Abstract
To investigate the characteristics and associated factors for Mycobacterium tuberculosis (TB) infection in patients with systemic lupus erythematosus (SLE) from Southern China. A retrospective study of 1108 patients admitted to the First Affiliated Hospital of Sun Yat-Sen University from January 2007 to December 2017 was performed. Demographic and clinical characteristics, laboratory data, and radiographic manifestations were recorded. A total of 59 (5.3%) lupus patients with active TB were included. Pulmonary TB occurred in 41 (69.5%) patients. Single lobe involvement was showed in 14 (34.1%) patients. Multi-lobar involvement, including miliary TB (36.6%), was presented in 27 (65.8%) patients. Lower lobe involvement accounted for 31 (75.6%) of the cases. Extrapulmonary TB occurred in 18 (30.5%) patients. Nearly one-third (35.6%) of the patients developed disseminated TB. T-SPOT.TB assay was performed in 23 patients and positive in 18 patients (78.3%). Nineteen patients (32.2%) had co-infection with TB and other pathogens, most of which were bacterial-associated (52.6%). Lymphopenia was predominant in TB-infected patients, especially in those with disseminated TB. Multivariate logistic regression analysis found that lymphopenia [odds ratio (OR) = 2.19, 95% confidence interval (CI) 1.03–4.63, P = 0.04] and the accumulated doses of glucocorticoid (GC) (OR = 2.32, 95% CI 1.69–3.20, P < 0.001) were associated with TB. TB infection is a common comorbidity in patients with SLE. Manifestations of pulmonary computed tomography (CT) scan are relatively atypical. Co-infection with TB and other pathogens is not rare. Lymphopenia and the accumulated doses of GC are associated with TB infection in lupus patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease caused by immune disorder. Survival rate and quality of life have been improved due to the progress in treatment strategy [1]. However, infection has arisen as one of the major causes of mortality in patients with SLE [2, 3]. Tuberculosis (TB) is an important public health issue worldwide. Estimated prevalence was reported as 1,908,212 in China in 2015 [4]. Patients with SLE are more susceptible to TB compared to the general population. Risks varied from 5- to 60-folds depending on different areas [5, 6]. Although TB burden is high, recent studies investigating its clinical spectrum and associated factors are scarce in lupus patients in China.
In this study, we performed a retrospective study, aiming to explore the prevalence, characteristics, and the associated factors of TB in patients with SLE from Southern China.
Methods
Study design and patients
A total of 1108 inpatients with SLE from the First Affiliated Hospital of Sun Yat-Sen University from January 1st, 2007 to December 31st, 2017 were investigated. Patients who met with the following criteria were included: (1) diagnosis of SLE according to the 1997 American College of Rheumatology (ACR) classification criteria [7], (2) diagnosis of active TB after SLE onset, and (3) complete data. Ethics committee of the First Affiliated Hospital of Sun Yat-sen University approved the research. This work was conducted according to the provisions of the Declaration of Helsinki.
Case definition
Active TB infection was defined as the presence of newly developed TB-associated symptoms and/or sign with (1) typical radiological manifestations, (2) positive M. tuberculosis culture, or (3) the presence of caseous necrosis in biopsied specimen. Disseminated TB was diagnosed if at least one of the following manifestations was presented: (1) M. tuberculosis detected from blood and/or bone marrow specimen, (2) chest radiography showing miliary TB, and (3) concomitant TB infection of ≥ 2 non-contiguous organs. Non-disseminated TB was defined as infection confined to a single site, excluding miliary pulmonary TB [8]. Lupus patients without infection caused by TB or other agents from the same period of time were chosen as controls. Cases and controls were matched 1:2 by age, gender, and the duration of SLE (from SLE onset to TB infection).
