Abstract
Objective
This retrospective clinical study aimed to examine the similarities and differences between connective tissue disease–associated interstitial lung disease (CTD-ILD) and interstitial pneumonia with autoimmune features (IPAF) and to identify the influencing factors of CTD-ILD, with a goal of early detection and active treatment of the disease.
Methods
We conducted a retrospective study of 480 patients: 412 with CTD-ILD and 68 with IPAF. Demographic features, clinical characteristics, laboratory indicators, and chest high-resolution computed tomography (HRCT) imaging data were analyzed.
Results
Compared with the IPAF group, the CTD-ILD group contained more women, and the incidences of joint pain, dry mouth/dry eyes, and Raynaud’s phenomenon were higher; erythrocyte sedimentation rate (ESR) and D-dimer levels were higher; red blood cell (RBC) and hemoglobin (Hb) levels were lower; a high rheumatoid factor (RF) titer (> 2 times the normal upper limit) was observed, and anti-cyclic citrullinated peptide antibody (anti-CCP), anti-keratin antibody (AKA), antinuclear antibody (ANA), and anti-melanoma differentiation-associated gene 5 antibody (anti-MDA5) levels were higher. Compared with CTD-ILD patients, IPAF patients were more likely to present initially with respiratory symptoms, with higher rates of fever, cough and expectoration, dyspnea, and Velcro crackles; anti-Ro52 titers were higher; incidences of honeycombing opacity, reticulate opacity, patchy opacity, and pleural thickening were greater. Female sex, a high RF titer (> 2 times the normal upper limit), anti-CCP positivity, ANA positivity, and anti-MDA5 positivity were risk factors for CTD-ILD when the odds ratios were adjusted.
Conclusion
CTD-ILD and IPAF patients differed in demographic features, clinical characteristics, laboratory indicators, and chest HRCT imaging data. Female sex, a high RF titer (> 2 times the normal upper limit), anti-CCP positivity, ANA positivity, and anti-MDA5 positivity were risk factors for CTD-ILD.
Key Points • This retrospective clinical study comprehensively compared the demographic features, clinical characteristics, laboratory indicators, and chest HRCT imaging data of CTD-ILD and IPAF patients. • The evidence suggested that female sex, a high RF titer, anti-CCP positivity, ANA positivity, and anti-MDA5 positivity were risk factors for CTD-ILD. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Connective tissue disease (CTD) is an autoimmune disease characterized by autoimmune-mediated organ damage that affects multiple systems [1, 2]. When CTD involves the respiratory system, it can lead to interstitial lung disease (ILD), pleural disease, airway disease, vascular disease, and lymphoproliferative disease [2,3,4], with ILD the most common [5]. ILD is one of the most prominent clinical features of CTD [6]. CTD is closely related to ILD. ILD caused by CTD is referred to as connective tissue disease–associated interstitial lung disease (CTD-ILD), which occurs in patients who are diagnosed with both CTD and ILD [7]. The prevalence of ILD caused by CTD ranges from 12.4 to 67.1% [8, 9]. ILD is among the leading causes of mortality in patients with CTD. A study [10] showed a survival rate of only 43.4% within 5 years after diagnosis of ILD in CTD patients. Therefore, timely diagnosis and treatment of ILD are important for CTD patients’ prognosis [11].
ILD is a diffuse lung disease with varying degrees of inflammation and fibrosis in the interstitial region of the lung [7]. The causes of ILD include CTD, pulmonary infection, inhalation lung injury, environmental exposure, tobacco smoke, genetic disorders, and drugs [12, 13]. The most common cause is CTD [13], which can occur in the form of systemic sclerosis (SSc), primary Sjögren’s syndrome (pSS), rheumatoid arthritis (RA), polymyositis/dermatomyositis (PM/DM), mixed connective tissue disease (MCTD), systemic lupus erythematosus (SLE), undifferentiated connective tissue disease (UCTD), anti-neutrophil cytoplasmic antibody-associated vasculitis (ANCA-associated vasculitis), etc. [12]. In general, the relationship between CTD and ILD can be categorized in three groups: (1) ILD occurs in patients with confirmed CTD. (2) ILD is the first manifestation of CTD; about 15% of patients with CTD-ILD are first diagnosed with ILD, or ILD is the only manifestation of CTD, with patients possibly having only ILD symptoms within 5 years after diagnosis of CTD [2, 3, 14, 15]. (3) ILD that has some autoimmune functions but does not meet the established CTD criteria was recently defined as interstitial pneumonia with autoimmune features (IPAF) [16].
