Abstract
Adiposity phenotypes, estimated by higher body adiposity index (BAI), lipid accumulation product (LAP), and cardiometabolic index (CMI), has conferred increased metabolic risk. The relative contribution of BAI, LAP, and CMI in hyperuricemia, however, is unknown. We hypothesized that these obesity indicators would refine identification of hyperuricemia. Information on serum uric acid (SUA), fasting lipid profiles, and body adiposity measures (BAI, LAP, and CMI) were recorded in a cross-sectional population-based sample of 11,102 participants (≥ 35 years old) from China. BAI, LAP, and CMI were strong independent predictors of SUA in both sexes after correction for potential confounders. In multivariable models, odds ratio (OR) for hyperuricemia for 1 SD increment in BAI, LAP, and CMI were 1.361 (95% CI, 1.224–1.513), 1.393 (95% CI, 1.273–1.525), and 1.332 (95% CI, 1.224–1.448) in females, respectively. For males, these adiposity indices corresponded to an increased hyperuricemia risk of 14, 47, and 33%, respectively. Additionally, compared to the bottom category, females with the top quartile of BAI, LAP, and CMI showed higher adjusted odds of having hyperuricemia, with ORs of 2.064, 7.500, and 4.944, respectively. ORs for hyperuricemia were statistically significant in the fourth quartile of BAI (1.622 [1.258–2.091]), LAP (5.549 [3.907–7.880]), and CMI (3.878 [2.830–5.313]) of male subgroup. Accumulation of ectopic adiposity in general (quantified by increased BAI), and of visceral adipose tissue in particular (reflected by elevated LAP and CMI), provided important insight regarding hyperuricemia risk and might potentially shed further light on our understanding of the metabolic sequelae of obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperuricemia is a major public health burden worldwide due to its high prevalence and clinical significance influence on a cluster of cardiometabolic abnormalities including diabetes, dyslipidemia, and hypertension [1, 2]. It also represents a key characteristic of gout and a strong predictor of cardiovascular disease (CVD) such as coronary heart disease, stroke, and heart failure [3, 4]. As a consequence of either urate overproduction or, more frequently, insufficient excretion from kidney, hyperuricemia has been identified as a mediator of insulin resistance, endothelial dysfunction, and vascular inflammation, all of which are clearly involved in the pathogenesis of CVD mortality and all-cause mortality [2, 4, 5]. In the view of its important pathophysiological role, the modifiable determinants of hyperuricemia need to be better understood for the early detection and aggressive prevention.
There is a wealth of evidence showing that obesity favors a spectrum of metabolic disorders in which the risk of developing adiposity-related hyperuricemia is aggravated. The positive association of excess body fat with impaired uric acid (UA) metabolism, or even particularly hyperuricemia, was attributable to its unfavorable impact on overproduction and poor excretion of UA [6, 7]. The value of body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WHtR) as obesity indicators for evaluating the risk of hyperuricemia has been investigated [8,9,10,11]. Current evidence has revealed that the clinical risk attributable to obesity depends not simply on the extent, but importantly, the distribution of the excess adiposity [12, 13]. Ectopic fat depots viscerally contribute to obesity-mediated hyperuricemia and are now regarded as a heterogeneous condition, with increased risk for developing UA metabolism disorders despite similar BMI among individuals [6, 14,15,16]. The incapability of these anthropometric indices to distinguish between visceral adipose tissue (VAT) and subcutaneous adiposity tissue (SAT) and the increasing recognition of the differential metabolic characteristics between these two fat depots have stimulated interest in exploring some nontraditional anthropometric indicators that can better capture visceral obesity and related dysmetabolic state independently of total adiposity [17, 18].
Recently, the body adiposity index (BAI), calculated from measurements of the hip circumference and height, has been recently proposed by Bergman et al. as a new method intended to substitute BMI to estimate the percentage of body fat and SAT [19, 20]. There has been no consensus on whether BAI is validated in its ability to determine the emerging cardiometabolic risk factors seen in adiposity [20,21,22,23,24]. The superior clinical utility and prognostic value of BAI in the context of obesity-related CVD were not firm enough to recommend [20, 21], whereas other studies still favored BAI in the evaluation of cardiovascular risk [22,23,24]. The cardiometabolic index (CMI) was posited as a new VAT distribution and dysfunction indicator to assess the presence of diabetes and atherosclerotic progression using triglyceride (TG)/high-density lipoprotein cholesterol (HDL-C) and WHtR [25,26,27]. As such, a novel sex-specific index based on WC and TG, termed lipid accumulation product (LAP), has been characterized as a pathogenic fat depot of VAT and suggested to detect insulin resistance, metabolic syndrome, and incident cardiovascular events [28,29,30]. Taken together, incorporating the concomitant of anthropometric and lipid parameters into a clinically simple and reproducible marker such as CMI and LAP, seems to be potentially able to discriminate the visceral compartment and cardiometabolic disease. Notably, knowledge of the properties of VAT and specific role of anthropometric indices has the potential to further our understanding of the hyperuricemia risk conferred by obesity. The prime effect of obesity on the development of hyperuricemia has been steadily established, but which parameters of adiposity that reflect the site-specific fat accumulation can underpin this association is unclear.
