Abstract
Objectives
To investigate the cross-sectional associations between suprapatellar pouch effusion-synovitis and serum levels of cartilage oligomeric matrix protein (COMP), high sensitivity C-reaction protein (hs-CRP), knee symptom, and structural changes in patients with symptomatic knee osteoarthritis (OA).
Method
A total of 173 subjects were included. The osteophytes, joint space narrowing (JSN), and radiographic severity of OA were determined using X-ray. Cartilage defects, bone marrow lesions (BMLs), and suprapatellar pouch effusion-synovitis were assessed using magnetic resonance imaging. Serum levels of COMP and hs-CRP were measured by enzyme-linked immunosorbent assay. The knee joint symptom was self-reported using visual analogue scale.
Results
In this OA cohort, after adjustment for age, sex, and BMI, the presence of pathological effusion-synovitis was associated with serum levels of COMP (β: 30.98, P = 0.018), and suprapatellar pouch effusion-synovitis maximum areas were associated with serum hs-CRP levels. Both suprapatellar pouch effusion-synovitis maximum area and grade were associated with osteophytes and Kellgren-Lawrence scores (ORs: 1.29–1.54, all P < 0.05). In patients with high tertile of hs-CRP, both suprapatellar pouch effusion-synovitis maximum area and grade were associated with cartilage defects at lateral and medial tibiofemoral sites (ORs: 3.01–8.41, all P < 0.05) after adjustment for covariates. In female patients, the significant associations were present between suprapatellar pouch effusion-synovitis and medial tibiofemoral BMLs (ORs: 1.43–1.53, all P < 0.05) after adjustment for covariates.
Conclusions
Suprapatellar pouch effusion-synovitis was associated with serum levels of COMP as well as hs-CRP and knee structural abnormalities in patients with knee OA. These suggested that effusion-synovitis may play a role in knee OA.
Key Points • Suprapatellar pouch effusion-synovitis is associated with serum levels of COMP in patients with knee OA. • Suprapatellar pouch effusion-synovitis is associated with cartilage defects in knee OA patients with high systemic inflammation. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA), the most common joint disease, is not only characterized by cartilage destruction but also by alteration of subchondral bone and synovial tissue metabolism [1]. There are four stages of the progressive trajectory of OA, which are the stages with biochemical changes, magnetic resonance imaging (MRI) evident changes, radiographically evident changes, and joint failure [1]. Unfortunately, almost all patients with OA are definitively diagnosed based on symptoms and radiographic images only when the destruction of joint tissue is irreversible [2]. MRI and serum biomarkers can allow us to understand the earlier stages of this disease.
Synovial activation, namely, hyperplasia and inflammation, has proved as a critical component of OA and a potential predictor of disease progression [3]. OA patients have shown low-grade chronic synovitis with a production of proinflammatory cytokines [4, 5]. It has been demonstrated that inflamed synovium produces catabolic and pro-inflammatory mediators such as interleukin 17, nitric oxide, prostaglandin E2, and neuropeptides, which lead to excess production of the proteolytic enzymes responsible for cartilage breakdown and disturbing the homeostasis of cartilage matrix degradation and repair [6, 7]. These suggested that synovial inflammation may be associated with knee structural changes; however, the relationship between them was still debated [8,9,10]. Effusion-synovitis are typically assessed with qualitative description, while in OA research the current standard is semi-quantitative scales such as the Whole Organ Magnetic Resonance Imaging Score (WORMS) [11]. Effusion-synovitis was distinguished in four subregions according to the anatomy of the knee joint synovial cavity, which are suprapatellar pouch, central portion, posterior femoral recess, and subpopliteal recess [12]. 89.2% of knees exhibited at least one subregion with a minimum grade 2 of effusion-synovitis in patients with knee OA [13]. In addition, Wang et al. reported that knee effusion-synovitis, particularly in suprapatellar pouch, may be causally related to cartilage defects [14].
