Abstract
We hypothesized that constant compression of the knee would mobilize residual synovial fluid and promote successful arthrocentesis. Two hundred and ten knees with grade II–III osteoarthritis were included in this paired design study: (1) conventional arthrocentesis was performed with manual compression and success and volume (milliliters) determined; and (2) the intra-articular needle was left in place, and a circumferential elastomeric brace was tightened on the knee to provide constant compression. Arthrocentesis was attempted again and additional fluid volume was determined. Diagnostic procedural cost-effectiveness was determined using 2017 US Medicare costs. No serious adverse events were noted in 210 subjects. In the 158 noneffusive (dry) knees, sufficient synovial fluid for diagnostic purposes (≥ 2 ml) was obtained in 5.0% (8/158) without compression and 22.8% (36/158) with compression (p = 0.0001, z for 95% CI = 1.96), and the absolute volume of arthrocentesis fluid obtained without compression was 0.28 ± 0.79 versus 1.10 ± 1.81 ml with compression (293% increase, p = 0.0001). In the 52 effusive knees, diagnostic synovial fluid (≥ 2 ml) was obtained in 75% (39/52) without compression and 100% (52/52) with compression (p = 0.0001, z for 95% CI = 1.96), and the absolute volume of arthrocentesis without compression was 14.7 ± 13.8 versus 25.3 ± 15.5 ml with compression (72.1% increase, p = 0.0002). Diagnostic procedural cost-effectiveness was $655/sample without compression and $387/sample with compression. The new technique of constant compression via circumferential mechanical compression mobilizes residual synovial fluid beyond manual compression improving the success, cost-effectiveness, and yield of diagnostic and therapeutic arthrocentesis in both the effusive and noneffusive knee.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arthrocentesis is essential for the diagnosis of inflammatory and septic arthritis and is the basic procedure for intra-articular therapy, including therapeutic arthrocentesis, needle lavage, and intra-articular injection [1,2,3,4,5]. Complete arthrocentesis before injection of corticosteroid or hyaluronan confirms the diagnosis, reduces the possibility of superimposed infection, reduces patient pain, verifies the true intra-articular positioning of the needle tip, and improves the response to the injected drug [5,6,7,8,9,10,11]. Other than the introduction of ultrasound guidance and recent research into the most accurate needle approach to access a knee effusion, there have been few advances in the basic technique of arthrocentesis since the 1950s [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
As a quality improvement process within the Division of Rheumatology, we introduced a constant compression procedure during arthrocentesis of the knee to mobilize residual synovial fluid and remove the operator’s hands from potential needlestick, and determined how this modified procedure influenced quality measures, including pain, safety, serious adverse events, aspiration success, fluid volume, and diagnostic procedural cost-effectiveness.
Materials and methods
This quality improvement program and data analysis formalized in the Division of Rheumatology, Department of Internal Medicine, University of New Mexico Health Science Center was approved by the institutional review board (IRB) and was in compliance with the Helsinki Declaration and subsequent revisions. This project assessed quality improvement of knee arthrocentesis in terms of overall success, outcome, and quality among individual patients who underwent arthrocentesis with and without constant compression applied by a compressive brace intended to remove the operator’s hands from the operative field and prevent potential needlestick, yet still provide robust compression of the knee during arthrocentesis and injection procedures [26]. The quality intervention was designed as a paired study in the same knee: that is, first conventional arthrocentesis was performed in an individual knee and quality and outcome measures were obtained, then immediately after conventional arthrocentesis, constant compression was applied to the same knee in the same individual, and quality measures were obtained once again. Two hundred and ten consecutive knees with grade II–III osteoarthritis were included in this study. Inclusion criteria for the quality program for arthrocentesis and joint injection included (1) painful symptomatic knee osteoarthritis with the patient requesting a knee arthrocentesis and injection, (2) indications for therapeutic-diagnostic arthrocentesis and/or injection, (3) indication for corticosteroid injection, and (4) formal signed consent of the patient to undergo the procedure. Prior to the procedure, the presence or absence of clinical effusion was confirmed by physical examination (manual palpation of a distended suprapatellar bursa, ballottement of a “floating” patella, and fluid shift with asymmetric compression), and the knee was then classified as “effusive” (swollen) or “noneffusive” (dry). Thus, all subjects regardless of clinic effusion received both arthrocentesis and injection.