IPAF [17] was defined by the European Respiratory Society and the American Thoracic Society (ERS/ATS) in 2015 as diseases characterized by idiopathic interstitial pneumonia in radiology or pathology, as well as autoimmunity without the confirmed criteria of CTD. The classification criteria of IPAF [18] are as follows: (1) High-resolution computed tomography (HRCT) or pulmonary biopsy confirming the presence of interstitial pneumonia. (2) Exclusion of other known causes. (3) Does not meet criteria of defined CTD. (4) At least one feature from two of the following domains: clinical, serologic, or morphologic.
In this study, we retrospectively studied 480 patients. The demographic features, clinical characteristics, laboratory indicators, and chest HRCT imaging data of CTD-ILD and IPAF patients were analyzed to determine the similarities and differences between the diseases, and to identify CTD-ILD risk factors, to help clinicians detect and diagnose the disease early, thereby improving prognosis and delaying progression.
Patients and methods
Clinical information
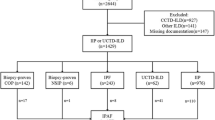
Inpatients with the diagnosis of CTD-ILD or IPAF in the Department of Rheumatology and Immunology of the Second Affiliated Hospital of Chongqing Medical University from January 2016 to January 2019 were selected in this study. Excluded were patients with confirmed and suspected pulmonary infections, lung tumors, asthma, tuberculosis, environmental exposure, and other known causes of ILD. All 480 patients were aware of and agree to participate in the study and gave written consent for their data to be statistically analyzed. The diagnosis of CTD patients complied with the criteria established by American College of Rheumatology [19,20,21]. The diagnosis of ILD patients complied with the criteria established by the Irish Thoracic Society and the Thoracic Society of Australia and New Zealand in 2009 [22]. The diagnosis of IPAF patients complied with the classification criteria established by ERS/ATS in 2015 [18].
Clinical, serological, and imaging data
Patient history, clinical characteristics, laboratory indicators, and imaging data were derived from patients’ medical records during the first clinical consultation. All patients completed a chest HRCT examination. HRCT classification complied with criteria established by ERS/ATS in 2002. All relevant reports were independently analyzed by two radiologists and a rheumatologist. Controversial cases were discussed. Imaging features were evaluated as honeycombing opacity, cystic opacity, reticulate opacity, patchy opacity, ground-glass opacity, traction bronchiectasis, and pleural thickening. Imaging classifications were non-specific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), desquamative interstitial pneumonia (DIP), cryptogenic organizing pneumonia (COP), acute interstitial pneumonia (AIP), and lymphocytic interstitial pneumonia (LIP).
Statistics
Measurement data that conformed to normal distributions were analyzed by independent sample t test and expressed as mean ± standard deviation. Continuous non-normally distributed data were analyzed by Mann-Whitney U test and expressed as the median (first quartile, third quartile). Categorical variables were analyzed by the chi-square test or Fisher’s exact test. Results were regarded as statistically significant when a p value was less than 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 21.0, Chicago, IL, USA).
Results
CTD types causing ILD
A total of 480 patients were included: 412 with CTD-ILD and 68 with IPAF. The types of CTD that caused ILD and the number of patients were RA (171, 41.5%), pSS (56, 13.6%), PM/DM (56, 12.6%), SSc (15, 3.6%), SLE (9, 2.2%), ANCA-associated vasculitis (6, 1.5%), MCTD (5, 1.2%), UCTD (15, 3.6%), and overlap syndrome (83, 20.1%).
Patient demographic features and clinical characteristics
The demographic features and clinical characteristics of the study participants are shown in Table 1. The mean age of all patients was 60.41 ± 12.40 years. Patients with CTD-ILD had ages similar to patients with IPAF. Compared with the IPAF group, female sex, joint pain, dry mouth/dry eyes, and Raynaud’s phenomenon were more common in the CTD-ILD group (p = 0.005, p < 0.001, p < 0.001, p < 0.001), which indicates that female ILD patients with those symptoms were more prone to be CTD-ILD. Compared with the CTD-ILD group, IPAF patients experienced higher rates of initial respiratory manifestations, fever, dyspnea, cough and expectoration, and Velcro crackles (all p < 0.001). Symptoms of Raynaud’s phenomenon and extremities ulcers were not seen in IPAF patients.