In this regard, it is likely to use indices of adiposity estimated as BAI, LAP, and CMI in larger epidemiological studies for that matter to gain mechanistic insight of hyperuricemia. However, no prior work has examined this premise comprehensively. Hence, the primary purpose of this study was to explore whether BAI, LAP, and CMI would provide incremental information on hyperuricemia risk in a large population-based sample of Chinese adults.
Materials and methods
Study population
This study was part of a large cross-sectional population-based epidemiological investigation that described the prevalence, incidence, and natural history of cardiovascular risk factors among 11,956 permanent residents (≥ 35 years of age) in rural areas of China from January 2012 to August 2013. The full details regarding the design and rationale of the study were extensively described elsewhere [31, 32]. Briefly, the study adopted a multistage, stratified random cluster-sampling scheme. In the first stage, three counties (Dawa, Zhangwu, and Liaoyang County) were selected from the eastern, southern, and northern region of Liaoning province. In the second stage, one town was randomly selected from each county (a total of three towns). In the third stage, 8 to 10 rural villages from each town were randomly selected (a total of 26 rural villages). The Ethics Committee of China Medical University (Shenyang, China) approved the study protocol. Subjects who passed the initial screening examination were enrolled in our study after providing written informed consent and the whole data and procedures conformed to the principles of ethical standards. In this report, 379 subjects with missing information on the variables analyzed in the study and 475 individuals with chronic kidney disease were excluded, leaving the eligible dataset of 11,102 participants.
Data collection and measurements
Prior reports have described the data collection and methods selection in detail [31, 32]. In brief, before the survey was performed, we invited all eligible investigators to attend an organized training session. The training included the purpose of this study, how to administer the questionnaire, the standard method of measurement, the importance of standardization, and the study procedures. A strict test was administered after this training, and only those who scored perfectly on the test were accepted as investigators in this study. Structured questionnaires were used to collect information regarding demographic characteristics, dietary habits, family income, sleep duration, health-related behaviors, and history of CVD (coronary heart disease, arrhythmia, and heart failure) by cardiologists and trained nurses during the interview.
Study participants waited for at least 5 min in a relaxed and sitting position. Then, blood pressure (BP) was measured by a standardized automatic electronic sphygmomanometer (HEM-907; Omron, Kyoto, Japan) using an appropriately sized cuff with the arm supported at the level of the heart. The mean readings of three replicate measurements were recorded for the present analysis.
Anthropometrics such as height, weight, hip circumference, and waist circumference were collected in all participants while lightly dressed. Briefly, their weight was quantified to the nearest 0.1 kg in the utility of a calibrated digital scale. A portable stadiometer was used for height measurement (rounded to the nearest 0.1 cm) in a standing position. After full expiration, WC was measured using a plastic measuring tape from the horizontal line at 1 cm above the belly button and hip circumference (HC) was measured at the maximum extension of the buttocks. Anthropometric indices were measured twice and then averaged. BMI was calculated as weight per height squared (kg/m2). WHtR was calculated as the ratio of WC divided by height in centimeters.
The subjects were reminded of keeping an overnight fasting with 12 h before the investigation. Venous blood specimens were designed to be obtained for assessing total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), HDL-C, fasting plasma glucose (FPG), blood urea nitrogen (BUN), serum creatinine (SCr), and serum uric acid (SUA). A full description of comprehensive storage process and standard laboratory measurement methods has been published in prior reports [31, 32]. Estimated glomerular filtration (eGFR) was calculated by virtue of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [33].
Definition
Adiposity phenotypes can be detected by anthropometric indexes such as BAI, LAP, and CMI. CMI was calculated using the equation suggested by Ichiro Wakabayashi et al.: CMI = TG/HDL-C × WHtR [25]. LAP was determined from WC (cm) plus TG (mmol/L) for men [(WC-65) × TG] and women [(WC-58) × TG] [28]. BAI was developed according to the formula proposed by Bergman et al.: BAI = [HC (cm)/height (m)1.5] − 18 [19]. The diagnosis of hypertension was established as BP level of at least 140/90 mmHg, individuals who were on antihypertensive medications or a prior diagnosis of hypertension. Diabetes was defined as the patient’s self-reported history of diabetes, use of diabetes medications, or a fasting plasma glucose ≥7 mmol/L. Hyperuricemia was defined as SUA ≥ 360 μmol/L for females and ≥ 420 μmol/L for males.
