Abstract
The safety and effect of physical therapy in adult patients with idiopathic inflammatory myopathies (IIMs) are currently unclear. Considering the muscle weakness resulting from disease activity as well as from the administered drugs, these patients could benefit from an evidence-based physical therapy program. To perform a systematic review to assess safety and effects of physical therapy on the functional outcome of patients with idiopathic inflammatory myopathies in both active and quiescent disease: Pubmed, Embase, and Cochrane. Patients with one of the following idiopathic inflammatory myopathies: polymyositis, dermatomyositis, immune-mediated necrotizing myopathy, and/or overlap myositis. The intervention included several types of rehabilitation programs, from strength and resistance training to endurance training, with a minimal duration of 1 month. Studies reporting intervention-related adverse events, disease activity, and functional outcomes were eligible. The risk of bias was assessed using the Cochrane guidelines. We included five randomized controlled and seven open-label non-randomized non-controlled trials. Data on statistical significance were extracted for all the trials. Included trials were of medium-quality evidence given the low number of patients and some risk of bias factors. Physical therapy does not have a negative effect on the disease activity of idiopathic inflammatory myopathies in quiescent disease and could improve functional outcome. The physical therapy program should minimally include endurance training. A combination with resistance training might be beneficial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic inflammatory myopathies (IIMs) can be divided into five major subtypes: dermatomyositis (DM), inclusion-body myositis (IBM), immune-mediated necrotizing myopathy (IMNM), overlap myositis, and polymyositis (PM) [1,2,3,4,5,6,7,8]. They are rare entities with incidence rates estimated between 4.27 and 7.89 per 100,000 person years and prevalence rates from 9.54 to 32.74 cases per 100,000 individuals [9, 10]. IIMs are characterized by muscle inflammation, which is the result of an important interplay between adaptive, innate immune, and non-immune mechanisms [11,12,13,14]. Clinical characteristics include muscle weakness (in proximal upper and lower limb, neck extensor, pharyngeal and respiratory muscles), muscle atrophy in severe cases, and extramuscular manifestations such as fever, weight loss, rash, cardiac arrhythmias or ventricular dysfunction, and pulmonary complications [1, 15]. The diagnosis is based on the combination of clinical history, tempo of disease progression, pattern of muscle involvement, muscle enzyme levels, electromyographic findings, muscle biopsy analysis, and an ever-increasing diagnostic role of myositis-specific antibodies [1, 16]. Treatment consists of glucocorticoids and/or immunosuppressive therapy such as methotrexate, azathioprine, mycophenolate mofetil and in selected cases, biologicals such as rituximab [1, 17,18,19,20]. Despite these treatment options, the disease course may be fatal, and many patients have sustained disability and poor quality of life [21,22,23,24,25].
Physical therapy may be an additional treatment method to improve functional outcome. Many cases have been described in which physical exercise positively affected several outcome parameters [26,27,28]. Exercise could improve muscle strength and performance, functional and aerobic capacity, and clinical disease activity in patients with IIMs [29,30,31,32,33,34,35]. The molecular mechanisms that lead to these effects are not fully understood but could partly be explained by downregulation of genes associated with inflammation and fibrosis and upregulation of genes associated with aerobic metabolism in muscle tissue [36].
The aim of this systematic review is to evaluate the safety and the effects of physical therapy on the functional outcome of patients with IIMs. We included randomized controlled trials (RCTs) and in extension also open-label non-randomized non-controlled trials. Additionally, we aim to evaluate the optimal type and timing of the training intervention(s).