Laboratory indicators
Laboratory indicators for the two groups are presented in Table 2. Erythrocyte sedimentation rate (ESR) (p = 0.001), D-dimer (p = 0.002), red blood cell (RBC) (p = 0.001), and hemoglobin (Hb) (p = 0.001) levels were significantly more abnormal in the CTD-ILD group compared to the IPAF group. We found no difference in the levels of fibrinogen (Fib), c-reactive protein (CRP), white blood cell (WBC), platelet (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), blood urea nitrogen (BUN), serum creatinine (Scr), creatine kinase (CK), or lactate dehydrogenase (LDH).
Antibody positivity
Table 3 shows the positive detection of antibodies for the two groups. Compared to the IPAF group, the CTD-ILD group had a higher RF titer (> 2 times the normal upper limit) (p < 0.001) and more abnormal levels of anti-cyclic citrullinated peptide antibody (anti-CCP) (p < 0.001), anti-keratin antibody (AKA) (> 1:10) (p = 0.008), antinuclear antibody (ANA) (≥ 1:320) (p < 0.001), and anti-melanoma differentiation–associated gene 5 antibody (anti-MDA5) (p = 0.002). Positivity for anti-Ro52 was higher in the IPAF group than the CTD-ILD group (p = 0.003). There was no difference in positivity for anti-ds-DNA, anti-RNP/Sm, anti-Sm, anti-SSB, anti-centromere, anti-Scl-70 antibody, anti-nucleosome, anti-synthetase, and anti-SRP between the two groups.
Chest HRCT imaging data
The chest HRCT imaging data of these patients are shown in Table 4. In the two groups, patchy opacity was the most frequently observed feature, followed by pleural thickening and reticulate opacity. A UIP pattern was the most common imaging classification observed in the two groups. However, there was no significant difference between the two groups in the image characteristics, except for honeycombing opacity (p < 0.001), reticulate opacity (p = 0.035), patchy opacity (p < 0.001), and pleural thickening (p = 0.015).
Risk factors for CTD-ILD
Based on the data presented in Tables 1, 2, 3, and 4, we used relatively significant variables to analyze independent risk factors for CTD-ILD using binary logistic regression (Table 5). Female sex (adjusted OR [95% CI] 2.940 (1.462–5.913), p = 0.002), a high RF titer (> 2 times the normal upper limit) (adjusted OR [95% CI] 7.071 (1.938–25.797), p = 0.003), anti-CCP positivity (adjusted OR [95% CI] 5.435 (1.711–17.271), p = 0.004), ANA positivity (adjusted OR [95% CI] 4.924 (1.804–13.437), p = 0.002), and anti-MDA5 positivity (adjusted OR [95% CI] 6.771 (2.030–22.221), p = 0.002) were risk factors for CTD-ILD.
Discussion
CTD often involves the respiratory system and can lead to ILD. ILD is often the first clinical manifestation of CTD and a factor in the poor prognosis of CTD [2, 8, 14]. Our results showed that the CTD-ILD group contained more women than the IPAF group. The age distribution was similar between the two groups, and the age of onset was around 60 years (CTD-ILD group 60.47 ± 12.48 years; IPAF group 60.07 ± 11.99 years). The clinical manifestations of the two groups were similar, but the incidences of joint pain, dry mouth/dry eyes and Raynaud’s phenomenon were higher in CTD-ILD patients than the IPAF patients (all p < 0.001). Compared with the CTD-ILD group, the IPAF group was more likely to present initially with respiratory manifestations, including cough and expectoration, dyspnea, and a high incidence of Velcro crackles (all p < 0.001). We believe this is because lung symptoms and signs, relative to other symptoms, tend to be more prominent in patients with IPAF [10]. Therefore, clinicians must evaluate extrapulmonary clinical symptoms and signs when identifying CTD-ILD and IPAF. When patients have extrapulmonary manifestations, clinicians should try to find the cause of ILD and determine whether CTD exists.