Arthrocentesis and joint injection technique
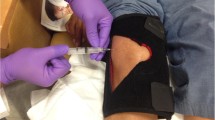
In all cases, the skin was first cleaned with chlorhexidine for antisepsis. Arthrocentesis was performed in a conventional manner, using the standard lateral approaches that have been shown to be superior to the medial approaches [12,13,14,15, 22, 24, 25]. The lateral portal was determined by palpation and marked with ink. The one-needle multiple-syringe technique was used where (1) one needle is placed for anesthesia, arthrocentesis, and intra-articular injection; (2) a first syringe or syringes are used to anesthetize the synovial membrane and completely aspirate effusion employing syringe exchanges if the effusion were large; and (3) a final syringe is used to inject the intra-articular therapy, in this case, a corticosteroid. A 22-gauge 2-in. needle (4710007050-22 GX2” (0.7 × 50 mm), FINE-JECT, Henke Sass Wolf, Kettenstrasse 1 D-78532 Tuttlingen, Germany) was mounted on a 3-ml syringe (3 ml Luer Lok syringe, BD, 1 Becton Drive, Franklin Lakes, NJ 07417, website: http://www.bd.com) filled with 3 ml of 1% lidocaine (Xylocaine® 1%, AstraZeneca Pharmaceuticals LP, 1800 Concord Pike, P.O. Box 15437, Wilmington, DE 19850-5437). Three milliliters of lidocaine was used to first anesthetize the skin, subcutaneous tissues, and synovial membrane as the 22-g needle was introduced through the skin into the lateral parapatellar recess of the suprapatellar bursa, and if there were no synovial fluid return, the needle was directed inferiorly under the patella into the patellofemoral joint toward the intercondylar notch. The knee was then compressed or “milked” by the operator’s free hand and a fully conventional arthrocentesis was performed [12,13,14,15,16,17,18,19]. After the conventional arthrocentesis was performed and fluid yield was recorded, the intra-articular needle was left in the joint, and an elastomeric knee brace (YooSoo Adjustable Knee brace, Shenzhen Shi Hai Xun Yun Wei Co., Ltd. No. 203, 69 Dong, Liyuan Xin Cun, Bantian Street, Longgang, 518000, Shenzhen, China, Amazon, https://www.amazon.com) was placed on the knee and modified so that the lateral suprapatellar bursa and patellofemoral joint could expand with fluid expressed due to constant compression from the brace on the medial and inferior knee (Fig. 1). The brace was tightened so that the patient felt considerable pressure, but the brace did not impede arterial flow in the leg. Placed this way, the brace applies constant compression without the use of human hands to the medial suprapatellar bursa and the synovial compartments of the medial and lateral inferior knee, collapsing these compartments, and forcing fluid to the lateral suprapatellar bursa and the patellofemoral joint where fluid could be accessed (Fig. 2). After the compressive brace was placed, arthrocentesis was again attempted repetitively at time 0, 30 s, 1 min, and 3 min and additional fluid yield if any was recorded. The syringe was then rotated off of the intra-articular needle, the needle was left in place in its intra-articular position, and a 3-ml syringe prefilled with 1 mg/kg triamcinolone acetonide suspension (maximum 80 mg) (Kenalog® 40, Westwood-Squibb Pharmaceuticals, Inc. (Bristol-Myers Squibb), 345 Park Ave, New York, NY 10154-0004, USA) and the medication was injected. The needle was then extracted, and firm pressure applied to the puncture site. To minimize potential procedural-related serious adverse events, the patients were screened for anesthesia and corticosteroid sensitivities before the procedure; the puncture site was compressed after needle extraction for at least 1 min and compressed for 3 min in individuals on anticoagulants; a small external diameter 22-g needle was used to reduce tissue trauma and hemorrhage; patients with known anxiety syndromes were given a small prescription of lorazepam and instructed on its emergency use for anxiety at discharge; the patients were educated to recognize postprocedural fever, infection, redness, pain, or unusual swelling and contact us if these were to occur, and were instructed to cover the puncture site for 3 days with daily bandage changes and not kneel in the garden or on the ground, use hot tubs, or otherwise contaminate the puncture site, and chlorhexidine for antisepsis was used instead of povidone. Diabetics were instructed to monitor their blood glucose postinjection and temporarily adjust antiglycemic therapy as necessary.