Materials and methods
Eligibility criteria
We included RCTs and non-randomized non-controlled trials studying patients diagnosed with IIMs (PM, DM, IMNM and/or overlap myositis) according to the Bohan and Peter criteria [37, 38] or the International Myositis Assessment and Clinical Studies Group (IMACS) criteria [3]. Trials including patients with juvenile DM and/or IBM were excluded. The intervention could be several types of rehabilitation programs, from strength and resistance training to endurance training. The rehabilitation program had to have a minimal duration of 1 month, thus excluding trials investigating a single exercise in order to examine long-term effects. To assess possible risk of increasing disease activity by physical therapy, one of the following disease activity measures was required: levels of C-reactive protein (CRP), creatine phosphokinase (CPK) and aldolase, erythrocyte sedimentation rate (ESR), patient’s and physician’s global disease activity on a visual analogue scale (PGA and PhGA), assessment of extraskeletal muscle disease activity in six organ systems using the Myositis Intent-to-Treat Activity Index (MITAX), 0 to 100 visual analogue scales (VAS) for assessing pain, and fatigue and the Borg CR-10 Scale [39]. Intervention-related adverse events were also eligible as safety measures but could not be found as outcome measures in the different trials. We accepted a broad range of functional outcome measures [40]: the Health Assessment Questionnaire Disability Index (HAQ-DI) [22, 24], the Myositis Activities Profile (MAP) [41], the Modified Functional Assessment Screening Questionnaire (MFASQ) [42, 43], the McMaster Toronto Arthritis Patient Preference Disability Questionnaire (MACTAR) [44], the Functional Independence Measure (FIM) [45], the Medical Outcomes Study 36-Item Short-Form Health Survey questionnaire (SF-36) [46], the Swedish version of the Nottingham Health Profile (NHP) [47], and the Kendall Manual Muscle Test (MMT); isometric/isokinetic assessments of muscle strength (peak isometric/isokinetic torque or PIT) [48,49,50]; the disease-specific functional index (FI) [51]; the distance covered in a 6- or 7-min walk test (6- or 7-min WT) [52]; 1, 5, 10 or 15 voluntary repetition maximum (VRM) measures of muscle strength; timed-stands test (TST) [53]; timed-up-and-go test (TUGT) [54]; quadriceps cross-sectional area (QCSA); grip strength (GS) [55]; and aerobic capacity (VO2 max and time to exhaustion). Only trials written in English were included.
Information sources
We searched the following databases: Pubmed, Embase, and Cochrane. The search was carried out between February 2018 and February 2019. Review articles were hand-searched for relevant references.
Search strategy
Based on the PICO search model (patients defined as patients diagnosed with IIMs, intervention being any form of physical therapy, comparison being conventional treatment and outcomes being disease activity measures, intervention-related adverse events and functional outcomes). We searched three databases (Pubmed, Embase, and Cochrane) for two of the four concepts, namely patients and intervention. We searched Pubmed using MeSH-terms (Medical Subject Headings) and terms in title and abstract to find articles that have been indexed during the last 6 months ([tiab]). We searched articles in Embase using Emtree-terms (Embase Subject Headings) and also terms in title and abstract (:ti,ab). In Cochrane, we used MeSH terms and terms in title and abstract (:ti,ab). For the full search, see S1.
Study selection
Studies were selected based on title, abstract, and/or full text. We used our eligibility criteria to rule out irrelevant articles. There were no limits for publication date.
Data collection process
The journal citation report with all the appraised articles was constructed in Word by one of the authors.
Data items
The data items are listed in Table S2.
Risk of bias in individual studies
The risk of bias was assessed for the six RCTs using the Cochrane guidelines. Every domain (selection bias, performance bias, detection bias, attrition bias, and reporting bias) was judged as having a low, high, or unclear risk of bias, and this judgment was further clarified and justified. For this purpose, we used Review Manager 5.3 (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Additional analyses
No additional analyses were performed.
Results
Study selection
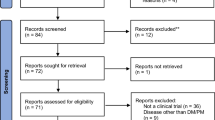
We identified 1349 articles: 476 articles in Pubmed, 779 articles in Embase, and 94 articles in Cochrane. No additional articles were found by hand searching review articles for relevant references. We excluded 419 Pubmed articles, 740 Embase articles, and 90 Cochrane articles based on patient population and/or study question, retaining a total of 100 articles. Removal of duplicates resulted in 74 retained articles that were screened based on abstract and/or full text. This resulted in the exclusion of 57 articles based on study design, outcome measure(s), or patient population (juvenile DM and/or IBM). Four conference abstracts were retrieved in Embase. Two were excluded because they were duplicates of published full articles. The remaining two were excluded because they did not contain sufficient methodologic information. Our study selection resulted in five RCTs [56,57,58,59,60] with one open-label extension [61] and seven non-randomized non-controlled trials [62,63,64,65,66,67,68]. The flow diagram of the study selection process is depicted in Fig. 1.
Study characteristics
Characteristics of the 12 individual studies are presented in Tables 1 and 2. Data on study size, study design, year of publication, inclusion criteria, exclusion criteria, intervention, comparison, and primary and secondary outcome measures and follow-up were extracted.
