, and anti-C1q antibodies were highly specific for the diagnosis of SLE, while IgA ANAs and IgM ANAs had no significant associations with SLE [25]. HA IgG ANA was consistent with the above indicators, which exhibited statistically significant differences among SLE patients, patients with other autoimmune diseases, and healthy controls. Then, we studied the performance characteristics of HA IgG ANA and other ANA isotypes in the context of disease activity in SLE patients. The results indicated that IgG ANAs, anti-dsDNA IgG, C3, and C4 presented significant differences between active and inactive SLE, which is consistent with a previous study [20]. Interestingly, the prevalence of HA IgG ANA was significantly higher in active cases than in inactive cases of SLE or LN. This finding suggested that HA IgG ANA presented in the active state, which gradually increased as antibody affinity maturation occurred [26]. However, we were not able to demonstrate a significant association of HA IgG ANA with kidney involvement. The positivity for HA IgG ANA in groups with or without renal involvement was high at 74.3% and 68.2%, respectively. This result was expected because the cross-reactive epitopes of ANA isotypes make it difficult to distinguish the kidney residency or blood circulation of antigen-antibody complexes. This finding is consistent with the results of previous studies on total anti-dsDNA antibodies, which were not associated with lupus nephritis in Brazilian patients [27].
Based on the data for the sensitivity and specificity of ANA isotypes in SLE patients, HA ANAs showed moderate sensitivity and specificity (80% and 81.3%), as did the combination of HA IgG ANA and total IgG ANAs, for distinguishing active and inactive SLE. Although IgM ANAs and anti-dsDNA antibodies showed good specificity (100%), they had low sensitivity (30.6% and 50.6%, respectively). This finding is consistent with the results of previous reports showing that the specificity of anti-dsDNA antibodies has been estimated to be approximately 50% in SLE patients [18, 19]. Since HA IgG ANA showed moderate sensitivity and specificity, we examined its correlations with other ANA isotypes to determine whether it may have some clinical significance in the evaluation of SLE. Unfortunately, HA IgG ANA showed no significant differences among the different clinical manifestations of SLE. Again, there was no correlation between dsDNA and clinical manifestations in SLE patients. This finding is controversial, as it was previously reported that high levels of anti-ds-DNA autoantibodies may or may not be associated with lupus nephritis [17, 27,28,29]. We speculate that our observation is limited by the absence of clinical data.
Some reports have shown that HA dsDNA IgG detected by ELISA is significantly correlated with disease activity [16, 17]. We performed HA ANA ELISA and HA dsDNA ELISA at different times after treatment in 17 patients with SLE who were followed and performed relative avidity index (RAI) calculations to analyze the differences among the patients. The results showed that the HA IgG ANA RAI decreased as the HA dsDNA IgG RAI decreased after treatment. Interestingly, the results showed that the HA IgG ANA RAI was positively related to the SLEDAI after treatment at three time points. However, there was no correlation between the HA dsDNA RAI and disease activity after treatment. We suspected that HA IgG ANA could compensate for the rate of missed dsDNA-seronegative patients because of the rich antigens targeted by ANAs in SLE. HA IgG ANA could better reflect the overall antigen spectrum status of patients and be more related to the activity of the disease than dsDNA IgG.
In our study, the results showed that low C3 levels were associated with the disease activity of SLE. In addition, combined with low C4 levels, low C3 levels showed good sensitivity and specificity. This finding is consistent with the results of previous reports, which were included in the immunodiagnostic criteria for SLE [30, 31]. Consistent with most research, anti-C1q antibodies play indispensable roles in the pathogenesis of SLE, especially in distinguishing LN from non-LN [28]. Conversely, a negative correlation between anti-C1q antibodies and arthritis was demonstrated in our study. We hypothesized that anti-C1q antibodies might play a protective role in arthritis in SLE. In addition, a negative correlation between low C3 levels and the hematologic classification was demonstrated in our study.
Our study has several limitations. The number of subjects in this experiment is not large enough, and we need a larger sample size to confirm our conclusions. The progressive decrease in the time elapsed between disease onset and diagnosis is one of the major contributors to improvements in the survival [32] and quality [33] of life of SLE patients, which means that additional efforts should be made to make the diagnosis of SLE as early as possible [34, 35]. However, we have not yet evaluated HA IgG ANA in terms of prognosis.