Serum biomarkers can reflect the breakdown of cartilage matrix proteins. Cartilage oligomeric matrix protein (COMP) is a pentameric protein of the thrombospondin family which can bind type I, II, and IX collagens [15]. It is synthesized by chondrocytes, synovial cells, and other cells of the skeleton and increased in chondrocytes and synovial cells when activated by proinflammatory cytokines [15]. COMP is a promising predictor of cartilage loss, active synovitis, as well as accelerated joint erosion [16, 17], and it may be useful for identifying patients at high risk of progression and assessing therapeutic response in OA because of its faster response compared with X rays [18, 19]. So far, the association between effusion-synovitis and serum COMP is largely unclear.
Therefore, this study aimed to investigate the associations between MRI-detected knee suprapatellar pouch effusion-synovitis and serum levels of COMP, hs-CRP, joint symptom, and knee structural changes including osteophytes (OP), joint space narrowing (JSN), cartilage defect, and bone marrow lesions (BMLs) in patients with knee OA.
Methods
Subjects
A total of 205 patients who fulfilled the American College of Rheumatology criteria for the classification of clinical knee OA [2] were consecutively recruited from the Department of Rheumatology and Immunology in the First Affiliated Hospital of Anhui Medical University, from January 2012 to November 2013, to the Anhui Osteoarthritis (AHOA) study. Participants with rheumatoid arthritis or other arthritis and having knee surgery recently or contraindications to MRI were excluded. Thirty-two patients were excluded from the study because of incomplete data, leaving 173 patients. The study was approved by the First Affiliated Hospital, Anhui Medical University Ethics Committee (ethics approval number: H1000589), and written informed consent were obtained from all participants.
Knee MRI assessment
The painful knee (the worse one if both were affected; the right knee if both knees were equally painful) of each patient was imaged on a 3.0-T whole-body MR unit (GE Signa 3.0 T HDXT, USA) and a T2-weighted fat suppressed fast spin echo (flip angle 90°, repetition time 3067 ms, echo time 112 ms, field of view 16 cm, 15 partitions, 228 × 256-pixel matrix) was used [20]. Sagittal images were obtained at a slice thickness of 4 mm with an interslice gap of 0.5 to 1.0 mm [20]. All the structural changes were determined using OsiriX software [21].
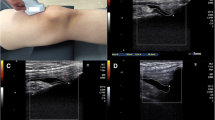
Knee suprapatellar pouch effusion-synovitis was assessed as the presence of an intra-articular fluid-equivalent signal which extends superiorly from the upper surface of the patellar, between the posterior suprapatellar fat pad and the anterior surface of the femur on T2-weighted MRI. Suprapatellar pouch effusion-synovitis grade was scored from 0 to 3 in terms of the estimated maximal distention of the synovial cavity individually according to WORMS [11]. Pathological effusion-synovitis was defined as a score of ≥ 2 [8]. The effusion-synovitis maximal area was selected from the effusion-synovitis area of each slice directly generated in the entire series of images. Two observers measured suprapatellar pouch effusion-synovitis maximal area. The intra-observer and inter-observer reproducibility was 0.81 and 0.60, respectively [22].
Cartilage defects were assessed on the T2-weighted MRIs at lateral tibial plateau (LT), medial tibial plateau (MT), lateral femoral plateau (LF), and medial femoral plateau (MF) sites [11] and scored 0–4 using the modified Outerbridge Score [23] as described in our previous study [21]. A cartilage defect had to be present in at least two consecutive slices. Lateral tibiofemoral cartilage defects were the sum of LT and LF cartilage defects. Medial tibiofemoral cartilage defects were the sum of MT and MF cartilage defects. The intraclass correlation coefficients (ICCs) for intra- and inter-observer reproducibility were 0.85–0.93 and 0.89–0.94, respectively [21].
Subchondral BMLs were assessed at the medial and lateral tibial, medial, and lateral femoral on T2-weighted and defined as the discrete region of increased signal intensity in the marrow space adjacent to subcortical bone. A semi-quantitative scoring system was used to evaluate the grade of BMLs from 0 to 3 based on the extent of regional involvement as previously described [14]. Lateral tibiofemoral BMLs were the sum of LT and LF BMLs. Medial tibiofemoral BMLs were the sum of MT and MF BMLs. Intra-observer repeatability was assessed in 50 subjects between two readers with ICCs from 0.89 to 1.00 at different sites [24].