The arthrocentesis is performed through the superiolateral approach with an elastomeric brace applying circumferential (radial) constant compression to the knee and forcing residual fluid where it can be accessed at the superiolateral portal. An absorbent impermeable drape can be placed in the access portal to protect the brace
Outcome measures
Patients were observed for serious adverse events including reaction to local anesthesia, postprocedure infections, postprocedural pain, dermal atrophy, postprocedure anxiety or other corticosteroid complications, clinical hemorrhage, deep venous thrombosis, or postinjection visits to emergency facilities, and all subjects were interviewed at 3–12 months whether any late complications had occurred. Patient pain was measured with the standardized and validated 0–10-cm Visual Analogue Pain Scale (VAS Pain Scale), where 0 cm = no pain and 10 cm = unbearable pain [11, 27, 28]. Pain by VAS was determined (1) prior to the procedure (baseline pain), (2) during arthrocentesis (procedural pain), and (3) immediately postprocedure (postprocedural pain). Aspirated fluid volume was quantified in milliliters (ml) with conventional arthrocentesis, and the additional and total fluid obtained after application of the constant compression brace. Trace fluid was defined as at least 0.25 ml but less than 2 ml. Diagnostic fluid was defined as greater or equal to 2.0 ml (1 ml for culture and 1 ml for cell counts and crystal examination). Fluid was evaluated for cell counts, crystals, and gram stain and sent for culture and sensitivity as appropriate.
Costs and diagnostic procedural cost-effectiveness
Costs of the procedure in US dollars ($) were defined as those costs reimbursed by 2017 Medicare (USA) national rates for HCPC/CPT 20610 code for a large joint arthrocentesis for a physician office ($68.97/procedure), 15 min outpatient encounter ($76.68), and compressive brace ($10.00/brace) [29]. With these data, a conventional arthrocentesis costs $146.65, and a constant compression procedure $156.65. Diagnostic procedural cost-effectiveness was defined as cost/sample (cost per adequate diagnostic synovial fluid (sample ≥ 2 ml) to permit at least 1 ml for culture and 1 ml for cell counts and crystal examination) [30, 31].
Statistical analysis
Data were entered into Excel (Version 5, Microsoft, Seattle, WA) and analyzed in SAS (SAS/STAT Software, Release 6.11, Cary, NC). A power calculation was made using preliminary data at this level where α = 0.0001, power = 0.9, and allocation ratio = 1.0 indicated that n = 100 in each group would provide statistical power at the p < 0.001 level and n = 200 in each group at the p < 0.0001 level. Pearson chi-squares two by two table analysis was performed on categorical data calculating both p values and confidence intervals with significance reported at the p < 0.05 level. Measurement data was analyzed using the paired Student’s t test calculating both p values and confidence intervals.
Results
The mean age of the cohort was 60.1 ± 12.1 years. The male:female ratio was 18:192, typical of studies of osteoarthritis of the knee demonstrating a female gender bias. Preprocedural pain according to the 10-cm VAS was 7.7 ± 1.7 cm, procedural pain was 2.5 ± 1.8 cm, and postprocedural pain was 1.0 ± 1.6 cm, similar to other pain data from studies of arthrocentesis of the osteoarthritic knee [11, 24, 31].
There were no serious adverse events encountered by the 210 patients in the cohort including but not limited to needlestick, infection, septic joint, hemarthrosis, deep venous thrombosis, dermal atrophy, significant bruising, or other hemorrhage.
The risk of any serious adverse events from similar needle procedures has been determined to be 1:74 (1.4%); in the present study, assuming the next subject would experience a complication, the risk of any serious adverse event was less than 1:211 (< 0.5%) [32]. Infection and septic joint rates for similar needle procedures have been reported to be 1:887 (0.1%); in the present study, assuming the next subject would experience an infection, the risk of infection and septic joint was less than 1:211 (< 0.5%) [32].
Trace synovial fluid (≥ 0.25 ml) was obtained in 33.8% (71/210) without compression and 56.2% (118/210) with compression (p = 0.0001, z for 95% CI = 1.96, Pearson). Diagnostic synovial fluid (≥ 2 ml) was obtained in 22.4% (47/210) without compression and 40.5% (85/210) with compression (p = 0.0001, z for 95% CI = 1.96, Pearson). Diagnostic procedural cost-effectiveness was $655/diagnostic sample without compression and $387/diagnostic sample with compression. Absolute volume of arthrocentesis fluid yield without compression was 2.62 ± 7.98 versus 5.32 ± 11.2 ml with compression (105% increase, CI of difference − 4.56 < − 2.7 < − 0.84, p = 0.002).
In the subset of 158 palpably noneffusive (dry) knees, trace synovial fluid (≥ 0.25 ml) was obtained in 17.7% (28/158) without compression and 45.6% (72/158) with compression (p = 0.0001, z for 95% CI = 1.96). Sufficient synovial fluid for diagnostic purposes (≥ 2 ml) was obtained in 5.0% (8/158) without compression and 22.8% (36/158) with compression (p = 0.0001, z for 95% CI = 1.96). Diagnostic procedural cost-effectiveness was $2896/diagnostic sample without compression and $688/diagnostic sample with compression. In the palpably dry knee, the absolute volume of arthrocentesis fluid obtained without compression was 0.28 ± 0.79 versus 1.10 ± 1.81 ml with compression (293% increase, 95% CI of difference − 1.1179 < − 0.81 < − 0.5021, p = 0.0001). These data are shown in Fig. 3 in graphic form.