Risk of bias within studies
The evaluated risk of bias of the five RCTs is presented in Table S3–7 with a judgment of low, high, or unclear risk of bias and the support for this judgment. These results are depicted as a risk of bias graph in Fig. 2 and a risk of bias summary in Fig. 3. Overall, there is a high risk of performance bias and detection bias for patient-reported outcome measures since it was impossible to blind patients for the intervention (given that it is a rehabilitation program). The risk for attrition and reporting bias is unclear. Since all included RCTs used adequate randomization methods, the risk of selection bias was interpreted as low.
Results of individual studies
The aim of this systematic review was to assess safety and effect of physical therapy on the functional outcome of patients with IIMs. In addition, we assessed the optimal training timing and intervention type.
We divided outcome measures used in the clinical trials in seven groups: activities of daily living, quality of life, muscle function, aerobic capacity, disease activity, pain, and fatigue. Safety could only be assessed by the evolution in disease activity measures since intervention-related adverse events were not reported as outcome measures in any of the trials. When drawing our conclusions, we put more emphasis on the results of the RCTs because of the higher level of evidence.
The results of the 12 trials and the open-label extension are presented in Table 3 as significant effect, non-significant effect, or no data provided. The table clearly visualizes the heterogeneity of outcome measures. For full data, see Table S8–13.
Safety
Physical therapy does not have a negative effect on the disease activity. In all appraised studies, disease activity measures remained stable or improved. As such, we conclude that physical therapy does not lead to disease flares.
Complications of the intervention (for example cardiovascular or musculoskeletal) were not specifically addressed as outcome measures. None of the trials mentioned any adverse event linked to the intervention. Nevertheless, the reasons for dropout were not always mentioned so it is not clear if it was intervention-related or not (see Table S3–7). Most of the trials included a statement that the program was well tolerated by all the patients.
Effect on functional outcome
A clinically significant improvement in the activities of daily living was seen in two trials [56, 60], measured by the HAQ-DI and the MFASQ, respectively. In one trial, pain significantly improved whereas fatigue did not [56]. The scales used to address quality of life (SF-36 and NHP) are divided into different subscales. None of the RCTs demonstrated significant improvement in all of the subscales and, when comparing the different RCTs, no single subscale improved consistently across all studies. Therefore, no uniform conclusions can be drawn about the effect on quality of life.
We considered muscle function and aerobic capacity to be important outcome measures to determine the effect on functional outcome. One group noted a significant improvement in muscle function, measured by the PIT [60], whereas another did not [56]. Furthermore, there was no consistent improvement in the MMT in this last trial [56]. In one trial, significance results were not provided for the MMT [58]. Five voluntary repetition maximum measures of muscle strength were only significant for the left side in another trial [59]. Regarding aerobic capacity, an improvement was found in VO2 max in three RCTs [58,59,60] and in time to exhaustion in one RCT [58]. One group did not find a significant improvement in aerobic capacity and FI, another outcome measure related to muscle function [57].
Components of the training program
The investigated rehabilitation programs consisted of endurance training, resistance training, or a combination of both. As written above, a clear improvement in muscle function and aerobic capacity was seen in three RCTs [58,59,60]. All three RCTs investigated an endurance training program which suggests that this is the most optimal training intervention. These findings are also in line with the open-label extension [61], which is an extension of the previous RCT [60]. These endurance programs had a frequency of two or three times a week, lasted approximately 1 h and covered a period between 6 and 12 weeks. They consisted of a period of warm-up (for example cycling at 50% of VO2max), more intense cycling with a gradual increase in intensity (for example aiming at 70% of VO2max), muscular endurance exercise, step aerobics, and/or a period of cool-down and stretching.
On the other hand, there could also be some beneficial effect of a combination of endurance and resistance training given the fact that there was a significant improvement in the HAQ-DI in one trial [56] even though there was no improvement in muscle function. This implies that adding resistance training improves self-perceived functionality or improves disabilities encountered by patients.
Six out of seven non-randomized non-controlled trials investigated a resistance training program [62, 64,65,66,67,68]. Although five trials reported significant improvements in some muscle function measures, we cannot generalize results due to low methodological study design and conduct, namely no control arm and few study participants.