Knee X-ray assessment
A standing anteroposterior semiflexed view of the painful knee (as previously defined) with 15° of fixed knee flexion was performed in all patients. Each knee joint was scored for osteophytes and joint space narrowing (JSN), each on a scale of 0–3, according to Osteoarthritis Research Society International (OARSI) atlas [25]. Kellgren-Lawrence (K-L) grading system (grades 0–4) was used to assess the radiographic severity of OA [26]. Radiographic OA (ROA) was defined as K-L grade of ≥ 2 [27]. Two investigators evaluated the grade of JSN and osteophytes. The intraclass correlation coefficient (ICC) was 0.95 for osteophytes and 0.93 for JSN. The interclass correlation coefficient was 0.90 for osteophytes and 0.88 for JSN.
Joint symptom assessments
Using visual analogue scale (VAS) 0–10, the knee joint pain and stiffness after first awakening were self-reported.
Serological indicators
Fasting blood samples were collected from patients. Serum was separated and aliquoted into plastic storage tubes stored at − 80 °C till analysis. Serum levels of COMP and hs-CRP were measured by enzyme-linked immunosorbent assay (R&D Systems, USA) kits according to manufacturer instructions. The optical density was measured at 450 nm using an automatic ELISA reader (Sunrise; Tecan, Mannedorf, Switzerland).
Statistical methods
Student’s t-tests, chi-square tests, and the Mann-Whitney U tests were used to compare means, proportions, and medians, respectively. We examined the associations between effusion-synovitis, knee joint symptom, hs-CRP, and COMP using linear regression. Ordinal logistic regression analyses were used to estimate associations between effusion-synovitis and JSN, osteophytes, cartilage defects, and BMLs after adjusted for age, gender, and BMI, and lateral or medial tibiofemoral osteophytes, JSN, cartilage defects or BMLs (where appropriate) were further adjusted in these analyses. All statistical analyses were performed on SPSS 13.0. Statistical significance was set as a P value of ≤ 0.05 (two-tailed).
Results
One hundred seventy-three subjects (85.7%) of females were included in this study. The average age was 55.3 years, and average serum levels of COMP were 127.1 ng/ml. The prevalence of knee suprapatellar pouch effusion-synovitis (≥ 2) was 52.0% (N = 90), and the average maximum area of suprapatellar pouch effusion was 1.220 cm2. As shown in Table 1, participants with or without pathological effusion-synovitis (score 0–1 vs 2–3) were similar in terms of gender, BMI, JSN, cartilage defects, and knee symptom; however, participants with pathological effusion-synovitis were older and had higher proportions of osteophytes and ROA, total BMLs scores, and serum levels of COMP.
Although suprapatellar pouch effusion-synovitis maximum areas and grades were not significantly associated with serum levels of COMP after adjustment for age, sex, and BMI, presence of pathological suprapatellar pouch effusion-synovitis was positively associated with serum levels of COMP after adjustment for confounders (β: 30.98, 95% CI 5.49–56.47, P = 0.018). Suprapatellar pouch effusion-synovitis maximum areas were associated with serum levels of hs-CRP after adjustment for age, sex, and BMI (β: 0.567, 95% CI 0.033–1.101, P = 0.037), while suprapatellar pouch effusion-synovitis grades were not associated with hs-CRP after adjustment for confounders (β: 0.504, 95% CI − 0.108–1.116, P = 0.106). Nonsignificant association between knee suprapatellar pouch effusion-synovitis and knee pain/stiffness was found in patients with knee OA in our study (data not shown).
Suprapatellar pouch effusion-synovitis maximum areas and grades were significantly associated with lateral and medial tibiofemoral of osteophyte and K-L scores after adjustment for age, sex, and BMI (Table 2). Suprapatellar pouch effusion-synovitis maximum areas were associated with medial JSN after adjustment for confounders (Table 2). Suprapatellar pouch effusion-synovitis grades were not associated with JSN after adjustment for confounders (Table 2).