This graph shows the significantly increased synovial fluid yield with constant compression (upper bars) and conventional (lower bars) in the entire 158 knee cohort with noneffusive (dry) knee, demonstrating a mean 293% increase in synovial fluid yield (p < 0.0001) and improvement in diagnostic arthrocentesis (≥ 2 ml) from 5.0 to 22.8% (p < 0.0001)
In the subset of 52 patients with a palpably effusive knee, trace synovial fluid (≥ 0.25 ml) was obtained in 96% (51/52) without compression and 100% (52/52) with compression (p = 0.22, z for 95% CI = 1.96). Diagnostic synovial fluid (≥ 2 ml) was obtained in 75% (39/52) without compression and 100% (52/52) with compression (p = 0.0001, z for 95% CI = 1.96). Diagnostic procedural cost-effectiveness was $195/diagnostic sample without compression and $156/diagnostic sample with compression. In the palpably effusive knee, absolute volume of arthrocentesis without compression was 14.7 ± 13.8 versus 25.3 ± 15.5 ml with compression (72.1% increase, 95% CI of mean difference − 16.2407 < − 10.6 < − 4.9593, p = 0.0002). These data are shown in Fig. 4 in graphic form.
This graph shows the significantly increased synovial fluid yield with constant compression (upper bars) and conventional (lower bars) in the entire 52 knee cohort with effusive (swollen) knee, demonstrating a mean 75% increase in synovial fluid yield (p < 0.0001) and improvement in diagnostic arthrocentesis (≥ 2 ml) from 75 to 100% (p < 0.0002)
Discussion
As part of a safety and quality improvement program, we introduced a new variation on the standard arthrocentesis procedure. The variation simply involves using an elastomeric knee brace to exert a constant circumferential compression on the soft tissues of the knee and thereby press out residual synovial fluid and cause it to pool in the lateral suprapatellar bursa and patellofemoral joint. The intent of this quality improvement was to reduce interoperator variability in manual compression, accentuate completeness of residual effusion removal, and minimize the use of the operator’s hands in the operative field to reduce the risk of an inadvertent needlestick to the operator and infection to patient [26]. We hypothesized that in addition to reducing the chances of needlestick by removing the hands from the procedural site, the constant compression of the knee by an external compressive device would provide more complete arthrocentesis by mobilizing residual synovial fluid. The present study demonstrates that the success, fluid yield, and cost-effectiveness of knee arthrocentesis can be significantly improved beyond that of manual compression by the application of a constant compression circumferential knee brace (Figs. 3 and 4).
The risk of any serious adverse events from similar needle procedures has been determined to be 1:74 (1.4%) and the risk of postprocedural septic joint to be 1:887 (0.1%) [32]. In the present study, the risks of serious adverse events were very similar, the risk of any serious adverse event was less than 1:211 (< 0.5%), and the risk of infection and septic joint was less than 1:211 (< 0.5%) [32]. The presence of a compressive brace could increase the possibility of infection by contaminating the puncture site or could decrease the possibility of infection by removing contamination associated with manual compression; in any event, no infections or septic joint occurred in 210 subjects. To demonstrate a statistical increase in infections (p < 0.05) beyond a risk of 1:887 (0.1%), at least 2.1 infections would have to occur in 210 subjects, yet no infections were observed in 210 subjects with use of the compression brace—thus, it is unlikely even with larger subject numbers that an increase in infection rate would be associated with the use of mechanical compression [32]. Unique theoretical complications of using a compressive brace could be ischemia to the involved limb if the brace were to impede arterial flow (this would happen if the physician forgot to remove the brace or put it on too tightly—we have never observed this and patients have worn compressive braces chronically for other purposes and ischemia does not occur), deep venous thrombosis from compressing the popliteal venous structures (this is also unlikely in that similar external compressive devices are used to prevent deep venous thrombosis), and a hematoma/bleeding from compression in a patient on anticoagulation therapy but manual compression is more likely to be traumatic because of unequal pressure points than the soft distributed compression by a elastomeric brace. In any event, none of these complications were observed. Thus, the use of a mechanical compressive device for arthrocentesis and joint injection appears to be safe and does not appear to increase complications of arthrocentesis and injection, although larger numbers of subjects and further experience are required to fully determine the definitive effects on outcomes.