In conclusion, our study confirmed the diagnostic value of HA IgG ANA with other ANS isotypes in SLE patients. HA IgG ANA, IgG ANAs, IgM ANAs, and anti-dsDNA IgG can assist in identifying the disease activity of SLE. In addition, HA IgG ANA was evaluated in determining the activity of LN. Furthermore, HA IgG ANA was regarded as having a moderate PPV, NPV, and OR for the diagnosis and disease activity prediction of SLE and correlated with the SLEDAI. Therefore, HA IgG ANA is more suitable than HA dsDNA in correlating with the SLEDAI in the treatment process and can better reflect the overall disease activity state of patients. Even so, HA IgG ANA has a high prevalence in SLE patients and may be an important biomarker of active disease after treatment.
References
Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, de la Torre IG, Herold M, Klotz W, Cruvinel WD, Mimori T, von Muhlen C, Satoh M, Chan EK (2019) Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis 78(7):879–889
Aringer M, Costenbader KH, Brinks R, Boumpas D, Daikh D, Jayne D, Kamen D, Mosca M, Ramseygoldman R, Smolen JS (2018) OP0020 Validation of new systemic lupus erythematosus classification criteria. Annual European Congress of Rheumatology, EULAR 2018, Amsterdam, 13–16 June 2018; 2018
Swaak T, Smeenk R (1985) Detection of anti-DSDNA as a diagnostic tool - a prospective study in 441 non-systemic lupus-erythematosus patients anti-DSDNA antibody (anti-DSDNA). Ann Rheum Dis 44(4):245–251
Lopez-Hoyos M, Cabeza R, Martinez-Taboada VM, Crespo J, SanSegundo D, Blanco R, Lopez-Escribano H, Pena M, Rodriguez-Valverde V (2005) Clinical disease activity and titers of anti-dsDNA antibodies measured by an automated immunofluorescence assay in patients with systemic lupus erythematosus. Lupus 14(7):505–509
Compagno M, Jacobsen S, Rekvig OP, Truedsson L, Heegaard NH, Nossent J, Jonsen A, Jacobsen RS, Eilertsen GO, Sturfelt G, Bengtsson AA (2013) Low diagnostic and predictive value of anti-dsDNA antibodies in unselected patients with recent onset of rheumatic symptoms: results from a long-term follow-up Scandinavian multicentre study. Scand J Rheumatol 42(4):311–316
Arbuckle MR, James JA, Kohlhase KF, Rubertone MV, Dennis GJ, Harley JB (2001) Development of anti-dsDNA autoantibodies prior to clinical diagnosis of systemic lupus erythematosus. Scand J Immunol 54(1–2):211–219
Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349(16):1526–1533
Nossent JC, Huysen V, Smeenk RJ, Swaak AJ (1989) Low avidity antibodies to double stranded DNA in systemic lupus erythematosus: a longitudinal study of their clinical significance. Ann Rheum Dis 48(8):677–682
Ghirardello A, Doria A, Zampieri S, Tarricone E, Tozzoli R, Villalta D, Bizzaro N, Piccoli A, Gambari PF (2004) Antinucleosome antibodies in SLE: a two-year follow-up study of 101 patients. J Autoimmun 22(3):235–240
Pisetsky DS (2016) Anti-DNA antibodies--quintessential biomarkers of SLE. Nat Rev Rheumatol 12(2):102–110
Berden JH (1997) Lupus nephritis. Kidney Int 52(2):538–558
Sui M, Lin Q, Xu Z, Han X, Xie R, Jia X, Guo X, Zhang W, Guan X, Ren H (2013) Simultaneous positivity for anti-DNA, anti-nucleosome and anti-histone antibodies is a marker for more severe lupus nephritis. J Clin Immunol 33(2):378–387
Cortes-Hernandez J, Ordi-Ros J, Labrador M, Bujan S, Balada E, Segarra A, Vilardell-Tarres M (2004) Antihistone and anti-double-stranded deoxyribonucleic acid antibodies are associated with renal disease in systemic lupus erythematosus. Am J Med 116(3):165–173
Steward MW (1981) The biolgoical significance of antibody affinity. Immunol Today 2(7):134–140
Hedman K, Hietala J, Tiilikainen A, Hartikainen-Sorri AL, Raiha K, Suni J, Vaananen P, Pietilainen M (1989) Maturation of immunoglobulin G avidity after rubella vaccination studied by an enzyme linked immunosorbent assay (avidity-ELISA) and by haemolysis typing. J Med Virol 27(4):293–298
Suh-Lailam BB, Chiaro TR, Davis KW, Wilson AR, Tebo AE (2011) Evaluation of a high avidity anti-dsDNA IgG enzyme-linked immunosorbent assay for the diagnosis of systemic lupus erythematosus. Int J Clin Exp Pathol 4(8):748–754