Demographic, clinical, laboratory, radiographic data, and therapeutic variables
Demographic and clinical data were collected from medical records. Clinical characteristics of TB included symptoms and sign, sites of involvement, and medication history. Laboratory data included routine blood tests, anti-nuclear antibodies (ANA), anti-double-stranded DNA (dsDNA) antibodies, erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP), and procalcitonin (PCT) levels. Anemia was defined according to the World Health Organization (WHO) guidelines as hemoglobin < 13 g/dL in males and hemoglobin < 12 g/dL in females [9]. Hypoalbuminemia was defined according to clinical laboratory threshold of albumin < 3.5 g/dL. ESR was evaluated using the Westergren method (Oumeng Hangzhou, China). The value > 15 mm/h was considered as positive in male and > 20 mm/h in female. Serum CRP levels were measured with nephelometry immunoassay (Dade Behring Diagnostics, USA). Value < 3 mg/L was considered as normal. Serum PCT levels were measured with electrochemiluminescence (ECL) immunoassay (Roche Diagnostics, Germany), and value > 0.5 ng/mL was considered as positive. The tuberculin skin test (TST) was performed with a 5-TU dose of tuberculin-purified protein derivative using Mantoux method [10]. Strong response is defined as diameter of the induration after 48 to 72 h ≥ 10 mm [11, 12]. Interferon gamma release assays (IGRAs) were carried out by using the commercially available T-SPOT.TB Assay (Oxford Immunotec, Oxford, UK) according to the manufacturer’s instructions. The SLE Disease Activity Index (SLEDAI) was used to evaluate SLE activity. Scores > 4 were considered as active SLE [13]. Assessment of organ damage was made using the Systemic Lupus International Collaborating Clinic/American College Rheumatology (SLICC/ACR) damage index (SDI) [14]. The SDI ≥ 1 was considered to reflect a long-term inflammatory burden [15]. Radiographic manifestations and biopsy findings were recorded if available. Medication history included the daily dose of glucocorticoid (GC) and the use of immunosuppressive agents during the 3 months prior to TB onset. The dose of GC was converted using the following equation: 1 mg of prednisone = 0.8 mg of methylprednisolone = 0.15 mg of dexamethasone.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Continuous data were presented as mean ± SD or median (interquartile range, IQR). Categorical variables were presented as frequency and percentage. Between-group comparisons were evaluated with the Chi-square test or the Fisher exact test for categorical variables and the Student’s t test for continuous variables with normal distribution. Between-group comparisons were evaluated using the Mann-Whitney U test for continuous variables with non-normal distribution. Clinically significant variables with a P < 0.1 in univariate logistic regression analysis were adjusted by multivariate logistic regression analyses to identify the factors associated with TB or disseminated TB. The backward procedure was applied in the multivariate logistic regression analysis. Odds ratio (OR) and corresponding 95% confidence interval (CI) were calculated.
Results
Demographic data
A total of 1108 patients fulfilled the 1997 ACR classification criteria for SLE. All cases were of Chinese Han ethnicity. Active TB was diagnosed in 59 patients (43 females, 16 males) with a prevalence of 5.3% (59/1108). Mean age at TB onset was 34.4 ± 12.7 years old (range, 14–73 years old). One hundred and eighteen lupus patients without infection were selected as controls. Symptoms of SLE at enrollment included hematologic disorders (20.3%), active nephritis (16.9%), polyarthralgia (10.2%), rash (6.8%), neuro-psychiatric lupus (3.4%), and oral ulcer (1.7%). Twenty-nine (64.3%) patients developed TB infection during the first 3 years after SLE diagnosis and 27 during the chronic stage of SLE. During the 3 months prior to TB onset, 47 (79.7%) patients received GC treatment. The median (IQR)-accumulated dose of GC was 2250 mg (900–3125 mg). Cyclophosphamide (CYC), mycophenolate mofetil (MMF), methotrexate (MTX), and cyclosporine A (CsA) were prescribed to 12, 10, 7, and 5 patients, respectively. No patient received biologic treatment.
Characteristics of TB infection in patients with SLE
Symptoms of TB infection included fever (41/59, 69.5%), cough (25/59, 42.4%), sputum (21/59, 35.6%), weight loss (15/59, 25.4%), chest pain (10/59, 16.9%), headache (10/59, 16.9%), chest distress (7/59, 11.9%), dyspnea (6/59, 10.2%), night sweats (6/59, 10.2%), abdominal pain (2/59, 3.2%), and hemoptysis (1/59, 1.7%). TST was conducted in 26 patients and T-SPOT.TB in 23. Four patients were screened by both TST and T-SPOT.TB. The positive rates were 26.9% (7/26) for TST and 78.3% (18/23) for T-SPOT.TB. Ten patients were diagnosed with TB by biopsy. Specimens were obtained from the pleura (three cases), lung (two cases), bronchus (one case), lymph nodes (two cases), and fallopian tube (one case). One patient received biopsy in the pleura and lung. Specimens submitted for M. tuberculosis culture included sputum (33 specimens), bronchoalveolar lavage fluid (4 specimens), secretion from the ulcer in the lower limb (1 specimen), and bone marrow (1 specimen). M. tuberculosis culture was positive in 12 (18.6%) patients.