Complete arthrocentesis before injection of corticosteroid or hyaluronan confirms the diagnosis, ensures proper needle placement, excludes superimposed infection, reduces patient pain, and improves the response to the injected drug [6,7,8,9,10,11]. Weitoft and Uddenfeldt and Sibbitt et al. demonstrated that complete arthrocentesis before injection of corticosteroid resulted in a significantly sustained response, indicating that complete arthrocentesis before injection of corticosteroid was clinically important [6, 11]. Similarly, Zhang et al., Waddell and Marino, and Tananka et al. have reported occult effusion and/or significantly better response and persistence of response to hyaluronan if complete arthrocentesis is performed prior to intra-articular injection of hyaluronan into the knee [8,9,10].
The mechanisms of improved outcomes with more complete arthrocentesis are unclear, but increased intra-articular concentrations of injected medication, the removal of fluid so that the synovial membrane is in direct proximity to cartilage, and removal of pathologic cytokines and cells in synovial fluid have been postulated to be important [6,7,8,9,10,11]. The concept of intentionally removing and debulking cells and excess fluid that reduce or consume beneficial cytokines—the reduction of the “cytokine sink”—is growing in immunology [33,34,35]. In this immunologic construct applied to arthritis, the excessive synovial fluid and cells in a joint effusion become a reservoir for proinflammatory cytokines (for example IL-1β, TNF-α, IL-6, IL-15, IL-17, and IL-18) and a “sink” for anti-inflammatory cytokines (for example IL-4, IL-10, and IL-13), thus effectively increasing intra-articular residence time of proinflammatory cytokines while at the same time reducing concentrations of anti-inflammatory cytokines to the detriment of cartilage and synovial membrane [36, 37]. Thus, removal of as much synovial fluid as possible by complete arthrocentesis would result in reduction of the pathologic synovial fluid cytokine “sink” and thus be beneficial to the joint by restoring cytokine balance and increasing anti-inflammatory cytokines and injected drug concentrations [6, 36, 37].
Despite the importance of arthrocentesis to the diagnosis and management of arthritis, arthrocentesis with conventional methods is often unsuccessful in the absence of a significant effusion and, thus, can be unnecessarily traumatic and painful to patients [12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Ultrasound is increasingly used to detect synovial effusions and to guide the needle for arthrocentesis and intra-articular injection [11, 20, 23, 30, 31, 38, 39]. Ultrasound enables improved accuracy of needle placement, improved fluid aspiration, and greater overall success of arthrocentesis compared to traditional palpation-guided anatomic landmark methods [11, 30, 39]. However, ultrasound-guided arthrocentesis requires training, the equipment is costly, and the procedure is time-consuming. Moreover, the procedure is expensive, and many insurance carriers and health plans do not reimburse for ultrasound-guided knee procedures, despite the improved outcomes [30, 31]. Although ultrasound improves the detection of intra-articular sites where joint fluid may be pooling, it does not move fluid to a collection space where it is more accessible. In addition, ultrasound may not detect small amounts of fluid that are layered over the synovial membrane and cartilage surfaces [23]. However, a constant compression brace can be combined with ultrasound to confer the benefits of both techniques, and we have used this combination for particularly difficult knee arthrocentesis.
For anatomic landmark-guided knee arthrocentesis and injection, many experts recommend that the patient be supine and the knee be mostly extended with 0–25° of flexion [12,13,14,15,16]. The needle is directed inferiorly from the lateral or medial side to enter the skin 1 cm superior to the superior third of the patella, and the needle is then directed under the patella into the patellofemoral joint toward the intracondylar notch. Mounting evidence indicates that the lateral approach for arthrocentesis and injection is the most successful and accurate [12,13,14,15,16,17,18,19,20,21,22,23,24,25]. This is believed to be a consequence of the medial muscle effect and because local anatomy tends to make synovial fluid naturally pool more laterally. Hirsch et al. and Chen et al. have recently demonstrated that fluid preferentially pools in the lateral recess of the suprapatellar bursa where it can be accessed, indicating that the lateral approach has natural advantages related to regional synovial fluid pooling [20, 40]. When using the medial approach, the needle may be obstructed by medial plica, the medial triangular fat pad, cumulative particulate matter (gelatinous masses) that pools on the medial side, compressed dense hypertrophied synovial tissues, or fat-replaced replaced soft tissues (lipoma arborenscens) of the medial knee compartment [12, 15, 22,23,24]. As pointed out by Roberts, these anatomic and structural challenges have less influence with the lateral approach, thus providing the rationale for this approach in the present study [12, 13].