This study found that the CTD-ILD group had faster ESR (p = 0.001), higher D-dimer (p = 0.002), lower RBC (p = 0.001), and lower Hb (p = 0.001) levels than the IPAF group. ESR reflects the severity of systemic inflammatory conditions, and D-dimer is involved in the acute phase of inflammation [23]. These indicators reflect inflammation in the body [24, 25]. In the acute phase of disease, CTD-ILD may affect organ systems due to autoimmune reactions, increasing the systemic inflammatory response compared to IPAF patients and causing faster ESR and higher D-dimer levels. Studies [26,27,28,29,30] show that CTD can cause iron deficiency anemia and autoimmune hemolytic anemia, resulting in decreased RBC and Hb levels. ESR, D-dimer, RBC, and Hb are relatively simple and inexpensive clinical indicators and should be used by clinicians to determine if CTD exists in ILD patients in cases of early changes. When these indicators are abnormal, for early diagnosis and treatment, clinicians need to be alert about whether ILD is a manifestation of CTD.
Our report showed that a high RF titer (> 2 times the normal upper limit), anti-CCP positivity, AKA positivity, and ANA positivity were significantly higher in the CTD-ILD group compared to the IPAF group. Our analysis indicated that a high RF titer (> 2 times the normal upper limit) (adjusted OR [95% CI] 7.07 1 (1.938-25.797), p = 0.003), anti-CCP positivity (adjusted OR [95% CI] 5.435 (1.711-17.271), p = 0.004), and ANA positivity (adjusted OR [95% CI] 4.924 (1.804-13.437), p = 0.002) were risk factors for CTD-ILD after adjusting for other variables at baseline. RF, anti-CCP antibody, and AKA are key factors in RA diagnosis [31]. In this study, RA-ILD patients accounted for a high proportion (41.5%) of study participants; we concluded that a high RF titer (> 2 times the normal upper limit), anti-CCP antibody positivity, and AKA positivity were high risk factors for CTD-ILD. Further studies with larger sample sizes are required to confirm these findings. Previous research [32] found that 95.7% of ILD patients with autoimmune features and 89.1% of other ILD patients have detectable autoantibodies, suggesting that the lungs may be involved in autoimmunity. Some scholars [7] suggested that the association of the lungs with autoimmunity may be due to local inflammation of the lungs, inducing the expression of autoantigens. Autoantibodies in the lungs bind to antigen, causing lung inflammation that aggravates and eventually leads to pulmonary fibrosis. This mechanism clarifies why anti-CCP antibody was detected in the serum of RA patient years before symptom onset [33]. It also explained why anti-CCP positivity increases ILD risk in RA patients [34]. Gudrun Reynisdottir et al. [35] found that common citrullination targets are present in the lungs and joints of RA patients, demonstrating that anti-CCP antibody is associated with the lungs and joints. This finding not only explains why the incidence of joint pain in patients with CTD-ILD was higher than in patients without pulmonary involvement [36], but it also explains why a higher incidence of anti-CCP positivity in patients was associated with higher incidence of joint pain. This finding is consistent with our results—the incidences of anti-CCP positivity and joint pain were higher in the CTD-ILD group compared to the IPAF group. This result may also be because CTD-ILD was in the active phase of CTD, and clinical manifestations were more obvious. We also found that ANA positivity was a risk factor for CTD-ILD, consistent with another study [37], showing that ANA is a characteristic laboratory indicator for most CTD patients. In conclusion, patients with CTD who have a high RF titer (> 2 times the normal upper limit), anti-CCP positivity, AKA positivity, and ANA positivity need to be screened for ILD. Patients with ILD who have a high RF titer (> 2 times the normal upper limit), anti-CCP positivity, AKA positivity, and ANA positivity need to be studied to find the cause of ILD.
According to our study, positivity for anti-Ro52 was significantly higher in the IPAF group compared to the CTD-ILD group (p = 0.003). Marie I et al. [38] reported that anti-Ro52 positivity is associated with cough symptoms, consistent with the conclusion that IPAF is more likely to manifest initially as respiratory symptoms. The report also showed that anti-Ro52 is prone to exhibiting acute symptomatic ILD and lead to progressive deterioration of ILD with increased mortality. Another study [39] indicated that patients with anti-Ro52 and anti-Jo-1 double positivity are more severe than patients with anti-Jo-1 single positivity. Our previous report [40] found that patients with anti-Ro52 and anti-MDA5 double positivity have a worse prognosis than patients with anti-MDA5 single positivity. The studies [38,39,40] demonstrated that anti-Ro52 antibody may be related to the severity of ILD. Therefore, when we find clinically anti-Ro52 positivity but undefined CTD, a chest HRCT is crucial for early detection of lung lesions.