Statistical analyses
Descriptive statistics for all covariates were sex-specific stratified by hyperuricemia and summarized as mean (SD) or median (interquartile range), when appropriate, for continuous variables and frequency (percentage) for categorical variables. Stratified by sex, demographic, biochemical, and clinical characteristics between the study subgroups (hyperuricemia vs. non-hyperuricemia) were analyzed using unpaired Student’s t test to compare mean values and the chi-squared test to evaluate differences in prevalence rates. Data sets that did not have equal variances were examined using the nonparametric Mann–Whitney test. Pearson’s Correlation was used to explore correlation among anthropometric indicators. To test linear trends of variables across the BAI, LAP, and CMI quartiles, one-way analysis of variance (ANOVA) was used for mean SUA level and Cochran Armitage trend test was used for proportion of hyperuricemia. We constructed sex-specific multivariable linear regression models predicting changes in SUA. Both nonstandardized (B) with 95% confidence interval (CI) and standardized (β) coefficient estimates are given per 1 SD increment in BAI, LAP, and CMI. The proportion of explained variability incremental to each measure of adiposity (BAI, LAP, and CMI) was estimated by the change in R2. Both categorical (quartiles) and continuous analyzes were carried out. BAI, LAP, and CMI were standardized by dividing each adiposity index by its own distribution-specific SD. Multivariable logistic regression models were constructed to compute standardized odds ratios (ORs) with 95% CIs for 1 SD increase in BAI, LAP, and CMI and for sex-specific adiposity exposure categories from BAI, LAP, and CMI with prevalent hyperuricemia as outcome. The analyses were performed first in an unadjusted model (model 1), then adjusting for age, race, educational status, family annual income, physical activity, diet score, sleep duration, cigarette smoking, and alcohol consumption (model 2), and then further adjusting for BMI, BUN, eGFR, diabetes, hypertension, and history of CVD (model 3). For avoiding over adjustment, we did not include BMI in the multivariable analysis of BAI and hyperuricemia because both BAI and BMI are indicators of overall adiposity. SPSS 21.0 software (IBM Corp) was applied for all calculations, and statistical significance was assessed for a two-tailed P < 0.05.
Results
Of the 11,102 eligible participants, the mean age was 53.5 years and 53.5% were females (Table 1). The prevalence of hyperuricemia was given to 5.4% of females and 13.1% of males, respectively. Regardless of sex, anthropometric indices of obesity, including BMI, WC, HC, WHtR, BAI, LAP, and CMI, were dramatically increased in the hyperuricemia group (all P < 0.001). Concordantly, adults with hyperuricemia exhibited higher levels of systolic BP and diastolic BP together with increased FPG, BUN, SCr, and SUA, as well as decreased eGFR, with the exception of systolic BP, FPG, and BUN in males, which were unaltered (P < 0.01 for all cases). Regarding lipid profiles, TC, TG, LDL-C, and TG/HDL-C were remarkably augmented in hyperuricemia females and males compared with their counterparts, while HDL-C concentrations were greatly reduced in hyperuricemic subjects (all P < 0.001). As expected, participants with hyperuricemia constituted a higher percentage of hypertension and history of CVD relative to the non-hyperuricemia ones.
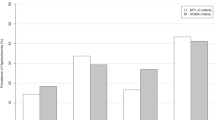
In sex-specific quartile analysis, there was a graded dose-response observed relationships of BAI, LAP, and CMI with the mean values of SUA and risk of developing hyperuricemia (Fig. 1 and Fig. 2). In females, the rates of hyperuricemia were 1.9-, 11.5-, and 9.3-fold higher across quartiles of BAI, LAP, and CMI, respectively. We found that among males, in comparison with the top versus bottom quartiles of adiposity phenotypes, the probability of hyperuricemia increased by 1.6-fold in BAI, 5.8-fold in LAP, and 5.0-fold in CMI.
Sex-specific mean values (± SD) of serum uric acid (SUA) stratified by quartiles of BAI, LAP, and CMI. Data are presented as means ± SDs. SUA demonstrated a significant increasing trend among the four BAI, LAP, and CMI quartiles (all P for trend < 0.001). BAI, body adiposity index; LAP, lipid accumulation product; CMI, cardiometabolic index; SUA, serum uric acid. *P < 0.001; **P < 0.05
The prevalence of hyperuricemia by quartiles of BAI, LAP, and CMI. The proportion of prevalent hyperuricemia demonstrated a significant trend with stepwise increase across ascending quartiles of BAI, LAP, and CMI (all P for linear trend < 0.001). BAI, body adiposity index; LAP, lipid accumulation product; CMI, cardiometabolic index
All anthropometric parameters presented positive and statistically significant associations detected by Pearson’s coefficients with the strongest relationship observed between BAI and BMI in females and males (Table 2). Although significant, LAP and CMI had lower correlation with BMI and WC, and there were no differences among females and males.