Timing
All RCTs were performed in the stable stage of the disease [56,57,58,59,60]. Three of the seven non-randomized non-controlled trials were carried out during the active stage of the disease or following acute exacerbation [63, 65, 66]. There were no drop outs in these trials. The first trial consisted of only three patients and as such could not provide any data on statistical significance [63]. The second trial only showed a significant improvement in muscle strength in a part of the muscle groups [65]. The last trial showed significant improvements in the FI score, but the relative impacts of the exercise program and the medical treatment could not be separated [66]. This would probably be inherent to any physical therapy intervention in an active phase of the disease where patients need regular pharmacological treatment adaptations and ongoing disease activity is still affecting functional evolution.
Additional analysis
No additional analyses were performed.
Discussion
In conclusion, physical therapy does not lead to disease flares, at least in patients medically treated and with stable disease course. However, the lack of elaboration on the reasons for dropouts does not allow firm conclusions as potential intervention-related adverse events could have been missed. There is also a possibility of inclusion bias to consider because if muscle damage and trainability is too low, inclusion in these trials is probably not always possible.
Current evidence supports the use of endurance training while the benefit of resistance training or combination of both remains unclear. Our results apply only to patients with a diagnosis of PM or DM. However, many patients now recognized as IMNM or overlap myositis were previously classified as PM, currently a diagnosis of exclusion [7].
Regarding the timing of intervention, evidence supports that physical therapy has a beneficial effect during the stable stage of the disease. We cannot draw clear conclusions about a beneficial effect during the active stage.
There are a number of limitations that we have to consider
First of all, an important limiting factor is that IIMs are rare diseases and it is difficult to find an adequate number of patients to include in clinical trials. As a consequence, many trials have a lack of power, recruitment targets were not always achieved, and baseline characteristics were not always completely comparable due to random chance mechanisms.
Secondly, there are some risk of bias factors (see Table S3–7). One of the main problems is that patients were not blinded because the intervention consisted of a rehabilitation program. Therefore, performance bias could not be excluded. This problem could be solved by comparing a light rehabilitation program (instead of placebo) with an active rehabilitation program. Patients were also not blinded when they had to complete patient-reported outcome measures (such as filling in a questionnaire) which could introduce a form of detection bias. On the other hand, independent assessors who had to assess objective outcome measures were blinded in most studies, which reduces the magnitude of detection bias. The randomization methods in the six RCTs were carried out thoroughly, which makes selection bias unlikely. Due to missing data and the fact that not all data were always reported, there is an unclear risk of attrition and reporting bias.
Finally, comparison between trials was frequently not possible due to the heterogeneity of outcome measures. These issues precluded a meta-analysis.
Conclusions
Physical therapy does not have a negative effect on the disease activity of patients with IIMs, and it could improve the functional outcome of these patients. We recommend physical therapy in the form of endurance training such as cycling or step aerobics at a frequency of three times a week. Addition of resistance training is safe, though no clear conclusion on effect could be drawn. Physical therapy seems to be safe during the stable stage of disease and possibly also in the active stage, though at the moment, only a favorable effect in the stable stage can be supported by evidence from multiple randomized clinical trials.
Future research should focus on the effects of physical therapy during the active stage of the disease, the added value of resistance training alongside endurance training, and the possible differences in rehabilitation programs for the different subtypes of IIMs. Trials investigating effects during the active stage of the disease pose specific methodological challenges in terms of efficacy evaluation given the concurrent effect of the natural evolution of the disease and pharmacological treatment. To take care of this, larger sample sizes will be needed and pharmacological treatment will have to be standardized as much as possible, while medication and evolution of disease activity will have to be included as confounders during statistical evaluation.