Arthrocentesis success can be improved by “milking” or compressing the superior knee or opposing sides of the knee with the operator’s nonsyringe hand, or by having a medical assistant use two hands to force fluid toward one side or the other, around obstructing masses, or into patellofemoral joint where the fluid can most easily be aspirated [12,13,14,15,16,17,18,19,20]. However, when a single operator applies pressure, it results in one-handed operation of the syringe, and one-handed compression of the adult knee cannot compress all the synovial compartments simultaneously. When a second operator is available to compress the knee, although two hands can compress the knee better than one, it is also generally impossible to get the complete 360° of compression. There is also an increased expense with the addition of a second assistant or operator. Further, bringing hands into the operative field increases the chance of a contaminated needlestick to the operator or assistant and increases the risk of a blood-borne infection, thus is to preferably be avoided [26]. Finally, certain operators may compress the knee vigorously and effectively and others ineffectively, factors that may contribute to interoperator variability in arthrocentesis success.
In 2008, Meehan proposed the use of an external pneumatic compression device for joint aspiration, but no published studies followed to support the usefulness of this method [41]. The present study utilized a largely conventional nonpneumatic elastomeric knee brace that provided constant circumferential compression to the joint via stretched elastomeric compression, forcing joint fluid toward a predefined portal, from which the fluid could be more easily accessed (Figs. 1 and 2). The modified arthrocentesis technique reported here is also intended to minimize interoperator variability by providing the same circumferential pressure to all knees. As the results demonstrate, constant compression significantly improves joint aspiration success and the volume of fluid collected in both the clinically effusive and noneffusive (dry) knees (Figs. 3 and 4). The current study did not evaluate whether removal of this extra 75–293% of synovial fluid beyond conventional arthrocentesis improves injection outcome. However, prior studies focusing on the completeness of aspiration by arthrocentesis have demonstrated a significant improvement in joint injection outcomes after complete aspiration of the joint [6,7,8,9,10].
Our quality intervention was designed such that we first completed conventional arthrocentesis, and then in a second step, we applied constant circumferential compression for a second round of residual synovial fluid removal permitting a paired sample analysis. The first observation is the considerable amount of residual synovial fluid that remains hidden in the joint after conventional arthrocentesis that can be mobilized and extracted by constant compression (Figs. 3 and 4). Interestingly, we found that the increased fluid return was not always immediate, but in some cases, required 30 s to 3 min to allow fluid movement and pooling before the additional fluid could be extracted. This delay in fluid movement after constant compression may be a result of the gel-like semisolid nature of synovial fluid and its non-Newtonian viscosity and elasticity [42,43,44,45]. Because of synovial fluid’s rheological properties, a constant low circumferential pressure as supplied by the brace is best suited to force the viscoelastic synovial fluid to move from one area of the joint to another. Sharp compressive force on the joint as provided by hand squeezing or “milking” increases the elastic properties of synovial fluid over its viscous properties and causes increased resistance to flow [45]. In addition, the constant low-pressure radial force of the brace may compress synovial membrane and adjacent structures, thereby forcing interstitial fluid from the tissues into the joint space where it can then be aspirated. Another less likely possibility is that the constant compression causes more synovial fluid to be secreted from the synovial membrane—though unlikely during the timescale we studied here (up to 3 min), the mechanism of the increased volume of aspirate we report here remains an important avenue for future research.
Intra-articular pressure and synovial fluid movement are dynamic throughout the phases of the arthrocentesis procedure when an external compressive brace is applied to a knee. There are three dynamic phases: (1) when the brace is first applied (creating an intra-articular pressure differential), (2) when equilibrium (intra-articular pressure homogeneity) is established after fluid has moved from the compressed tissues into the synovial fluid space at the access portal, and (3) when arthrocentesis is performed (creating an intra-articular pressure differential again between the synovial fluid compartment and the needle opening). When the brace is first applied, the low-pressure area occurs at the portal access point so fluid flows from high-pressure areas compressed by the brace to low-pressure areas not compressed by the brace. However, after the intra-articular fluid has moved within the joint from high-pressure areas to low-pressure areas, the intra-articular pressure reaches a new equilibrium so that the fluid pressures at the portal access point and compressed areas covered by the brace are equal—this is the new steady state. However, once the fluid is aspirated with a needle at the portal, a low-pressure region at the access point is created, and the pressure gradient forces more intra-articular fluid from the high-pressure areas compressed by the brace to the low-pressure access point until flow ceases when all of the effusion has been removed.