In multivariable analyses, suprapatellar pouch effusion-synovitis maximum areas or grades were not significantly associated with cartilage defects and BMLs (data not shown). However, suprapatellar pouch effusion-synovitis maximum areas and grades were significantly and positively associated with cartilage defects in patients with high tertile of serum levels of hs-CRP (> 2.02 mg/L) and remained significant after further adjustment for lateral or medial tibiofemoral osteophytes, JSN and BMLs (Table 3), but not in patients with low or middle levels of hs-CRP (data not shown). Suprapatellar pouch effusion-synovitis maximum areas were associated with medial and lateral tibiofemoral BMLs in female after adjustment for age, sex, and BMI, while suprapatellar pouch effusion-synovitis grades were associated with medial tibiofemoral but not with lateral tibiofemoral BMLs in female (Table 4). The significant associations apart from that in lateral tibiofemoral portion remained significant after further adjustment for lateral or medial tibiofemoral osteophytes, JSN, and cartilage defects (Table 4). By contrast, suprapatellar pouch effusion-synovitis was not associated with BMLs in male at any site (data not shown).
Discussion
To the best of our knowledge, this is the first study to report the significant associations between suprapatellar pouch effusion-synovitis and serum COMP in patient with knee OA. Our results suggested that suprapatellar pouch effusion-synovitis not only associated with osteophyte, K-L grade, cartilage defect in patients with high tertile of hs-CRP levels, and BMLs in female patients but also associated with serum COMP and hs-CRP which could respectively reflect cartilage degradation and local inflammation. These suggest that suprapatellar pouch effusion-synovitis may be associated with early changes of cartilage by knee OA patients with inflammatory phenotype.
Synovitis has traditionally been considered as a hallmark of inflammatory arthritis but is less relevant to clinical OA, while there is emerging evidence linking synovial inflammation and both early and late OA [4, 28]. The synovial tissues of early OA with hydrarthrosis showed moderate proliferation of fibroblast-like synoviocytes similarly to RA and were immunohistochemically positive for matrix metalloproteinase 3, tumor necrosis factor α, and interleukin 6 [29]. Catabolic and proinflammatory mediators are produced by the inflamed synovium and alter the balance of cartilage matrix degradation and repair, leading to excess production of the proteolytic enzymes responsible for cartilage breakdown [6, 7]. Cartilage alteration in turn amplifies synovial inflammation, creating a vicious circle [7].
MRI has many advantages in visualizing the joint and is useful to investigate early “preclinical” disease before radiographic changes occur [30]. Homogeneous hyperintensity within the joint cavity on MRI proton density (PD)-weighted (w) fat suppressed images represent both effusion and synovial thickening [13]. For this reason, some researchers suggested a combined score of assessing joint effusion and synovitis [11]. Roemer et al. reported that synovitis in the suprapatellar region was shown in 59.5% of 111 patients with knee OA [13], and the prevalence of moderate or larger effusions was 54.6% in patients with knee pain or radiographic OA [31]. Similarly, we found that the prevalence of knee suprapatellar pouch effusion-synovitis of ≥ 2 was 52.1% in patients with symptomatic knee OA.
COMP is an important degradation product of articular cartilage which is synthesized and secreted not only by articular chondrocytes but also by synovial fibroblasts. Increased serum level of COMP has been associated with accelerated joint damage in patients with OA [17]. Compared with JSN assessed by X-ray, or cartilage defects assessed by MRI, cartilage damage could be reflected much earlier through serum COMP. Previous studies of COMP were usually limited to its correlations with osteophytes, cartilage loss, and severity of knee OA [32,33,34]. The relationship between effusion-synovitis and COMP is still on debate. El-Arman et al. observed no significant correlations between COMP levels and joint effusion as well as synovial thickening in patients with knee OA [35], while suprapatellar effusion was related directly to serum COMP levels in women [36]. COMP biomarker can be an indicator of the appearance of effusion (sensitivity = 59%, specificity = 50%), while the connection was not proved between the concentration of COMP with the size of effusion and synovitis [37]. Our study reported that the presence of pathological suprapatellar pouch effusion-synovitis was associated with serum COMP. This weak but significant association suggesting that suprapatellar pouch effusion-synovitis may be associated with early cartilage degradation before irreversible damage has occurred. The inflamed synovium may provide an additional source for the elevated levels of COMP observed in OA [17].