Oliveira RC, Oliveira IS, Santiago MB, Atta M, Atta AM (2015) High avidity dsDNA autoantibodies in Brazilian women with systemic lupus erythematosus: correlation with active disease and renal dysfunction. J Immunol Res. https://doi.org/10.1155/2015/814748
Kim HA, Jeon JY, Choi GS, Sung JM, Kim MJ, Yun JM, Suh CF (2008) The antichromatin antibodies can be useful as a diagnostic tool and disease activity marker of systemic lupus erythematosus in Koreans. Clin Immunol 128(2):277–283
Atta AM, Santiago MB, Guerra FG, Pereira MM, Sousa Atta ML (2010) Autoimmune response of IgE antibodies to cellular self-antigens in systemic lupus erythematosus. Int Arch Allergy Immunol 152(4):401–406
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35(6):630–640
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25(11):1271–1277
Walravens M (1987) Systemic diseases and the detection of antinuclear and anticytoplasmic antibodies. An historical review. Clin Rheumatol 6(Suppl 1):9–17
Kumar Y, Bhatia A, Minz RW (2009) Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: a journey revisited. Diagn Pathol 4:1
Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sanchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64(8):2677–2686
Ray SK, Putterman C, Diamond B (1996) Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci U S A 93(5):2019–2024
Atta AM, Pereira MM, Santiago M, Sousa-Atta ML (2009) Anti-dsDNA antibodies in Brazilian patients of mainly African descent with systemic lupus erythematosus: lack of association with lupus nephritis. Clin Rheumatol 28(6):693–697
Jia Y, Zhao LL, Wang CY, Shang J, Miao Y, Dong YY, Zhao ZZ (2018) Anti-Double-Stranded DNA Isotypes and anti-C1q antibody improve the diagnostic specificity of systemic lupus erythematosus. Dis Markers. https://doi.org/10.1155/2018/4528547
Wang W, Li JM (2011) Predominance of IgG1 and IgG3 subclasses of autoantibodies to peptidylarginine deiminase 4 in rheumatoid arthritis. Clin Rheumatol 30(4):563–567
Kim AHJ, Strand V, Sen DP, Fu Q, Mathis NL, Schmidt MJ, Bruchas RR, Staten NR, Olson PK, Stiening CM, Atkinson JP (2019) Association of blood concentrations of complement split product iC3b and serum C3 with systemic lupus erythematosus disease activity. Arthritis Rheum 71(3):420–430
Birmingham DJ, Irshaid F, Nagaraja HN, Zou X, Tsao BP, Wu H, Yu CY, Hebert LA, Rovin BH (2010) The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus 19(11):1272–1280
Doria A, Iaccarino L, Ghirardello A, Zampieri S, Arienti S, Sarzi-Puttini P, Atzeni F, Piccoli A, Todesco S (2006) Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med 119(8):700–706
Doria A, Rinaldi S, Ermani M, Salaffi F, Iaccarino L, Ghirardello A, Zampieri S, Della Libera S, Perini G, Todesco S (2004) Health-related quality of life in Italian patients with systemic lupus erythematosus. II. Role of clinical, immunological and psychological determinants. Rheumatology (Oxford) 43(12):1580–1586
Doria A, Arienti S, Rampudda M, Canova M, Tonon M, Sarzi-Puttini P (2008) Preventive strategies in systemic lupus erythematosus. Autoimmun Rev 7(3):192–197
Doria A, Briani C (2008) Lupus: improving long-term prognosis. Lupus 17(3):166–170
Funding
This work is supported by Fujian Provincial Department of Science and Technology (2014-CXB-44) and the Natural Science Foundation of Fujian Province (2017J01378).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Y-LZ, YX, Y-QL. Performed the experiments: X-LW, YZ, FP, J-JW, C-LJ, S-QL. Analyzed the data: Y-LZ, YX. Contributed reagents/materials/analysis tools: Y-QL, L-CJ. Wrote the paper: YX, Y-LZ. Revised the manuscript critically for important intellectual content: Y-LZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent
The study was approved by the Institutional Ethics Committee of Zhongshan Hospital, School of Medicine, Xiamen University, and conforms to the ethical guidelines of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, Y., Lin, Y., Wang, X. et al. Assessment of a high-avidity IgG ANAs for the diagnosis and activity prediction of systemic lupus erythematosus. Clin Rheumatol 39, 2619–2629 (2020). https://doi.org/10.1007/s10067-020-05040-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05040-4