As listed in Table 3, in the univariate linear regression model (model 1), the strong positive associations of BAI, LAP, and CMI with SUA were noted. After multivariable adjustment (model 2), BAI, LAP, and CMI consistently remained as robust and independent determinants of SUA among both sexes (β 0.118, 0.206, and 0.175 in females; β 0.043, 0.203, and 0.164 in males, respectively; all P < 0.001). For females, per 1 SD increment in BAI, SUA increased on average 7.61 μmol/L, whereas CMI and LAP were 11.30 and 13.28 μmol/L higher. Likewise, the magnitude of the association of the average SUA increase per 1 SD increment in LAP was larger than that for CMI and BAI (16.51 versus 13.32 and 3.51 μmol/L, respectively) in males. Indeed, the proportion of hyperuricemia variability that can be attributed to the variation in the adiposity measures was 3.1% for LAP, 2.6% for CMI, and 1.3% for BAI in females. For males, the variability by the measures of adiposity was 3.2% for LAP, 2.3% for CMI, and 0.2% for BAI.
ORs and 95% CI for prevalent hyperuricemia according to sex-specific BAI, LAP, and CMI quartiles and BAI, LAP, and CMI as continuous variables were shown in Table 4. It was worthy of noting that raised BAI, LAP, and CMI, either expressed as sex-specific quartiles or expressed as a continuous variable, had significantly greater risks of hyperuricemia in all of the models. There was a significant linear trend among the four BAI, LAP, and CMI groups for hyperuricemia even after multivariable adjustment (all P < 0.001). In the fully adjusted multivariable model (model 3), the risk of prevalent hyperuricemia increased robustly with higher CMI, LAP, and BAI quartiles (> 206%, 750%, and 494% increase in OR from the 1st to the 4th BAI, LAP, and CMI quartile, respectively) in females. For males, the odds of hyperuricemia increased 1.62, 5.55, and 3.88 times at the 75th percentile of BAI (OR 1.622, 95% CI 1.258–2.091), LAP (OR 5.549, 95% CI 3.907–7.880), and CMI (OR 3.878, 95% CI 2.830–5.313). When BAI, LAP, and CMI were entered as a continuous variable in the adjusted model above (model 3), we revealed a 36%, 39 and 33% higher risk for hyperuricemia with each SD increment in BAI, LAP, and CMI levels in females, respectively. Similar results were acquired for the independent effect of BAI, LAP, and CMI on hyperuricemia in males (ORs for each BAI, LAP, and CMI SD increase 1.141 [1.045–1.245], 1.469 [1.347–1.601], and 1.327 [1.231–1.431], respectively).
Discussion
Novel findings in our study highlighted that BAI, LAP, and CMI could be applied to assess body composition as such or to derive some insight into the risk of hyperuricemia linked with either excess adiposity or specific patterns of adipose tissue distribution. This study may further support the prominent role of VAT (quantified by LAP and CMI) that is intrinsically different than overall adiposity (indicated by BMI) in the manifestation of cardiometabolic disease such as hyperuricemia. Therapeutic strategies targeting at the modifiable adiposity indices, such as BAI, LAP, and CMI, may have a beneficial effect in preventing or delaying the future development of hyperuricemia.
Obesity constitutes a global health issue and is increasingly recognized as a heterogeneous condition with a cluster of metabolic derangements postulated to explain its association with hyperuricemia and CVD [8,9,10,11]. Anthropometric markers of obesity, as proxies for more direct measurement of total body fat (BMI) and fat stored in specific body depots (WC and WHtR), such as the abdomen, have been the subjects of numerous previous studies to interpret the influences of obesity on hyperuricemia risk [8,9,10,11, 34]. However, BMI is criticized for its single estimate of the degree of body adipose tissue and does neither differentiate between a higher body fat content and increased lean mass nor fully reflect the amount and distribution of body fat, among which VAT is correlated with several deleterious pathophysiological processes resulting in a dysfunctional cardiometabolic phenotype than SAT [35, 36]. Additionally, given that Asians have an apparently “healthy” body weight yet are predisposed to visceral fat accumulation and insulin resistance than Western populations, it was conceivable that BMI was not suitable for diagnosing metabolic disorders, especially in Asians [37]. As well, WC and WHtR are imprecise parameters because they are functions of both the SAT and VAT compartments [17, 18]. Therefore, it is important to find obtainable and accurate clinical tools for determination of total body adipose tissue masses and diagnosis of visceral obesity in the setting of the variation in metabolic risk among obese subjects. It is worth mentioning that a better understanding of the risks linked with distinct adipose depots facilitates the explanation of obesity heterogeneity.