Our results showed that anti-MDA5 positivity in the CTD-ILD group was significantly higher than in the IPAF group (p = 0.002), and anti-MDA5 positivity was a risk factor for CTD-ILD (adjusted OR [95% CI] 6.771 (2.030–22.221), p = 0.002). Anti-MDA5 antibody is closely related to PM/DM [41], often leading to rapid progressive interstitial pneumonia (RP-ILD) [42], with a 6-month survival rate of only 50% and a poor prognosis [43]. Therefore, when clinicians detect anti-MDA5 positivity, they need to be alert to the presence or absence of defined CTD. In addition, a chest HRCT is needed as soon as possible for active treatment and improved prognosis.
This report also found higher incidences of honeycombing opacity (p < 0.001), mesh opacity (p = 0.035), patchy opacity (p < 0.001), and pleural thickening (p = 0.015) on chest HRCT in the IPAF group compared with the CTD-ILD group. A previous study [44] showed that honeycombing opacities in a UIP pattern are more common, and Oldham et al. [45] observed that 54.6% of IPAF patients had UIP patterns from chest HRCT images. These studies showed that IPAF patients exhibited more honeycombing opacity, consistent with our results. In conclusion, when clinicians find chest HRCT images with these features in patients with ILD, they should find the cause of ILD and the underlying disease as soon as possible.
This study has several limitations. First, we selected only inpatients in the Department of Rheumatology and Immunology, where some patients with mild symptoms were treated by our outpatient service. There were also some patients with CTD-ILD or IPAF who were treated by the pneumology department as they presented initially with respiratory symptoms. These factors may have an impact on the outcome. Second, this was a retrospective study that failed to track patient prognoses. Third, this study conducted in only one institute, which caused bias.
In conclusion, this report showed that female sex, joint pain, dry mouth/dry eyes, Raynaud’s phenomenon, high ESR, high D-dimer, low RBC, low Hb, high RF titer (> 2 times the normal upper limit), anti-CCP antibody positivity, ANA positivity, and anti-MDA5 positivity were associated with CTD-ILD. Presenting initially with respiratory manifestations, fever, cough and expectoration, dyspnea, Velcro crackles, anti-Ro52 positivity, honeycombing opacity, mesh opacity, patchy opacity, and pleural thickening on chest HRCT were associated with IPAF. Therefore, when patients with rheumatic immune disease have these manifestations, screening for ILD is necessary. In patients with ILD, clinicians should look for rheumatic immune disease to detect and diagnose underlying diseases earlier. At present, few studies have reported on the association between CTD-ILD and IPAF. In future studies, we will increase our sample size, conduct multicenter studies, and design forward-looking research to better guide clinical work.
References
Antoniou KM, Margaritopoulos G, Economidou F, Siafakas NM (2009) Pivotal clinical dilemmas in collagen vascular diseases associated with interstitial lung involvement. Eur Respir J 33:882–896
Tsuchiya Y, Fischer A, Solomon JJ, Lynch DA (2015) Connective tissue disease-related thoracic disease. Clin Chest Med 36(283-297):ix
Vij R, Strek ME (2013) Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest 143:814–824
Koo SM, Uh ST (2017) Treatment of connective tissue disease-associated interstitial lung disease: the pulmonologist’s point of view. Korean J Intern Med 32:600–610
Swigris JJ, Yorke J, Sprunger DB, Swearingen C, Pincus T, du Bois RM, Brown KK, Fischer A (2010) Assessing dyspnea and its impact on patients with connective tissue disease-related interstitial lung disease. Respir Med 104:1350–1355
Khanna D, Mittoo S, Aggarwal R, Proudman SM, Dalbeth N, Matteson EL, Brown K, Flaherty K, Wells AU, Seibold JR, Strand V (2015) Connective tissue disease-associated interstitial lung diseases (CTD-ILD) - report from OMERACT CTD-ILD Working Group. J Rheumatol 42:2168–2171