Radiographic manifestations of TB infection in patients with SLE
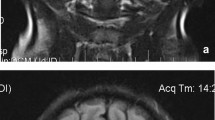
Pulmonary computed tomography (CT) scan showed TB-associated lesions in 41 (69.5%) patients (Table 1). Multi-lobar involvement, including miliary TB (36.6%), was presented in 27 (65.8%) of the cases. Approximately 31 (75.6%) patients showed TB-associated lesions in the lower lobes. Fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) was performed in one patient, who was diagnosed with miliary TB according to pulmonary CT imaging and positive sputum culture for M. tuberculosis. PET-CT found remarkably focal increased 18F-FDG uptake in the left supraclavicular as well as retroperitoneal lymph nodes (SUVmax: 12.3) (Fig. 1). Bone marrow smear foracid-fast bacillus (AFB) was positive. Taken together, the patient was diagnosed with disseminated TB, involving lungs, lymph nodes, and bone marrow. Due to the rapid progression of the disease, the patient died even though anti-tuberculosis therapy was applied. Mortality due to TB infection in lupus patients was 1.7% (1/59).
Distribution of TB infection in patients with SLE
Pulmonary TB accounted for 41 (69.5%) of the cases, while extrapulmonary TB accounted for 18 (30.5%). Among extrapulmonary TB, TB pleuritis (10.2%) was the most common. Twelve (20.3%) patients had concurrent TB infection in two organs or more. The distribution of TB infection is shown in Table 1.
Co-infection with TB and other pathogens in patients with SLE
Co-infection of TB and other pathogens occurred in 19 patients (32.2%) (Table 2). Lung was the most commonly affected (12/19, 63.2%). Pathogens were isolated in 18 patients. The left case was diagnosed with acute gastroenteritis clinically. Bacteria-associated infection accounted for 52.6% (10/19) of the cases, while fungi for 26.3% (5/19) and virus for 15.8% (3/19). Mixed infection with two pathogens or more was found in one case (5.3%). Most of the pathogens isolated were conditioned pathogens.
Comparison between disseminated TB and non-disseminated TB in patients with SLE
A comparison of demographic and clinical characteristics between patients with disseminated or non-disseminated TB infection is shown in Table 3. Lymphopenia was more predominant in patients with disseminated TB (85.7%) than those without (55.3%). Univariate logistic regression showed lymphopenia (OR = 4.38, 95% CI 1.10–17.42, P = 0.04) was associated with disseminated TB in lupus patients. Variables with a P value < 0.1 were added into the multivariate logistic regression model. However, neither lymphopenia (P = 0.06) nor PCT level (P = 0.20) was associated with disseminated TB.
TB-associated factors in patients with SLE
A comparison of demographic and clinical characteristics between cases and controls is shown in Table 4. The prevalence of lymphopenia (66.1% vs 36.1%, P < 0.001) and anemia (66.1% vs 48.3%, P = 0.03) was significantly higher in patients with TB than those without. ESR (median (mm/h), 48 vs 24, P < 0.001), CRP (median (mg/L), 27.5 vs 2, P < 0.001), and PCT (median (ng/mL), 0.2 vs 0.1, P = 0.002) levels elevated remarkably in patients with TB. The accumulated doses of GC (median (g), 2.3 vs 0.2, P < 0.001) were higher in TB-infected group than the control group. Results of univariate logistic regression are presented in Table 5. Variables with a P value < 0.1 were included into multivariate logistic regression. Considering that the elevation of ESR, CRP, and PCT was secondary to TB infection, only lymphopenia, anemia, and accumulated GC use were included into the model. Lymphopenia (OR = 2.19, 95% CI 1.03–4.63, P = 0.04) and the accumulated doses of GC (OR = 2.32, 95% CI 1.69–3.20, P < 0.001) were associated with TB infection in lupus patients.
Discussion
In this study, we explored the clinical characteristics of TB infection in lupus patients. Our results showed that manifestations of TB in lupus patients were relatively atypical. Concomitant infection with other organisms was not rare. Remarkable decrease of lymphocytes was predominant in patients with TB, especially with disseminated TB infection.