Recently, hs-CRP assays, which can detect CRP levels that are an order of magnitude lower than traditional assays, were used to measure low-level CRP in OA, where there is a local, low-grade inflammatory component. A cross-sectional study observed a trend that CRP had a positive association with effusion among individuals with a normal weight [38]. Our study found that suprapatellar pouch effusion-synovitis maximum areas were significantly associated with serum hs-CRP, which reflects synovial inflammation in OA patients perhaps by means of synovial interleukin 6 production [39, 40]. It was further verified that effusion-synovitis which represents both effusion and synovial thickening reflected local inflammation within the joint in OA patient and suggests that elevated hs-CRP may identify a potential inflammatory osteoarthritic phenotype [39, 40].
The clinical symptoms of OA include pain, stiffness, and dysfunction. As one of the most important symptoms of OA, knee pain is multifactorial and has many risk factors which may include body mass index [41] and knee structural abnormalities such as cartilage defects and bone marrow lesions [42, 43], as well as inflammation [14]. Previous studies revealed inconsistent associations between knee effusion and pain: some reported that knee effusion was associated with pain in OA [31, 44, 45], while some did not [10, 46]. The reasons underlying these discrepancies are unclear, but they may be due to the variations in study design, sample size, study populations, the nature of the study sample (e.g., age, sex distribution), individual psychological factors, and/or duration of follow-up [47]. Indeed, the acquisition and record of clinical symptoms are relatively simple in our study, and the evaluation of pain and morning stiffness using VAS (0–10) may not be a good approach to reflect the clinical manifestations of OA patients. Above reasons may explain why we did not find significant association between clinical symptoms and effusion in this study.
Effusion-synovitis strongly predicted the development of knee ROA [48]. Atukorala et al. found that pro-inflammatory cytokines, such as tumor necrosis factor alpha and interleukin 6, contributed to OA pathogenesis by increasing cartilage degradation and indicated that synovial membrane inflammation played a role in ROA [48, 49]. Roemer et al. found that more intra-articular sites were affected by synovitis and higher grades of joint effusion were associated with higher grades of radiographic tibiofemoral OA [13], and MRI-detected joint effusion was associated with a higher incidence of ROA [50]. JSN and osteophyte scores were positively associated with the change in effusion-synovitis volume [51]. Our study found that suprapatellar pouch effusion-synovitis maximum areas and grades were significantly associated with lateral and medial tibiofemoral OP and K-L scores. These suggested that suprapatellar pouch effusion-synovitis could have a link with structural changes in OA.
Whether knee joint effusion-synovitis was associated with increased knee cartilage defects was still on debate. Hill et al. reported that despite cartilage loss occurring in over 50% of patients with knee OA, synovitis was not associated with cartilage loss in either tibiofemoral or patellofemoral compartment [10]. While Roemer et al. found that synovitis and effusion might increase the risk of cartilage loss in 347 individuals with a statistically borderline significance (P = 0.07) [9]. Joint effusion-synovitis in suprapatellar pouch was consistently associated with cartilage defects and reduced cartilage volume in older adults both cross-sectionally and longitudinally [14]. In patient with OA, proinflammatory mediators are produced by the inflamed synovium and alter the balance of cartilage matrix degradation and repair [6, 7]. The associations between effusion-synovitis and cartilage alteration may be different in patients with different OA phenotypes. Serum high sensitivity C-reactive protein has been shown to correlate well with CRP in synovial fluid and reflects synovial inflammation in OA patients [39, 40]. The median C reactive protein level was 2.03 mg/l in patients with hip osteoarthritis observed by Conrozier et al. [52]. So we choose high tertile of hs-CRP (> 2.02 mg/l) as the cutoff value. Our study found that knee suprapatellar pouch effusion-synovitis was associated with cartilage defects in patients with high tertile of hs-CRP levels, indicating that joint effusion-synovitis was associated with knee cartilage damage by knee OA patients with inflammatory phenotype.