Demoruelle MK, Mittoo S, Solomon JJ (2016) Connective tissue disease-related interstitial lung disease. Best Pract Res Clin Rheumatol 30:39–52
Nascimento ECTD, Baldi BG, Sawamura MVY, Dolhnikoff M (2018) Morphologic aspects of interstitial pneumonia with autoimmune features. Arch Pathol Lab Med 142:1080–1089
Hu Y, Wang LS, Wei YR, Du SS, Du YK, He X, Li N, Zhou Y, Li QH, Su YL, Zhang F, Shen L, Weng D, Brown KK, Li HP (2016) Clinical characteristics of connective tissue disease-associated interstitial lung disease in 1,044 Chinese patients. Chest 149:201–208
Kocheril SV, Appleton BE, Somers EC, Kazerooni EA, Flaherty KR, Martinez FJ, Gross BH, Crofford LJ (2005) Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum 53:549–557
Suzuki A, Kondoh Y, Fischer A (2017) Recent advances in connective tissue disease related interstitial lung disease. Expert Rev Respir Med 11:591–603
Fischer A, du Bois R (2012) Interstitial lung disease in connective tissue disorders. Lancet 380:689–698
Mira-Avendano I, Abril A, Burger CD, Dellaripa PF, Fischer A, Gotway MB, Lee AS, Lee JS, Matteson EL, Yi ES, Ryu JH (2019) Interstitial lung disease and other pulmonary manifestations in connective tissue diseases. Mayo Clin Proc 94:309–325
De Langhe E, Lenaerts J, Bossuyt X, Westhovens R, Wuyts WA (2015) Mechanic’s hands in a woman with undifferentiated connective tissue disease and interstitial lung disease--anti-PL7 positive antisynthetase syndrome: a case report. J Med Case Rep 9:82
Strange C, Highland KB (2004) Interstitial lung disease in the patient who has connective tissue disease. Clin Chest Med 25:549–559 vii
Jee AS, Adelstein S, Bleasel J, Keir GJ, Nguyen M, Sahhar J, Youssef P, Corte TJ (2017) Role of autoantibodies in the diagnosis of connective-tissue disease ILD (CTD-ILD) and interstitial pneumonia with autoimmune features (IPAF). J Clin Med 6:51
Chartrand S, Swigris JJ, Stanchev L, Lee JS, Brown KK, Fischer A (2016) Clinical features and natural history of interstitial pneumonia with autoimmune features: a single center experience. Respir Med 119:150–154
Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, Lee JS, Leslie KO, Lynch DA, Matteson EL, Mosca M, Noth I, Richeldi L, Strek ME, Swigris JJ, Wells AU, West SG, Collard HR, Cottin V (2015) An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 46:976–987
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, Mariette X, International Sjögren’s Syndrome Criteria Working Group (2017) 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 76:9–16
Aggarwal R, Rider LG, Ruperto N, Bayat N, Erman B, Feldman BM, Oddis CV, Amato AA, Chinoy H, Cooper RG, Dastmalchi M, Fiorentino D, Isenberg D, Katz JD, Mammen A, de Visser M, Ytterberg SR, Lundberg IE, Chung L, Danko K, García-De la Torre I, Song YW, Villa L, Rinaldi M, Rockette H, Lachenbruch PA, Miller FW, Vencovsky J, International Myositis Assessment and Clinical Studies Group and the Paediatric Rheumatology International Trials Organisation (2017) 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis 76:792–801
Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, Hirani N, Hubbard R, Lake F, Millar AB, Wallace WA, Wells AU, Whyte MK, Wilsher ML, British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee; Thoracic Society of Australia; New Zealand Thoracic Society; Irish Thoracic Society (2008) Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 63(Suppl 5):v1–v58
Wannamethee SG, Whincup PH, Lennon L, Papacosta O, Lowe GD (2014) Associations between fibrin D-dimer, markers of inflammation, incident self-reported mobility limitation, and all-cause mortality in older men. J Am Geriatr Soc 62:2357–2362
Hargett CW, Tapson VF (2008) Clinical probability and D-dimer testing: how should we use them in clinical practice? Semin Respir Crit Care Med 29:15–24
Paydar-Darian N, Kimia AA, Monuteaux MC, Michelson KA, Landschaft A, Maulden AB, Chenard RL, Nigrovic LE (2018) C-reactive protein or erythrocyte sedimentation rate results reliably exclude invasive bacterial infections. Am J Emerg Med S0735-6757:30919–30917