Radiological manifestations of TB in lupus patients were different from those in the general population. TB-associated lesions usually locate in the upper lobes of the lungs. However, TB in lupus patients usually involved the middle and the lower lobes. In our study, multi-lobar involvement was presented in 65.8% of the cases. And 31 (75.6%) patients showed TB-associated lesions in the lower lobes. Consistently, Yun et al. reported that two-third of the TB infection presented as multi-lobar lesions in lupus patients [16]. Except for atypical radiographic manifestations, contradiction lay between laboratory results and clinical diagnosis. Both TST and T-SPOT.TB are used to identify latent TB infection, while their predictive value for active TB is controversial [17]. Enhanced interferon-gamma response could be associated with active TB [18]. In our study, positive rate of T-SPOT.TB (78.3%) was higher than that of TST (26.9%). Our results implied that T-SPOT.TB could be a more efficient method to distinguish active TB from other infection. However, we do not deny that the use of immunosuppressant would lead to false-negative of TST. Since radiological and laboratory results could be atypical, TB infection should be considered when lupus patients showed insufficient response to conventional antibiotics.
Patients with SLE were prone to develop non-respiratory TB [19]. The incidence of extrapulmonary TB in our study (30.5%) was comparable to that in kidney transplantation recipients (21.8%) [20]. Such an increase could be attributed to the inability to contain M. tuberculosis, and hence dissemination. Mortality was high in immunodeficient patients with disseminated TB [21, 22]. However, scattered cases of disseminated TB were reported in lupus patients until recently [22, 23]. In our study, the prevalence of disseminated TB was 35.6%, significantly higher than that in the general population (1–3%) [24], and comparable to that in patients receiving solid organ transplantation (23–39%) [25, 26]. Disseminated TB was found in even half of the lupus patients who developed TB in previous work [27]. Our findings suggested that disseminated TB was a crucial issue that needs close attention.
Although concomitant HIV infection was widely studied in patients with TB, concomitant infection with other pathogens was less established. Of note, 19 lupus patients were confirmed to have concomitant infection with other pathogens except M. tuberculosis in our study. Since the symptoms of infection caused by bacteria, fungi or virus resemble those by TB, co-infection was diagnosed only if specific pathogens were isolated. Pneumonia accounted for more than half of the cases (63.2%). About half of the infected cases (52.6%) were bacteria-associated. Fungal infection was not rare (26.3%). Most of them were opportunistic pathogens. Our results suggested that lupus patients were prone to have co-infection with various pathogens due to severe immunodeficiency. Physicians should be vigilant against opportunistic infection even though diagnosis of TB was confirmed.
In our research, lymphopenia was predominant in lupus patients with TB, especially in those with disseminated TB. Low peripheral blood total lymphocyte counts predicted poor prognosis in patients with TB [28]. The etiology of lymphopenia is multifactorial. Lymphopenia, as a pattern of hematological abnormality secondary to SLE, is frequent. The incidence was reported as high as 56.4% [29]. Besides, previous research found that CD4+T cells from lupus-prone mice displayed impaired defensive response against intracellular infection [30]. Immunosuppressive therapy including GC contributes to the host susceptibility to TB as well. GC inhibited T cell proliferation by suppressing a number of pro-inflammatory cytokines, including IL-2, IL-4, and IL-6 [31]. GC also induced apoptosis of the thymocytes and affected homeostasis of peripheral T cells [32]. Furthermore, the number of CD4+T cells increased after anti-tuberculosis treatment [33, 34], which implied that TB per se may exert inhibitory effect on lymphogenesis.