BMLs have been reported to play a pivotal role in knee OA [28, 42], but the causes of the BMLs are still uncertain. There have been only a few studies reporting the association between joint effusion-synovitis and BMLs. A cross-sectional study reported that synovial membrane volume was significantly associated with subchondral BMLs [43]. Wang et al. reported the significant association between effusion-synovitis and BMLs in most joint regions; however, these significant associations disappeared after adjustment for cartilage defects, indicating joint effusion may lead to BMLs via cartilage defects [14]. Our study found suprapatellar pouch effusion-synovitis was associated with medial tibiofemoral BMLs in female patients. It may be that hormonal difference plays a role in explaining sex differences. It has previously been shown that estrogen can act on subchondral bone via receptors and second messengers such as the regulatory polypeptides TGF-α, interfering with osteoclast and osteoblast coupling [53]. Moreover, estrogen and progesterone receptors were found in the lining cells of human synovium [54]. In females, estrogen levels increase at puberty and decrease to levels that are lower than circulating levels in men at menopause [54]. The change of estrogen levels may activate synovial abnormalities and eventually increase cartilage degradation by producing inflammatory mediators. Low serum levels of endogenous estradiol, progesterone, and testosterone are associated with increased knee effusion-synovitis in women with OA [55]. Knee effusion-synovitis may be causally related to cartilage defects, which would lead to BMLs over time [14]. The sex-specific nature of estrogen on articular cartilage is supported by several studies [56, 57]. The effect of estrogen on synovium may also appear to be sex-specific. The variations in sex hormones and other factors that may underlie the sex differences in knee effusion-synovitis and BMLs need to be further explored.
There are several potential limitations to our study. First, it was a cross-sectional study so the causal relationship was unknown. This needs to be determined by further cohort studies. Second, we did not have contrast-enhanced MRI to evaluate synovitis because of the risk of contrast agents and the high price. However, Roemer et al. reported that definite synovitis evaluated by contrast-enhanced MRI was present in 96.3% knees with an effusion [13], which suggested that joint effusion could almost represent synovitis. Third, the sample size was modest particularly in those with high levels of hs-CRP. It is possible that with larger sample size, more significant associations can be detected. Fourth, we did not assess the inter- or intra-observer reproducibility, so measurement error may influence results. However, given all measures were highly reproducible, this is considered unlikely. Last, all patients are Asian, so the results may not generalizable to patients with general knee OA.
In conclusion, suprapatellar pouch effusion-synovitis was associated with serum levels of COMP as well as knee structural abnormalities in patients with knee OA. These suggest that effusion-synovitis may play a role in knee OA.
References
Ding C, Zhang Y, Hunter D (2013) Use of imaging techniques to predict progression in osteoarthritis. Curr Opin Rheumatol 25:127–135
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049
Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M (2005) Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil 13:361–367
Benito MJ, Veale DJ, Fitzgerald O, van den Berg WB, Bresnihan B (2005) Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64:1263–1267
Smith MD, Wetherall M, Darby T, Esterman A, Slavotinek J, Roberts-Thomson P, Coleman M, Ahern MJ (2003) A randomized placebo-controlled trial of arthroscopic lavage versus lavage plus intra-articular corticosteroids in the management of symptomatic osteoarthritis of the knee. Rheumatology (Oxford) 42:1477–1485
Honorati MC, Bovara M, Cattini L, Piacentini A, Facchini A (2002) Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthr Cartil 10:799–807
Trumble TN, Billinghurst RC, Mcilwraith CW (2004) Correlation of prostaglandin E2 concentrations in synovial fluid with ground reaction forces and clinical variables for pain or inflammation in dogs with osteoarthritis induced by transection of the cranial cruciate ligament. Am J Vet Res 65:1269–1275
Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, Lynch JA, Lewis CE, Torner J, Zhang Y (2011) Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis 70:1804–1809
Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, Nevitt MC, Felson DT, Hughes LB, el-Khoury GY, Englund M, Guermazi A, Multicenter Osteoarthritis Study Investigators (2009) Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology 252:772–780
Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, Gale D, Grainger A, Conaghan P, Felson DT (2007) Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 66:1599–1603
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK (2004) Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil 12:177–190
Fenn S, Datir A, Saifuddin A (2009) Synovial recesses of the knee: MR imaging review of anatomical and pathological features. Skelet Radiol 38:317–328
Roemer FW, Javaid MK, Guermazi A et al (2010) Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthr Cartil 18:1269–1274
Wang X, Blizzard L, Halliday A, Han W, Jin X, Cicuttini F, Jones G, Ding C (2016) Association between MRI-detected knee joint regional effusion-synovitis and structural changes in older adults: a cohort study. Ann Rheum Dis 75:519–525
Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, Kraus VB (1999) Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County osteoarthritis project. Arthritis Rheum 42:2356–2364
Hunter DJ, Jiang L, Michael LV et al (2007) Cartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston osteoarthritis knee study. Arthritis Res Ther 9:1–8
Recklies AD, Baillargeon L, White C (1998) Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum 41:997–1006
Lohmander LS, Ionescu M, Jugessur H, Poole AR (1999) Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum 42:534–544
Poole RA (1994) Immunochemical markers of joint inflammation, skeletal damage and repair: where are we now? Ann Rheum Dis 53:3–5
Jones G, Ding C, Scott F, Glisson M, Cicuttini F (2004) Early radiographic osteoarthritis is associated with substantial changes in cartilage volume and tibial bone surface area in both males and females. Osteoarthr Cartil 12:169–174
Ding C, Garnero P, Cicuttini F, Scott F, Cooley H, Jones G (2005) Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthr Cartil 13:198–205
Wang X, Blizzard L, Jin X et al (2016) Quantitative assessment of knee effusion-synovitis in older adults: association with knee structural abnormalities. Arthritis Rheum 68:837–844
Recht MP, Kramer J, Marcelis S, Pathria MN, Trudell D, Haghighi P, Sartoris DJ, Resnick D (1993) Abnormalities of articular cartilage in the knee: analysis of available MR techniques. Radiology 187:473–478
Zhai G, Blizzard L, Srikanth V, Ding C, Cooley H, Cicuttini F, Jones G (2006) Correlates of knee pain in older adults: Tasmanian older adult cohort study. Arthritis Rheum 55:264–271
Altman RD, Gold GE (2007) Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthr Cartil 15 Suppl a: A1-56
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Ding C, Cicuttini F, Parameswaran V, Burgess J, Quinn S, Jones G (2009) Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum 60:1381–1389
Guermazi A, Hayashi D, Roemer FW, Zhu Y, Niu J, Crema MD, Javaid MK, Marra MD, Lynch JA, el-Khoury GY, Zhang Y, Nevitt MC, Felson DT (2014) Synovitis in knee osteoarthritis assessed by contrast-enhanced magnetic resonance imaging (MRI) is associated with radiographic tibiofemoral osteoarthritis and MRI-detected widespread cartilage damage: the MOST study. J Rheumatol 41:501–508
Kurose R, Kakizaki H, Akimoto H, Yagihashi N, Sawai T (2016) Pathological findings from synovium of early osteoarthritic knee joints with persistent hydrarthrosis. Int J Rheum Dis 19:465
Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio le Graverand-Gastineau MP, Keen H, Roemer FW (2008) Imaging in osteoarthritis. Rheum Dis Clin N Am 34:645–687
Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, Felson DT (2001) Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol 28:1330–1337
Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegård D, Dieppe PA (1995) Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol 34:306–310
Berry PA, Maciewicz RA, Wluka AE, Downey-Jones MD, Forbes A, Hellawell CJ, Cicuttini FM (2010) Relationship of serum markers of cartilage metabolism to imaging and clinical outcome measures of knee joint structure. Ann Rheum Dis 69:1816–1822
Eckstein F, Le GM, Charles HC et al (2011) Clinical, radiographic, molecular and MRI-based predictors of cartilage loss in knee osteoarthritis. Ann Rheum Dis 70:1223–1230