At present, BAI has the advantage of being applicable in the assessment of total body fat percentage and SAT, as well as performs well as a useful predictor of obesity [19, 20]. Considerable evidence has raised uncertainties regarding the performance of BAI in evaluating metabolic and cardiovascular health risk, with results both for and against the predictive utility of BAI on diabetes, hypertension, and all-cause and cardiovascular disease mortality [20,21,22,23,24]. Whether BAI can accurately characterize the impact of adiposity on hyperuricemia has not previously been explored. Our study showed for the first time that BAI was responsible for increased risk of hyperuricemia, whereas denoted an inferior predictability than other screening tools. Nevertheless, our study makes significant advances to the current literature by the extending the value of BAI as an alternative to established body adiposity measures for detecting the presence of hyperuricemia, particularly when a weight measurement is not available.
The heterogeneity of biological and clinical manifestations of obesity may result from the difference in adipose tissue distribution [12, 13]. It is evident that different fat compartments may be causally related to differential metabolic risk. The visceral fat component, the most metabolically active fat depot, warrants closer concern than general fat mass, in terms of affecting cardiometabolic profiles [13, 38]. There is growing evidence that VAT represents a pathological adipose tissue depot and has been prospectively linked to hyperuricemia risk [6, 14,15,16]. For instance, in a cross-sectional study of 699 Korean participants with diabetes, Kim et al. advocated that the mechanism of hyperuricemia in obesity was affected by the visceral fat area but not by the subcutaneous fat area [14]. Data from the Ohtori study concurred with the above results [15], where greater amounts of visceral fat measured by computed tomography (CT) were shown to be strongly correlated with hyperuricemia independently of other adipose depots. It was worth mentioning that methods for an exact quantification of body composition and fat distribution like CT and magnetic resonance imaging are not readily available in large epidemiological studies owing to a financial and time expenditure. In fact, there is an ongoing controversy as to which parameter of central obesity supposed to be used in everyday clinical practice to assess excess visceral deposition of body fat-related hyperuricemia risk [34, 39, 40]. The attempt to identify reliable visceral adiposity parameters able to assess VAT has fostered a growing enthusiasm. Lately, a novel parameter, named CMI, has been put forward by Ichiro Wakabayashi to assess the degree of body fat deposited viscerally and provide a more complete understanding of viscerally obese individuals at risk for diabetes and atherosclerotic progression [25,26,27], yet no information exists on its consequences of hyperuricemia. Conceptually, LAP is emerging as a valuable tool for health screening purposes, which has offered mechanistic insight into a well-known relation among the quantity of visceral fat and its physiopathological effects in the cardiometabolic system given its independent relationship with metabolic syndrome and incident cardiovascular events [28,29,30]. To the best of our knowledge, no study to date has addressed whether the specific mechanism behind the association of the influential adipose depot with the development of hyperuricemia is specifically accounted for by LAP. It should be noticed that much of the obesity-related risk for hyperuricemia was mainly mediated through ectopic visceral adiposity because adjustment for BMI basically could not eliminate the LAP and CMI-correlated hyperuricemia risk. In this sense, assessment of hyperuricemia could be improved with nontraditional indexes (LAP and CMI) associated with VAT.
In light of recent data about BAI, LAP, and CMI, the predictability of these tools in clinical practice for the quantitative evaluation of fat mass and for assessing excess visceral deposition of body fat subjects at risk for hyperuricemia would be of great public benefit. Under this scenario, our aims in a population-based design were to explore whether there exist corresponding differential relationships of these adiposity markers (BAI, LAP, and CMI) reflective to different degrees of abdominal obesity with the prevalence of hyperuricemia cross-sectionally. Due to metabolic differences in abdominal (particularly visceral) fat vs. general fat, it was known that body fat distribution, rather than the degree of overall adiposity, contributed to more cardiovascular risk [12, 13]. This was in accordance with our current findings, where the stronger association of hyperuricemia with LAP and CMI as compared to BAI indicated the hypothesized role of visceral fat as a unique, pathogenic fat depot. Our research built on previous investigations by reporting the intriguing patterns of LAP and CMI with hyperuricemia that are associated with VAT. Higher visceral fat accumulation, expressed by elevated LAP and CMI, had a greater influence on the uric acid metabolism than the less-specific BMI measure that included subcutaneous fat accumulation, suggesting that VAT was a primary driver in the process leading to hyperuricemia. Collectively, measurements of LAP, CMI, and BAI offered conceivable pathophysiologic explanations for the relationship of adiposity-related risks for hyperuricemia.