Kelkar AH, Shah AA, Yong SL, Ahmed Z (2017) An unusual association between hemophagocytic lymphohistiocytosis, mixed connective tissue disease, and autoimmune hemolytic anemia: a case report. Medicine (Baltimore) 96:e7488
Gormezano NW, Kern D, Pereira OL, Esteves GC, Sallum AM, Aikawa NE, Pereira RM, Silva CA, Bonfá E (2017) Autoimmune hemolytic anemia in systemic lupus erythematosus at diagnosis: differences between pediatric and adult patients. Lupus 26:426–430
Katsumata K (2006) A case of systemic sclerosis complicated by autoimmune hemolytic anemia. Mod Rheumatol 16:191–195
van Santen S, van Dongen-Lases EC, de Vegt F, Laarakkers CM, van Riel PL, van Ede AE, Swinkels DW (2011) Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum 63:3672–3680
Wen W, Liu Y, Zhao C, Sun X, Zhang C, Li Z (2015) Clinical and serologic features of primary Sjögren’s syndrome concomitant with autoimmune hemolytic anemia: a large-scale cross-sectional study. Clin Rheumatol 34:1877–1884
Bas S, Perneger TV, Seitz M, Tiercy JM, Roux-Lombard P, Guerne PA (2002) Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology (Oxford) 41:809–814
Jearn LH, Kim TY (2016) Autoantibodies are already found in connective tissue disease-associated interstitial lung disease. Chest 150:753–755
Kelmenson LB, Demoruelle MK, Deane KD (2016) The complex role of the lung in the pathogenesis and clinical outcomes of rheumatoid arthritis. Curr Rheumatol Rep 18:69
Inui N, Enomoto N, Suda T, Kageyama Y, Watanabe H, Chida K (2008) Anti-cyclic citrullinated peptide antibodies in lung diseases associated with rheumatoid arthritis. Clin Biochem 41:1074–1077
Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, Engström M, Grunewald J, Nyren S, Eklund A, Klareskog L, Sköld CM, Catrina AI (2014) Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum 66:31–39
Tzelepis GE, Toya SP, Moutsopoulos HM (2008) Occult connective tissue diseases mimicking idiopathic interstitial pneumonias. Eur Respir J 31:11–20
Tipu HN, Bashir MM (2018) Determination of specificity and pattern of antinuclear antibodies (ANA) in systemic rheumatic disease patients positive for ANA testing. J Coll Physicians Surg Pak 28:40–43
Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF, Levesque H, Jouen F (2012) Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody. Semin Arthritis Rheum 41:890–899
La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F (2006) In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 39:249–253
Huang W, Ren F, Wang Q, Luo L, Zhou J, Huang D, Pan Z, Tang L (2019) Clinical features of thirty-two patients with anti-melanoma differentiation-associated gene 5 antibodies. Clin Exp Rheumatol 2019 published on 11 Feb 2019
Palawisuth S, Kantikosum K, Patiyasikunt M, Sriprasart T, Asawanonda P, Rerknimitr P (2019) A bad sign: dermatomyositis with interstitial lung disease. Am J Med 132:182–186
Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R (2017) Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol 44:319–325
Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, Christopher-Stine L (2013) Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res 65:1307–1315
Chung JH, Chawla A, Peljto AL, Cool CD, Groshong SD, Talbert JL, McKean DF, Brown KK, Fingerlin TE, Schwarz MI, Schwartz DA, Lynch DA (2015) CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest 147:450–459
Oldham JM, Adegunsoye A, Valenzi E, Lee C, Witt L, Chen L, Husain AN, Montner S, Chung JH, Cottin V, Fischer A, Noth I, Vij R, Strek ME (2016) Characterisation of patients with interstitial pneumonia with autoimmune features. Eur Respir J 47:1767–1775
Funding
This work was supported by the National Natural Science Foundation of China (grant No. 81771738).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mengxue Tian is the first author.
Rights and permissions
About this article
Cite this article
Tian, M., Huang, W., Ren, F. et al. Comparative analysis of connective tissue disease–associated interstitial lung disease and interstitial pneumonia with autoimmune features. Clin Rheumatol 39, 575–583 (2020). https://doi.org/10.1007/s10067-019-04836-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04836-3