T lymphocytes, especially CD4+T cells, are essential for host defense against TB [35]. T cell response was reported to eliminate ~ 95% of the bacterial load of TB [36]. Similar to SLE, active TB infection is proportional to the number of peripheral blood CD4+T cells in HIV-infected patients [37]. In HIV−/TB+ population, low CD4+T lymphocyte count was also associated with TB severity [38]. In our research, lymphocyte counts were as low as 870,000/mm3 in patients with TB, while IgG and IgM levels were comparable between TB-infected group and the control group. Our results implied that the susceptibility to TB infection could be partially attributed to T cell dysfunction in lupus patients. In our research, lymphopenia was especially profound in patients with disseminated TB. A small case-control study revealed that lower lymphocyte count, detected at baseline and after treatment, was observed in patients with disseminated TB than those with localized disease [39]. The tendency of developing disseminated TB also resulted from the disproportion of Treg cells. The number of Treg cells was high in local disease site. Treg cells suppressed effector T cell proliferation by secreting IL-10, leading to miliary TB [40]. Only 21 patients were included in the subgroup analysis in our research. Although multivariate logistic regression analysis did not reveal significant association between lymphopenia and disseminated TB (P = 0.06), further research is still worthy to explore the underlying relation.
The interpretation of this study is subject to limitations. Firstly, as several clinical data were not recorded in the original database, we could not adjust for the important variables such as socioeconomic status, CD4+ T cell counts, and the accumulated doses of CYC. Secondly, data in this study were generated from one tertiary hospital, and only inpatients were included. The generalizability is limited due to patient selection bias.
In conclusion, patients with SLE from Southern China are at high risk of TB, especially extrapulmonary and disseminated TB. Chest manifestations in such population are relatively atypical. Co-infection with other pathogens is not rare. Lymphopenia and the accumulated doses of GC are associated with TB in lupus patients. Lymphopenia is also predominant in disseminated TB.
References
Fessler BJ (2002) Infectious diseases in systemic lupus erythematosus: risk factors, management and prophylaxis. Best Pract Res Clin Rheumatol 16(2):281–291
Cervera R, Abarca-Costalago M, Abramovicz D, Allegri F, Annunziata P, Aydintug AO, Bacarelli MR, Bellisai F, Bernardino I, Biernat-Kaluza E, Blockmans D, Boki K, Bracci L, Campanella V, Camps MT, Carcassi C, Cattaneo R, Cauli A, Cervera R, Chwalinska-Sadowska H, Contu L, Cosyns JP, Danieli MG, Cruz D, Depresseux G, Direskeneli H, Domènech I, Espinosa G, Fernández-Nebro A, Ferrara GB, Font J, Frutos MA, Galeazzi M, Garcìa-Carrasco M, García Iglesias MF, García-Tobaruela A, George J, Gil A, González-Santos P, Grana M, Gül A, Haga HJ, de Haro-Liger M, Houssiau F, Hughes GR, Ingelmo M, Jedryka-Góral A, Khamashta MA, Lavilla P, Levi Y, López-Dulpa M, López-Soto A, Maldykowa H, Marcolongo R, Mathieu A, Morozzi G, Nicolopoulou N, Papasteriades C, Passiu G, Perelló I, Petera P, Petrovic R, Piette JC, Pintado V, de Pita O, Popovic R, Pucci G, Puddu P, de Ramón E, Ramos-Casals M, Rodríguez-Andreu J, Ruiz-Irastorza G, Sanchez-Lora J, Sanna G, Scorza R, Sebastiani GD, Sherer Y, Shoenfeld Y, Simpatico A, Sinico RA, Smolen J, Tincani A, Tokgöz G, Urbano-Márquez A, Vasconcelos C, Vázquez JJ, Veronesi J, Vianna J, Vivancos J, European Working Party on Systemic Lupus Erythematosus (2006) Systemic lupuserythematosus in Europe at the change of the millennium: lessons from the BEuro-lupus project^. Autoimmun Rev 5(3):180–186
Jakes RW, Bae SC, Louthrenoo W, Mok CC, Navarra SV, Kwon N (2012) Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res (Hoboken) 64(2):159–168
GBD Tuberculosis Collaborators (2017) The global burden of tuberculosis: results from the global burden of disease study 2015. Lancet Infect Dis 18(3):261–284