References
Dalakas MC (2015) Inflammatory muscle diseases. N Engl J Med 372:1734–1747. https://doi.org/10.1056/NEJMra1402225
Dalakas MC (1991) Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med 325:1487–1498. https://doi.org/10.1056/NEJM199111213252107
Dalakas MC, Hohlfeld R (2003) Polymyositis and dermatomyositis. Lancet (London, England) 362:971–982. https://doi.org/10.1016/S0140-6736(03)14368-1
Dalakas MC (2011) Review: an update on inflammatory and autoimmune myopathies. Neuropathol Appl Neurobiol 37:226–242. https://doi.org/10.1111/j.1365-2990.2010.01153.x
Luo Y-B, Mastaglia FL (2015) Dermatomyositis, polymyositis and immune-mediated necrotising myopathies. Biochim Biophys Acta 1852:622–632. https://doi.org/10.1016/j.bbadis.2014.05.034
Rider LG, Miller FW (2011) Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA 305:183–190. https://doi.org/10.1001/jama.2010.1977
Mariampillai K, Granger B, Amelin D, Guiguet M, Hachulla E, Maurier F, Meyer A, Tohmé A, Charuel JL, Musset L, Allenbach Y, Benveniste O (2018) Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol 75:1528. https://doi.org/10.1001/jamaneurol.2018.2598
Lundberg IE, Tjarnlund A, Bottai M et al (2017) 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol (Hoboken, NJ) 69:2271–2282. https://doi.org/10.1002/art.40320
Findlay AR, Goyal NA, Mozaffar T (2015) An overview of polymyositis and dermatomyositis. Muscle Nerve 51:638–656. https://doi.org/10.1002/mus.24566
Svensson J, Arkema EV, Lundberg IE, Holmqvist M (2017) Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology (Oxford) 56:802–810. https://doi.org/10.1093/rheumatology/kew503
Rayavarapu S, Coley W, Nagaraju K (2011) An update on pathogenic mechanisms of inflammatory myopathies. Curr Opin Rheumatol 23:579–584. https://doi.org/10.1097/BOR.0b013e32834b41d2
Dalakas MC (2011) Pathophysiology of inflammatory and autoimmune myopathies. Presse Med 40:e237–e247. https://doi.org/10.1016/j.lpm.2011.01.005
Lundberg IE (2000) The role of cytokines, chemokines, and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep 2:216–224
Zong M, Lundberg IE (2011) Pathogenesis, classification and treatment of inflammatory myopathies. Nat Rev Rheumatol 7:297–306. https://doi.org/10.1038/nrrheum.2011.39
Ernste FC, Reed AM (2013) Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc 88:83–105. https://doi.org/10.1016/j.mayocp.2012.10.017
Casciola-Rosen L, Mammen AL (2012) Myositis autoantibodies. Curr Opin Rheumatol 24:602–608. https://doi.org/10.1097/BOR.0b013e328358bd85
Lundberg IE, Vencovsky J, Alexanderson H (2014) Therapy of myositis: biological and physical. Curr Opin Rheumatol 26:704–711. https://doi.org/10.1097/BOR.0000000000000109
Hengstman GJD, van den Hoogen FHJ, van Engelen BGM (2009) Treatment of the inflammatory myopathies: update and practical recommendations. Expert Opin Pharmacother 10:1183–1190. https://doi.org/10.1517/14656560902913815
Dalakas MC (2010) Immunotherapy of myositis: issues, concerns and future prospects. Nat Rev Rheumatol 6:129–137. https://doi.org/10.1038/nrrheum.2010.2
Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, Barohn RJ, Feldman BM, Harris-Love MO, Koontz DC, Fertig N, Kelley SS, Pryber SL, Miller FW, Rockette HE, and the RIM Study Group (2013) Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 65:314–324. https://doi.org/10.1002/art.37754
Marie I, Hachulla E, Hatron PY et al (2001) Polymyositis and dermatomyositis: short term and longterm outcome, and predictive factors of prognosis. J Rheumatol 28:2230–2237
Ponyi A, Borgulya G, Constantin T et al (2005) Functional outcome and quality of life in adult patients with idiopathic inflammatory myositis. Rheumatology (Oxford) 44:83–88. https://doi.org/10.1093/rheumatology/keh404
Sultan SM, Ioannou Y, Moss K, Isenberg DA (2002) Outcome in patients with idiopathic inflammatory myositis: morbidity and mortality. Rheumatology (Oxford) 41:22–26