El-Arman MM, El-Fayoumi G, El-Shal EW, El-Boghdady I, El-Ghaweet A (2010) Aggrecan and cartilage oligomeric matrix protein in serum and synovial fluid of patients with knee osteoarthritis. HSSJ 6:171–176
Kumm J, Tamm A, Lintrop M, Tamm A (2009) Association between ultrasonographic findings and bone/cartilage biomarkers in patients with early-stage knee osteoarthritis. Calcif Tissue Int 85:514–522
Zivanović S, Rackov LP, Zivanović A, Jevtić M, Nikolić S, Kocić S (2011) Cartilage oligomeric matrix protein - inflammation biomarker in knee osteoarthritis. Bosn J Basic Med Sci 11:27–32
Stout AC, Barbe MF, Eaton CB et al (2018) Inflammation and glucose homeostasis are associated with specific structural features among adults without knee osteoarthritis: a cross-sectional study from the osteoarthritis initiative. BMC Musculoskelet Disord 19:1
Stürmer T, Brenner H, Koenig W, Günther K-P (2004) Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann Rheum Dis 63:200–205
Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo E, Peterson M, Sculco TP, Crow MK (2007) Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr Cartil 15:516–523
Ding C, Cicuttini F, Scott F, Cooley H, Jones G (2005) Knee structural alteration and BMI: a cross-sectional study. Obes Res 13:350–361
Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, Kazis L, Gale DR (2001) The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 134:541–549
Sowers M, Hayes C, Jamadar D, Capul D, Lachance L, Jannausch M, Welch G (2003) Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthr Cartil 11:387–393
Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, le Graverand MP, Hunter DJ, OAI Investigators Group (2009) Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr Cartil 17:1562–1569
Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M (2011) Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis 70:60–67
Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, Roemer F, McCulloch C, Felson DT (2011) Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum 63:691–699
Neogi T (2017) Structural correlates of pain in osteoarthritis. Clin Exp Rheumatol 35(Suppl 107):75–78
Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, Hunter DJ (2016) Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis 75:390–395
Orita S, Koshi T, Mitsuka T et al (2011) Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord 12:144
Roemer FW, Kwoh CK, Hannon MJ et al (2015) What comes first? Multi-tissue involvement leading to radiographic osteoarthritis: MRI-based trajectory analysis over 4 years in the osteoarthritis initiative. Arthritis Rheum 67:2085–2096
Wang X, Jin X, Blizzard L, Antony B, Han W, Zhu Z, Cicuttini F, Wluka AE, Winzenberg T, Jones G, Ding C (2017) Associations between knee effusion-synovitis and joint structural changes in patients with knee osteoarthritis. J Rheumatol 44:1644–1651
Conrozier T, Carlier MC, Mathieu P, Colson F, Debard AL, Richard S, Favret H, Bienvenu J, Vignon E (2000) Serum levels of YKL-40 and C reactive protein in patients with hip osteoarthritis and healthy subjects: a cross sectional study. Ann Rheum Dis 59:828–831
Seyedin SM, Thompson AY, Benz H, Rosen DM, Piez KA (1986) Cartilage factor-a. J Biol Chem 261:5693–5695
Boyan BD, Hart DA, Enoka RM et al (2013) Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol Sex Differ 4:3
Jin X, Wang BH, Wang X, Antony B, Zhu Z, Han W, Cicuttini F, Wluka AE, Winzenberg T, Blizzard L, Jones G, Ding C (2017) Associations between endogenous sex hormones and MRI structural changes in patients with symptomatic knee osteoarthritis. Osteoarthr Cartil 25:1100–1106
Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS (2007) Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr Cartil 15:695–700
Kinney RC, Week K, Boyan BD (2005) Human articular chondrocytes exhibit sexual dimorphism in their responses to 17beta-estradiol. Osteoarthr Cartil 13:330–337
Acknowledgments
Special thanks go to the subjects who made this study possible.
Funding
This work was supported by National Natural Science Foundation of China (no.81172865).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, X., Ruan, G., Xu, J. et al. Associations between suprapatellar pouch effusion-synovitis, serum cartilage oligomeric matrix protein, high sensitivity C-reaction protein, knee symptom, and joint structural changes in patients with knee osteoarthritis. Clin Rheumatol 39, 1663–1670 (2020). https://doi.org/10.1007/s10067-019-04905-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04905-7