Agrawal PN, Gupta D, Aggarwal AN, Behera D (2000) Incidence of tuberculosis among patients receiving treatment with oral corticosteroids. J Assoc Physicians India 48(9):881–884
Yang Y, Thumboo J, Tan BH, Tan TT, Fong CHJ, Ng HS, Fong KY (2017) The risk of tuberculosis in SLE patients from an Asian tertiary hospital. Rheumatol Int 37(6):1027–1033
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725
Kawai K, Villamor E, Mugusi FM, Saathoff E, Urassa W, Bosch RJ, Spiegelman D, Fawzi WW (2011) Predictors of change in nutritional and hemoglobin status among adults treated for tuberculosis in Tanzania. Int J Tuberc Lung Dis 15(10):1380–1389
Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK Jr (2006) Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis 10(11):1224–1230
Ponce de León D, Acevedo-Vásquez E, Sánchez-Torres A, Cucho M, Alfaro J, Perich R, Pastor C, Harrison J, Sánchez-Schwartz C (2005) Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis 64(9):1360–1361
Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, Drobniewski F, Lalvani A (2007) A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 11(3):1–196
Drobniewski F, Caws M, Gibson A, Young D (2003) Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis 3(3):141–147
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35(6):630–640
Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, Bacon P, Bombardieri S, Hanly J, Hay E, Isenberg D, Jones J, Kalunian K, Maddison P, Nived O, Petri M, Richter M, Sanchez-Guerrero J, Snaith M, Sturfelt G, Symmons D, Zoma A (1996) The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 39(3):363–369
Shang Q, Yip GW, Tam LS, Zhang Q, Sanderson JE, Lam YY, Li CM, Wang T, Li EK, Yu CM (2012) SLICC/ACR damage index independently associated with left ventricular diastolic dysfunction in patients with systemic lupus erythematosus. Lupus 21(10):1057–1062
Yun JE, Lee SW, Kim TH, Jun JB, Jung S, Bae SC, Kim TY, Yoo DH (2002) The incidence and clinical characteristics of Mycobacterium tuberculosis infection among systemic lupus erythematosus and rheumatoid arthritis patients in Korea. Clin Exp Rheumatol 20(2):127–132
Ling DI, Nicol MP, Pai M, Pienaar S, Dendukuri N, Zar HJ (2013) Incremental value of T-SPOT.TB for diagnosis of active pulmonary tuberculosis in children in a high-burden setting: a multivariable analysis. Thorax 68(9):860–866
Metcalfe JZ, Cattamanchi A, Vittinghoff E, Ho C, Grinsdale J, Hopewell PC, Kawamura LM, Nahid P (2010) Evaluation of quantitative IFN-gamma response for risk stratification of active tuberculosis suspects. Am J Respir Crit Care Med 181(1):87–93
Ramagopalan SV, Goldacre R, Skingsley A, Conlon C, Goldacre MJ (2013) Associations between selected immune-mediated diseases and tuberculosis: record-linkage studies. BMC Med 11:97
Meinerz G, da Silva CK, Goldani JC, Garcia VD, Keitel E (2016) Epidemiology of tuberculosis after kidney transplantation in a developing country. Transpl Infect Dis 18(2):176–182
Marcy O, Laureillard D, Madec Y, Chan S, Mayaud C, Borand L, Prak N, Kim C, Lak KK, Hak C, Dim B, Sok T, Delfraissy JF, Goldfeld AE, Blanc FX, CAMELIA (ANRS 1295-CIPRA KH001) Study Team (2014) Causes and determinants of mortality in HIV-infected adults with tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001 randomized trial. Clin Infect Dis 59(3):435–445
Chen D, Yang Z, Yang Y, Zhan Z, Yang X (2016) A rare case of disseminated tuberculosis of the bone marrow in systemic lupus erythematosus: case report. Medicine (Baltimore) 95(18):e3552
Li JC, Fong W, Wijaya L, Leung YY (2018) Disseminated tuberculosis masquerading as a presentation of systemic lupus erythematosus. Int J Rheum Dis 21(1):352–355
Kim JH, Langston AA, Gallis HA (1990) Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis 12(4):583–590
Benito N, García-Vázquez E, Horcajada JP, González J, Oppenheimer F, Cofán F, Ricart MJ, Rimola A, Navasa M, Rovira M, Roig E, Pérez-Villa F, Cervera C, Moreno A (2015) Clinical features and outcomes of tuberculosis in transplant recipients as compared with the general population: a retrospective matched cohort study. Clin Microbiol Infect 21(7):651–658