Clarke AE, Bloch DA, Medsger TAJ, Oddis CV (1995) A longitudinal study of functional disability in a national cohort of patients with polymyositis/dermatomyositis. Arthritis Rheum 38:1218–1224
Bronner IM, van der Meulen MFG, de Visser M, Kalmijn S, van Venrooij WJ, Voskuyl AE, Dinant HJ, Linssen WHJP, Wokke JHJ, Hoogendijk JE (2006) Long-term outcome in polymyositis and dermatomyositis. Ann Rheum Dis 65:1456–1461. https://doi.org/10.1136/ard.2005.045690
Hicks JE, Miller F, Plotz P et al (1993) Isometric exercise increases strength and does not produce sustained creatinine phosphokinase increases in a patient with polymyositis. J Rheumatol 20:1399–1401
Jorgensen AN, Aagaard P, Nielsen JL et al (2016) Effects of blood-flow-restricted resistance training on muscle function in a 74-year-old male with sporadic inclusion body myositis: a case report. Clin Physiol Funct Imaging 36:504–509
Hejazi SMA, Engkasan JP, Qomi MSM (2012) Intensive exercise and a patient in acute phase of polymyositis. J Back Musculoskelet Rehabil 25:231–234. https://doi.org/10.3233/BMR-2012-0340
Nader GA, Lundberg IE (2009) Exercise as an anti-inflammatory intervention to combat inflammatory diseases of muscle. Curr Opin Rheumatol 21:599–603. https://doi.org/10.1097/BOR.0b013e3283319d53
Johnson LG, Collier KE, Edwards DJ, Philippe DL, Eastwood PR, Walters SE, Thickbroom GW, Mastaglia FL (2009) Improvement in aerobic capacity after an exercise program in sporadic inclusion body myositis. J Clin Neuromuscul Dis 10:178–184. https://doi.org/10.1097/CND.0b013e3181a23c86
Alexanderson H (2009) Exercise effects in patients with adult idiopathic inflammatory myopathies. Curr Opin Rheumatol 21:158–163. https://doi.org/10.1097/BOR.0b013e328324e700
de Salles Painelli V, Gualano B, Artioli GG, de Sá Pinto AL, Bonfá E, Lancha Junior AH, Lima FR (2009) The possible role of physical exercise on the treatment of idiopathic inflammatory myopathies. Autoimmun Rev 8:355–359. https://doi.org/10.1016/j.autrev.2008.11.008
Habers GEA, Takken T (2011) Safety and efficacy of exercise training in patients with an idiopathic inflammatory myopathy--a systematic review. Rheumatology (Oxford) 50:2113–2124. https://doi.org/10.1093/rheumatology/ker292
Alexanderson H, Lundberg IE (2012) Exercise as a therapeutic modality in patients with idiopathic inflammatory myopathies. Curr Opin Rheumatol 24:201–207. https://doi.org/10.1097/BOR.0b013e32834f19f5
Alemo Munters L, Alexanderson H, Crofford LJ, Lundberg IE (2014) New insights into the benefits of exercise for muscle health in patients with idiopathic inflammatory myositis. Curr Rheumatol Rep 16(429):429. https://doi.org/10.1007/s11926-014-0429-4
Nader GA, Dastmalchi M, Alexanderson H, Grundtman C, Gernapudi R, Esbjörnsson M, Wang Z, Rönnelid J, Hoffman EP, Nagaraju K, Lundberg IE (2010) A longitudinal, integrated, clinical, histological and mRNA profiling study of resistance exercise in myositis. Mol Med 16:455–464. https://doi.org/10.2119/molmed.2010.00016
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292:344–347. https://doi.org/10.1056/NEJM197502132920706
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292:403–407. https://doi.org/10.1056/NEJM197502202920807
Isenberg DA, Allen E, Farewell V, Ehrenstein MR, Hanna MG, Lundberg IE, Oddis C, Pilkington C, Plotz P, Scott D, Vencovsky J, Cooper R, Rider L, Miller F (2004) International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology (Oxford) 43:49–54. https://doi.org/10.1093/rheumatology/keg427
Alexanderson H, Lundberg IE, Stenstrom CH (2002) Development of the myositis activities profile--validity and reliability of a self-administered questionnaire to assess activity limitations in patients with polymyositis/dermatomyositis. J Rheumatol 29:2386–2392
Seltzer GB, Granger CV, Wineberg DE (1982) Functional assessment: bridge between family and rehabilitation medicine within an ambulatory practice. Arch Phys Med Rehabil 63:453–457