It remains a matter of considerable interest as to the mechanisms of the responsible drivers (BAI, LAP, and CMI) for hyperuricemia in this context. First, owing to its role as an endocrine organ secreting adipocytokines such as leptin and adiponectin, which contribute to inflammation, insulin resistance, and the risk of developing poor metabolic traits, the VAT compartment has been assumed to be a unique pathogenic fat depot [12, 41]. The manifestation of hyperuricemia associated with ectopic visceral adiposity is the effect of insulin on proximal tubular urate transport of the kidney [2]. It is considered that altered renal handling of uric acid due to insulin resistance or hyperinsulinemia effect, characterizing a state of increased uric acid and sodium reabsorption from the renal tubules which in turn can reduce urinary uric acid excretion, is one of the pathophysiological causes for the existence of hyperuricemia [42, 43]. This hypothesis is supported by a population-based study showing a causal positive association of weight and fat mass explained by genetic variants with SUA both in females and males, while no evidence to suggest that SUA causally impacts on adiposity [44]. Thus, it is possible that elevated SUA is a result of rather than a cause of adiposity. Furthermore, lipogenesis and lipolysis are frequent findings in persons with raised visceral abdominal fat accumulation in which high content of free fatty acids (FFA) may flow into the liver through the portal vein, a process that involves the overproduction of very low-density lipoprotein and enhancement of TG synthesis, resulting in hypertriglyceridemia [6]. The increased need for NADPH attributable to the excessive inflow of FFA could mediate de novo purine synthesis by means of the pentose phosphate pathway, an effect that is possibly related to the acceleration in hepatic production of uric acid [45, 46]. This explanation is concordant with prior findings, which disclose the association between VAT fat mass and serum TG levels was significant and positive. As expected, a statistically positive relationship is observed between TG and SUA levels after adjustment for age, sex and other confounders, which mirrors the value of TG synthesis in the context of UA production attributed to VAT [14, 45].
Some limitations should be acknowledged when interpreting this study. Due to the cross-sectional nature of our study, we are unable to assign causality to our findings. Further studies are needed to determine whether modification of adipose tissue distribution (quantified by BAI, LAP, and CMI) can lead to improvement of diagnostic and therapeutic strategies for hyperuricemia. Also, given that our results are predominantly applicable to Chinese adults, generalizability to other racial or ethnic populations is limited. Moreover, in the absence of urinary uric acid data, the extent to which adiposity phenotypes affect uric acid metabolism or the type of hyperuricemia could not be analyzed in the current study. Despite these limitations, our study had important clinical and public health implications.
This is the first large-scale study to reveal that BAI, LAP, and CMI were robust independent predictors of hyperuricemia in the general population. Indeed, the hyperuricemia risk worsened with a greater degree of BAI, supporting the idea that early accumulation of overall adiposity was associated with an adverse orientation of metabolic profile. Our results also underlined the implications of LAP and CMI, measures of adiposity that take into account the function of VAT compartments, in the aggregation of cardiovascular risk factors that ultimately drive the occurrence of hyperuricemia. In this sense, a more precise identification of the hyperuricemia state together with the function and expansion capacity of adipose tissue, getting insight into the broad cluster of adiposity phenotypes (BAI, LAP, and CMI), is of paramount importance for early and effective prevention of chronic metabolic diseases. It should be stressed that the adiposity–hyperuricemia pathway, evaluated by three different indices, may be a key target for efforts to compress CVD mortality on the basis of the adverse health and functional consequences of hyperuricemia.
Conclusion
Our findings provided a unique, novel framework by which to interpret hyperuricemia risk associated with raised BAI, LAP, and CMI, as hallmarks of percent body fat and visceral abdominal fat, and thereby add further impetus to understand the heterogeneity of clinical outcomes in obesity. This study may be clinically relevant in terms of primary preventive strategies for hyperuricemia which may necessitate the need to monitor excess VAT fat mass or whole body fat, namely higher LAP, CMI, and BAI as potential predictive factors.
References
Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G (2015) Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 33 (9):1729–1741; discussion 1741. doi:https://doi.org/10.1097/hjh.0000000000000701
Li C, Hsieh MC, Chang SJ (2013) Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol 25(2):210–216. https://doi.org/10.1097/BOR.0b013e32835d951e
Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Kramer BK, Ritz E, Marz W (2015) Uric Acid and cardiovascular events: a Mendelian randomization study. Journal of the American Society of Nephrology : JASN 26(11):2831–2838. https://doi.org/10.1681/asn.2014070660
Zhao G, Huang L, Song M, Song Y (2013) Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis 231(1):61–68. https://doi.org/10.1016/j.atherosclerosis.2013.08.023
Kanellis J, Kang DH (2005) Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol 25(1):39–42
Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, Matsuzawa Y (1998) Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metab Clin Exp 47(8):929–933
Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I (2013) Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 288(38):27138–27149. https://doi.org/10.1074/jbc.M113.485094
Ishizaka N, Ishizaka Y, Toda A, Tani M, Koike K, Yamakado M, Nagai R (2010) Changes in waist circumference and body mass index in relation to changes in serum uric acid in Japanese individuals. J Rheumatol 37(2):410–416. https://doi.org/10.3899/jrheum.090736
McAdams-DeMarco MA, Law A, Maynard JW, Coresh J, Baer AN (2013) Risk factors for incident hyperuricemia during mid-adulthood in African American and white men and women enrolled in the ARIC cohort study. BMC Musculoskelet Disord 14:347. https://doi.org/10.1186/1471-2474-14-347
Wang J, Chen RP, Lei L, Song QQ, Zhang RY, Li YB, Yang C, Lin SD, Chen LS, Wang YL, Zhao F, Liang GX, Hu BC, Lin JC, Cai DH (2013) Prevalence and determinants of hyperuricemia in type 2 diabetes mellitus patients with central obesity in Guangdong Province in China. Asia Pac J Clin Nutr 22(4):590–598. https://doi.org/10.6133/apjcn.2013.22.4.16
Suma S, Naito M, Okada R, Kawai S, Yin G, Morita E, Wakai K, Matsuo H, Hamajima N (2014) associations between body mass index and serum uric acid levels in a japanese population were significantly modified by LRP2 rs2544390. Nagoya J Med Sci 76(3–4):333–339
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB Sr, O'Donnell CJ (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116(1):39–48. https://doi.org/10.1161/circulationaha.106.675355
Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, Buchan I, Day N, Khaw KT (2007) Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation 116(25):2933–2943. https://doi.org/10.1161/circulationaha.106.673756
Kim TH, Lee SS, Yoo JH, Kim SR, Yoo SJ, Song HC, Kim YS, Choi EJ, Kim YK (2012) The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetology & metabolic syndrome 4(1):3. https://doi.org/10.1186/1758-5996-4-3
Yamada A, Sato KK, Kinuhata S, Uehara S, Endo G, Hikita Y, Fujimoto WY, Boyko EJ, Hayashi T (2016) Association of visceral fat and Liver fat with hyperuricemia. Arthritis care & research 68(4):553–561. https://doi.org/10.1002/acr.22729
Hikita M, Ohno I, Mori Y, Ichida K, Yokose T, Hosoya T (2007) Relationship between hyperuricemia and body fat distribution. Internal medicine (Tokyo, Japan) 46(17):1353–1358
Britton KA, Fox CS (2011) Ectopic fat depots and cardiovascular disease. Circulation 124(24):e837–e841. https://doi.org/10.1161/circulationaha.111.077602
Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P (2008) Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28(6):1039–1049. https://doi.org/10.1161/atvbaha.107.159228
Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, Xiang AH, Watanabe RM (2011) A better index of body adiposity. Obesity (Silver Spring, Md) 19(5):1083–1089. https://doi.org/10.1038/oby.2011.38
Melmer A, Lamina C, Tschoner A, Ress C, Kaser S, Laimer M, Sandhofer A, Paulweber B, Ebenbichler CF (2013) Body adiposity index and other indexes of body composition in the SAPHIR study: association with cardiovascular risk factors. Obesity (Silver Spring, Md) 21(4):775–781. https://doi.org/10.1002/oby.20289
Barreira TV, Staiano AE, Harrington DM, Heymsfield SB, Smith SR, Bouchard C, Katzmarzyk PT (2012) Anthropometric correlates of total body fat, abdominal adiposity, and cardiovascular disease risk factors in a biracial sample of men and women. Mayo Clin Proc 87(5):452–460. https://doi.org/10.1016/j.mayocp.2011.12.017
D'Elia L, Manfredi M, Sabino P, Strazzullo P, Galletti F (2016) The Olivetti Heart Study: Predictive value of a new adiposity index on risk of hypertension, blood pressure, and subclinical organ damage. Nutrition, metabolism, and cardiovascular diseases : NMCD 26(7):630–636. https://doi.org/10.1016/j.numecd.2016.03.009
Lopez AA, Cespedes ML, Vicente T, Tomas M, Bennasar-Veny M, Tauler P, Aguilo A (2012) Body adiposity index utilization in a Spanish Mediterranean population: comparison with the body mass index. PLoS One 7(4):e35281. https://doi.org/10.1371/journal.pone.0035281
Marcadenti A, Fuchs FD, Moreira LB, Gus M, Fuchs SC (2017) Adiposity phenotypes are associated with type-2 diabetes: LAP index, body adiposity index, and neck circumference. Atherosclerosis 266:145–150. https://doi.org/10.1016/j.atherosclerosis.2017.09.022
Wakabayashi I, Daimon T (2015) The “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clinica chimica acta; international journal of clinical chemistry 438:274-278. doi:https://doi.org/10.1016/j.cca.2014.08.042