García-Goez JF, Linares L, Benito N, Cervera C, Cofán F, Ricart MJ, Navasa M, Pérez-Villa F, González J, Moreno A (2009) Tuberculosis in solid organ transplant recipients at a tertiary hospital in the last 20 years in Barcelona, Spain. Transplant Proc 41(6):2268–2270
Ribeiro FM, Szyper-Kravitz M, Klumb EM, Lannes G, Ribeiro FR, Albuquerque EM, Shoenfeld Y (2010) Can lupus flares be associated with tuberculosis infection? Clin Rev Allergy Immunol 38(2–3):163–168
Podlekareva DN, Efsen AM, Schultze A, Post FA, Skrahina AM, Panteleev A, Furrer H, Miller RF, Losso MH, Toibaro J, Miro JM, Vassilenko A, Girardi E, Bruyand M, Obel N, Lundgren JD, Mocroft A, Kirk O, TB:HIV study group in EuroCoord (2016) Tuberculosis-related mortality in people living with HIV in Europe and Latin America: an international cohort study. Lancet HIV 3(3):e120–e131
González-Naranjo LA, Betancur OM, Alarcón GS, Ugarte-Gil MF, Jaramillo-Arroyave D, Wojdyla D, Pons-Estel GJ, Rondón-Herrera F, Vásquez-Duque GM, Quintana-López G, Da Silva NA, Tavares Brenol JC, Reyes-Llerena G, Pascual-Ramos V, Amigo MC, Massardo L, Alfaro-Lozano J, Segami MI, Esteva-Spinetti MH, Iglesias-Gamarra A, Pons-Estel BA (2016) Features associated with hematologic abnormalities and their impact in patients with systemic lupus erythematosus: data from a multiethnic Latin American cohort. Semin Arthritis Rheum 45(6):675–683
Lieberman LA, Tsokos GC (2014) Lupus-prone mice fail to raise antigen-specific T cell responses to intracellular infection. PLoS One 9(10):e111382
Leung DY, Bloom JW (2003) Update on glucocorticoid action and resistance. J Allergy Clin Immunol 111(1):3–22
Pazirandeh A, Xue Y, Prestegaard T, Jondal M, Okret S (2002) Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J 16(7):727–729
Skogmar S, Schön T, Balcha TT, Jemal ZH, Tibesso G, Björk J, Björkman P (2013) CD4 cell levels during treatment for tuberculosis (TB) in Ethiopian adults and clinical markers associated with CD4 lymphocytopenia. PLoS One 8(12):e83270
Andersen AB, Range NS, Changalucha J, Praygod G, Kidola J, Faurholt-Jepsen D, Krarup H, Grewal HM, Friis H (2012) CD4 lymphocyte dynamics in Tanzanian pulmonary tuberculosis patients with and without HIV co-infection. BMC Infect Dis 12:66
Bold TD, Ernst JD (2012) CD4+ T cell-dependent IFN-γ production by CD8+ effector T cells in Mycobacterium tuberculosis infection. J Immunol 189(5):2530–2536
Boggiano C, Eichelberg K, Ramachandra L, Shea J, Ramakrishnan L, Behar S, Ernst JD, Porcelli SA, Maeurer M, Kornfeld H (2017) "the impact of Mycobacterium tuberculosis immune evasion on protective immunity: implications for TB vaccine design" - meeting report. Vaccine 35(27):3433–3440
Kwan CK, Ernst JD (2011) HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 24(2):351–376
Skogmar S, Schön T, Balcha TT, Jemal ZH, Tibesso G, Björk J, Björkman P (2013) CD4 cell levels during treatment for tuberculosis (TB) in Ethiopian adults and clinical markers associated with CD4 lymphocytopenia. PLoS One 16;8(12):e83270
Al-Aska A, Al-Anazi AR, Al-Subaei SS, Al-Hedaithy MA, Barry MA, Somily AM, Buba F, Yusuf U, Al Anazi NA (2011) CD4+ T-lymphopenia in HIV negative tuberculous patients at King Khalid University Hospital in Riyadh, Saudi Arabia. Eur J Med Res 16(6):285–288
Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK (2009) FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med 179(11):1061–1070
Funding
This project was supported by grants from the Guangdong Technology Project (2016A020215043) and grants from the National Natural Science Foundation of China (81603435, 81601403).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics committee of the First Affiliated Hospital of Sun Yat-sen University approved the research. This work was conducted according to the provisions of the Declaration of Helsinki.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Lao, M., Chen, D., Wu, X. et al. Active tuberculosis in patients with systemic lupus erythematosus from Southern China: a retrospective study. Clin Rheumatol 38, 535–543 (2019). https://doi.org/10.1007/s10067-018-4303-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4303-z