Millard RW (1989) The functional assessment screening questionnaire: application for evaluating pain-related disability. Arch Phys Med Rehabil 70:303–307
Alemo Munters L, van Vollenhoven RF, Alexanderson H (2011) Patient preference assessment reveals disease aspects not covered by recommended outcomes in polymyositis and dermatomyositis. ISRN Rheumatol 2011:463124. https://doi.org/10.5402/2011/463124, 1, 5
Keith RA, Granger CV, Hamilton BB, Sherwin FS (1987) The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil 1:6–18
Gandek B, Sinclair SJ, Kosinski M, Ware JEJ (2004) Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev 25:5–25
Grimby A, Wiklund I (1994) Health-related quality of life in old age. A study among 76-year-old Swedish urban citizens. Scand J Soc Med 22:7–14
Tiffreau V, Ledoux I, Eymard B, Thévenon A, Hogrel JY (2007) Isokinetic muscle testing for weak patients suffering from neuromuscular disorders: a reliability study. Neuromuscul Disord 17:524–531. https://doi.org/10.1016/j.nmd.2007.03.014
Backman E (1988) Methods for measurement of muscle function. Methodological aspects, reference values for children, and clinical applications. Scand J Rehabil Med Suppl 20:9–95
Stoll T, Bruhlmann P, Stucki G et al (1995) Muscle strength assessment in polymyositis and dermatomyositis evaluation of the reliability and clinical use of a new, quantitative, easily applicable method. J Rheumatol 22:473–477
Josefson A, Romanus E, Carlsson J (1996) A functional index in myositis. J Rheumatol 23:1380–1384
Brooks D, Solway S, Gibbons WJ (2003) ATS statement on six-minute walk test. Am J Respir Crit Care Med 167:1287
Newcomer KL, Krug HE, Mahowald ML (1993) Validity and reliability of the timed-stands test for patients with rheumatoid arthritis and other chronic diseases. J Rheumatol 20:21–27
Podsiadlo D, Richardson S (1991) The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148
Nordenskiold UM, Grimby G (1993) Grip force in patients with rheumatoid arthritis and fibromyalgia and in healthy subjects. A study with the Grippit instrument. Scand J Rheumatol 22:14–19
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Tiffreau V, Rannou F, Kopciuch F, Hachulla E, Mouthon L, Thoumie P, Sibilia J, Drumez E, Thevenon A (2017) Postrehabilitation functional improvements in patients with inflammatory myopathies: the results of a randomized controlled trial. Arch Phys Med Rehabil 98:227–234. https://doi.org/10.1016/j.apmr.2016.09.125
Alexanderson H, Munters LA, Dastmalchi M, Loell I, Heimbürger M, Opava CH, Lundberg IE (2014) Resistive home exercise in patients with recent-onset polymyositis and dermatomyositis -- a randomized controlled single-blinded study with a 2-year followup. J Rheumatol 41:1124–1132. https://doi.org/10.3899/jrheum.131145
Alemo Munters L, Dastmalchi M, Katz A, Esbjörnsson M, Loell I, Hanna B, Lidén M, Westerblad H, Lundberg IE, Alexanderson H (2013) Improved exercise performance and increased aerobic capacity after endurance training of patients with stable polymyositis and dermatomyositis. Arthritis Res Ther 15:R83. https://doi.org/10.1186/ar4263
Alemo Munters L, Dastmalchi M, Andgren V, Emilson C, Bergegård J, Regardt M, Johansson A, Orefelt Tholander I, Hanna B, Lidén M, Esbjörnsson M, Alexanderson H (2013) Improvement in health and possible reduction in disease activity using endurance exercise in patients with established polymyositis and dermatomyositis: a multicenter randomized controlled trial with a 1-year open extension followup. Arthritis Care Res (Hoboken) 65:1959–1968. https://doi.org/10.1002/acr.22068
Wiesinger GF, Quittan M, Aringer M, Seeber A, Volc-Platzer B, Smolen J, Graninger W (1998) Improvement of physical fitness and muscle strength in polymyositis/dermatomyositis patients by a training programme. Br J Rheumatol 37:196–200
Wiesinger GF, Quittan M, Graninger M et al (1998) Benefit of 6 months long-term physical training in polymyositis/dermatomyositis patients. Br J Rheumatol 37:1338–1342