Wakabayashi I, Sotoda Y, Hirooka S, Orita H (2015) Association between cardiometabolic index and atherosclerotic progression in patients with peripheral arterial disease. Clinica Chimica Acta; Int J Clin Chem 446:231–236. https://doi.org/10.1016/j.cca.2015.04.020
Wakabayashi I (2016) A U-shaped relationship between alcohol consumption and cardiometabolic index in middle-aged men. Lipids Health Dis 15:50. https://doi.org/10.1186/s12944-016-0217-4
Ioachimescu AG, Brennan DM, Hoar BM, Hoogwerf BJ (2010) The lipid accumulation product and all-cause mortality in patients at high cardiovascular risk: a PreCIS database study. Obesity (Silver Spring, Md) 18(9):1836–1844. https://doi.org/10.1038/oby.2009.453
Nascimento-Ferreira MV, Rendo-Urteaga T, Vilanova-Campelo RC, Carvalho HB, da Paz OG, Paes Landim MB, Torres-Leal FL (2017) The lipid accumulation product is a powerful tool to predict metabolic syndrome in undiagnosed Brazilian adults. Clinical nutrition (Edinburgh, Scotland) 36(6):1693–1700. https://doi.org/10.1016/j.clnu.2016.12.020
Bozorgmanesh M, Hadaegh F, Azizi F (2010) Diabetes prediction, lipid accumulation product, and adiposity measures; 6-year follow-up: Tehran lipid and glucose study. Lipids Health Dis 9:45. https://doi.org/10.1186/1476-511x-9-45
Guo X, Li Z, Guo L, Zheng L, Yu S, Yang H, Zou L, Zhou Y, Zhang Y, Zhu L, Zhang Y, Sun Y (2014) An update on overweight and obesity in rural Northeast China: from lifestyle risk factors to cardiometabolic comorbidities. BMC Public Health 14:1046. https://doi.org/10.1186/1471-2458-14-1046
Li Z, Bai Y, Guo X, Zheng L, Sun Y, Roselle AM (2016) Alcohol consumption and cardiovascular diseases in rural China. Int J Cardiol 215:257–262. https://doi.org/10.1016/j.ijcard.2016.04.095
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Dong J, Ni YQ, Chu X, Liu YQ, Liu GX, Zhao J, Yang YB, Yan YX (2016) Association between the abdominal obesity anthropometric indicators and metabolic disorders in a Chinese population. Public Health 131:3–10. https://doi.org/10.1016/j.puhe.2015.08.001
Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F (2010) Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (2005) 34(5):791–799. https://doi.org/10.1038/ijo.2010.5
Gomez-Ambrosi J, Silva C, Galofre JC, Escalada J, Santos S, Millan D, Vila N, Ibanez P, Gil MJ, Valenti V, Rotellar F, Ramirez B, Salvador J, Fruhbeck G (2012) Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (2005) 36 (2):286–294. doi:https://doi.org/10.1038/ijo.2011.100
Deurenberg P, Deurenberg-Yap M, Guricci S (2002) Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obesity reviews : an official journal of the International Association for the Study of Obesity 3(3):141–146
Seven E, Thuesen BH, Linneberg A, Jeppesen JL (2016) Abdominal adiposity distribution quantified by ultrasound imaging and incident hypertension in a general population. Hypertension (Dallas, Tex : 1979) 68(5):1115–1122. https://doi.org/10.1161/hypertensionaha.116.07306
Dong H, Xu Y, Zhang X, Tian S (2017) Visceral adiposity index is strongly associated with hyperuricemia independently of metabolic health and obesity phenotypes. Sci Rep 7(1):8822. https://doi.org/10.1038/s41598-017-09455-z
Zhang N, Chang Y, Guo X, Chen Y, Ye N, Sun Y (2016) A Body Shape Index and Body Roundness Index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. European journal of internal medicine 29:32–36. https://doi.org/10.1016/j.ejim.2016.01.019
Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR (2004) Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care 27(6):1375–1380
Quinones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E (1995) Effect of insulin on uric acid excretion in humans. Am J Phys 268(1 Pt 1):E1–E5
Facchini F, Chen YD, Hollenbeck CB, Reaven GM (1991) Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 266(21):3008–3011
Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber G, Vollenweider P, Bochud M (2012) Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One 7(6):e39321. https://doi.org/10.1371/journal.pone.0039321
Seyed-Sadjadi N, Berg J, Bilgin AA, Grant R (2017) Visceral fat mass: is it the link between uric acid and diabetes risk? Lipids Health Dis 16(1):142. https://doi.org/10.1186/s12944-017-0532-4
Fabregat I, Revilla E, Machado A (1987) Short-term control of the pentose phosphate cycle by insulin could be modulated by the NADPH/NADP ratio in rat adipocytes and hepatocytes. Biochem Biophys Res Commun 146(2):920–925
Funding
This study was supported by grants from “Thirteenth Five-Year” program funds (The National Key Research and Development Program of China, Grant no. 2017YFC1307600) and “Twelfth Five-Year” project funds (The National Science and Technology Support Program of China, Grant no. 2012BAJ18B02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Wang, H., Sun, Y., Wang, S. et al. Body adiposity index, lipid accumulation product, and cardiometabolic index reveal the contribution of adiposity phenotypes in the risk of hyperuricemia among Chinese rural population. Clin Rheumatol 37, 2221–2231 (2018). https://doi.org/10.1007/s10067-018-4143-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4143-x