Mattar MA, Gualano B, Perandini LA, Shinjo SK, Lima FR, Sá-Pinto AL, Roschel H (2014) Safety and possible effects of low-intensity resistance training associated with partial blood flow restriction in polymyositis and dermatomyositis. Arthritis Res Ther 16(473). https://doi.org/10.1186/s13075-014-0473-5
Mattar MA, Gualano B, Roschel H, Perandini LA, Dassouki T, Lima FR, Shinjo SK, de Sá Pinto AL (2014) Exercise as an adjuvant treatment in persistent active polymyositis. J Clin Rheumatol 20:11–15. https://doi.org/10.1097/RHU.0000000000000056
Alexanderson H, Dastmalchi M, Esbjornsson-Liljedahl M et al (2007) Benefits of intensive resistance training in patients with chronic polymyositis or dermatomyositis. Arthritis Rheum 57:768–777. https://doi.org/10.1002/art.22780
Varju C, Petho E, Kutas R, Czirjak L (2003) The effect of physical exercise following acute disease exacerbation in patients with dermato/polymyositis. Clin Rehabil 17:83–87. https://doi.org/10.1191/0269215503cr572oa
Alexanderson H, Stenstrom CH, Jenner G, Lundberg I (2000) The safety of a resistive home exercise program in patients with recent onset active polymyositis or dermatomyositis. Scand J Rheumatol 29:295–301
Alexanderson H, Stenstrom CH, Lundberg I (1999) Safety of a home exercise programme in patients with polymyositis and dermatomyositis: a pilot study. Rheumatology (Oxford) 38:608–611
Escalante A, Miller L, Beardmore TD (1993) Resistive exercise in the rehabilitation of polymyositis/dermatomyositis. J Rheumatol 20:1340–1344
Acknowledgements
Jean-Baptiste Vulsteke was supported by the Fund Joel Hurlet and by a FWO SB Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 37 kb)
GLOSSARY OF ABBREVIATIONS AND ACRONYMS
- ADL
-
Activities of daily living
- CPK
-
Creatine phosphokinase
- CR
-
Category ratio/chronotropic reserve
- CRP
-
C-reactive protein
- DM
-
Dermatomyositis
- EMG
-
Electromyography
- Emtree
-
Embase subject headings
- ESM
-
Erythrocyte sedimentation rate
- FEF(25-75%)
-
Forced expiratory flow
- FEV1/FVC
-
Forced expiratory volume one second to forced vital capacity ratio
- FI
-
Functional index
- FIM
-
Functional independence measure
- FVC
-
Forced vital capacity
- GS
-
Grip strength
- HAQ-DI
-
Health assessment questionnaire disability index
- IBM
-
Inclusion-body myositis
- IIM
-
Idiopathic inflammatory myopathy
- IMACS
-
International myositis assessment and clinical studies group
- IMNM
-
Immune-mediated necrotizing myopathy
- MACTAR
-
McMaster Toronto arthritis patient preference disability questionnaire
- MAP
-
Myositis activities profile
- MeSH
-
Medical subject headings
- MFASQ
-
Modified functional assessment screening questionnaire
- MITAX
-
Myositis intent-to-treat activity index
- MMT
-
Manual muscle test
- MRI
-
Magnetic resonance imaging
- NHP
-
Nottingham health profile
- PGA
-
Patient’s global disease activity
- PhGA
-
Physician’s global disease activity
- PICO
-
Patient intervention comparison outcome
- PIT
-
Peak isometric/isokinetic torque
- PM
-
Polymyositis
- QCSA
-
Quadriceps cross-sectional area
- RCP
-
Respiratory compensation point
- RCT
-
Randomized controlled trial
- RM
-
Repetition maximum
- ROM
-
Range of motion
- SDGI
-
Subjective global disease impact
- SF-36
-
Medical outcomes study 36-item short-form health survey questionnaire
- TST
-
Timed-stands test
- TUGT
-
Timed-up-and-go test
- VAS
-
Visual analogue scale
- VAT
-
Ventilatory anaerobic threshold
- VRM
-
Voluntary repetition maximum
- WT
-
Walk test
- ΔHRR1
-
Heart rhythm at the first minute after the test
- ΔHRR2
-
Heart rhythm at the second minute after the test
Rights and permissions
About this article
Cite this article
Van Thillo, A., Vulsteke, JB., Van Assche, D. et al. Physical therapy in adult inflammatory myopathy patients: a systematic review. Clin Rheumatol 38, 2039–2051 (2019). https://doi.org/10.1007/s10067-019-04571-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04571-9