Ike et al. have demonstrated that voluntary quadriceps contraction can move otherwise occult fluid to the lateral recess of the suprapatellar bursa where the fluid can be accessed in a manner very like the compressive brace [23]. The reasons for this fluid movement are very similar to those for fluid movement when using the constant compression brace described here, that is contraction of the quadriceps muscle creates increased pressure in the inferior knee, the patellofemoral joint, and medial bursa, thereby forcing fluid to the lateral recess of the suprapatellar bursa. A disadvantage to the quadriceps contraction technique is that contraction of the quadriceps muscle forces the patella and quadriceps tendon firmly against the femur making the patellofemoral joint completely inaccessible if that were the intended target. Also, it is impossible for the patient to maintain constant contraction for several minutes (due to the muscle fibers contracting irregularly and intermittently and eventually tiring during contraction), so that the tissues “tremble” creating an unstable procedure environment with tissue movement against the procedure needle tip causing accentuated pain. The constant compression brace in essence provides all the same benefits as quadriceps contraction yet permits the patient to relax the muscles, and thus, creates a stable procedure environment, and permits ready access to the patellofemoral joint if that were the desired target. Further, constant compression increases the proportion of adequate diagnostic samples, improving diagnostic procedural cost-effectiveness by reducing costs from $655/diagnostic sample without compression to $387/diagnostic sample with compression [29,30,31, 46].
One limitation to this study is that the lateral approaches were used for arthrocentesis and injection; it is possible that the less preferred conventional medial approaches might be similarly successful by placing the brace in a reverse orientation to drive fluid from the lateral and inferior knee toward the medial knee where it could be accessed. Another important limitation of our study is that we did not evaluate whether the additional fluid expressed with compression was identical to the fluid obtained with the standard arthrocentesis procedure. Studies comparing the content of the synovial fluid collected during the first and second aspirations are currently planned. A final limitation to this study is the paired study design that, although providing a robust structure for determining improvements in individual residual arthrocentesis yield, could not determine effects or benefits of enhanced arthrocentesis on injection outcomes.
Conclusion
The quality, success, and cost-effectiveness of diagnostic and therapeutic knee arthrocentesis in both the clinically noneffusive and effusive knee can be significantly improved through the application of constant compression using a circumferential knee brace to mobilize residual synovial fluid. The improved diagnostic arthrocentesis success with respect to more consistently obtaining adequate fluid for analysis, the more complete decompression of the joint, the improved cost-effectiveness, and an improved rate of synovial fluid return confirming the accuracy of intra-articular needle placement prior to an injection procedure without an increase in serious adverse events all provide added value. As complete arthrocentesis reduces the pathologic “cytokine sink” created by the synovial effusion and has been demonstrated to improve subsequent intra-articular injection outcomes, constant compression with an elastomeric knee brace to achieve complete arthrocentesis is a simple and low-cost quality improvement technique that can readily be incorporated into clinical musculoskeletal practice and injection clinics.
References
Aceves-Avila FJ, Delgadillo-Ruano MA, Ramos-Remus C, Gomez-Vargas A, Gutierrez-Urena S (2003) The first descriptions of therapeutic arthrocentesis: a historical note. Rheumatology (Oxford) 42:180–183
Guggi V, Calame L (2002) Contribution of digit joint aspiration to the diagnosis of rheumatic diseases. Joint Bone Spine 69:58–61
Manadan AM, Block JA (2004) Daily needle aspiration versus surgical lavage for the treatment of bacterial septic arthritis in adults. Am J Ther 11:412–415
Schumacher HR, Chen LX (2005) Injectable corticosteroids in treatment of arthritis of the knee. Am J Med 118:1208–1214
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P, American College of Rheumatology (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 64:465–474
Weitoft T, Uddenfeldt P (2000) Importance of synovial fluid aspiration when injecting intra-articular corticosteroids. Ann Rheum Dis 59:233–235
Jones A, Regan M, Ledingham J, Patrick M, Manhire A, Doherty M (1993) Importance of placement of intra-articular steroid injections. BMJ 307:1329–1330
Waddell DD, Marino AA (2007) Chronic knee effusions in patients with advanced osteoarthritis: implications for functional outcome of viscosupplementation. J Knee Surg 20:181–184
Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S (2002) Intra-articular injection of high molecular weight hyaluronan after arthrocentesis as treatment for rheumatoid knees with joint effusion. Rheumatol Int 22:151–154
Zhang Q, Zhang T (2016) Effect on pain and symptoms of aspiration before hyaluronan injection for knee osteoarthritis: a prospective, randomized, single-blind study. Am J Phys Med Rehabil 95:366–371
Sibbitt WL Jr, Kettwich LG, Band PA, Chavez-Chiang NR, DeLea SL, Haseler LJ, Bankhurst AD (2012) Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol 41:66–72
Roberts WN, Hayes CW, Breitbach SA, Owen DS Jr (1996) Dry taps and what to do about them: a pictorial essay on failed arthrocentesis of the knee. Am J Med 100:461–464
Roberts WN (2007) Primer: pitfalls of aspiration and injection. Nat Clin Pract Rheumatol 3:464–472
Roberts WO (1998) Knee aspiration and injection. Phys Sports Med 26:93–94
Thomsen TW, Shen S, Shaffer RW, Setnik GS (2006) Videos in clinical medicine. Arthrocentesis of the knee. N Engl J Med 354(19):e19
Beran TN, McLaughlin K, Al Ansari A, Kassam A (2013) Conformity of behaviors among medical students: impact on performance of knee arthrocentesis in simulation. Adv Health Sci Educ Theory Pract 18:589–596
Neustadt DH (2006) Intra-articular injections for osteoarthritis of the knee. Cleve Clin J Med 73:897–898 901-4, 906-11
Zuber TJ (2002) Knee joint aspiration and injection. Am Fam Physician 66:1497–1500 1503–4, 1507
Johnson MW (2000) Acute knee effusions: a systematic approach to diagnosis. Am Fam Physician 61:2391–2400
Chen B, Lai LP, Putcha N, Stitik TP, Foye PM et al (2014) Optimal needle placement for ultrasound-guided knee joint injections or aspirations. J Trauma Treat 3:216. https://doi.org/10.4172/2167-1222.1000216
Gardner GC (2007) Teaching arthrocentesis and injection techniques: what is the best way to get our point across? J Rheumatol 34:1448–1450
Jackson DW, Evans NA, Thomas BM (2002) Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am 84-A:1522–1527
Ike RW, Somers EC, Arnold EL, Arnold WJ (2010) Ultrasound of the knee during voluntary quadriceps contraction: a technique for detecting otherwise occult effusions. Arthritis Care Res (Hoboken) 62:725–729
Chavez-Chiang CE, Sibbitt WL Jr, Band PA, Chavez-Chiang NR, Delea SL, Bankhurst AD (2011) The highly accurate anteriolateral portal for injecting the knee. Sports Med Arthrosc Rehabil Ther Technol 3:6. https://doi.org/10.1186/1758-2555-3-6
Dabke HV (2004) Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am 86-A:433–434
Sibbitt WL Jr, Band PA, Kettwich LG, Sibbitt CR, Sibbitt LJ, Bankhurst AD (2011) Safety syringes and anti-needlestick devices in orthopaedic surgery. J Bone Joint Surg Am 93:1641–1649
Myles PS, Troedel S, Boquest M, Reeves M (1999) The pain visual analogue scale. Is it linear or nonlinear? Anesth Analg 89:1517–1520
Katz J, Melzack R (1999) Measurement of pain. Surg Clin North Am 79:231–252
U.S. Department of Health & Human Services. Centers for Medicare & Medicaide Services (2017) Physician fee schedule look-up http://www.cms.hhs.gov/PFSlookup/
Sibbitt WL Jr, Band PA, Chavez-Chiang NR, Delea SL, Norton HE, Bankhurst AD (2011) A randomized controlled trial of the cost-effectiveness of ultrasound-guided intraarticular injection of inflammatory arthritis. J Rheumatol 38:252–263
Sibbitt WL Jr, Band PA, Kettwich LG, Chavez-Chiang NR, Delea SL, Bankhurst AD (2011) A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol 17:409–415
Taylor WJ, Fransen J, Dalbeth N et al (2016) Diagnostic arthrocentesis for suspicion of gout is safe and well tolerated. J Rheumatol 43:150–153
Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP (2005) Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol 26:111–117
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP (2005) Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202:907–912
Aranda F, Vacchelli E, Obrist F, Eggermont A, Galon J, Hervé Fridman W, Cremer I, Tartour E, Zitvogel L, Kroemer G, Galluzzi L (2014) Trial watch: adoptive cell transfer for anticancer immunotherapy. Oncoimmunology 3:e28344 eCollection 2014. Review
Hyc A, Osiecka-Iwan A, Niderla-Bielinska J, Jankowska-Steifer E, Moskalewski S (2009) Pro- and anti-inflammatory cytokines increase hyaluronan production by rat synovial membrane in vitro. Int J Mol Med 24:579–585
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D (2014) The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm 2014:561459
Cunnington J, Marshall N, Hide G, Bracewell C, Isaacs J, Platt P et al (2010) A randomised, controlled, double blinded study of ultrasound guided corticosteroid joint injection in patients with inflammatory arthritis. Arthritis Rheum 62:1862–1869
Wiler JL, Costantino TG, Filippone L, Satz W (2010) Comparison of ultrasound-guided and standard landmark techniques for knee arthrocentesis. J Emerg Med 39:76–82
Hirsch G, O’Neill T, Kitas G, Klocke R (2012) Distribution of effusion in knee arthritis as measured by high-resolution ultrasound. Clin Rheumatol 31:1243–1246
Meehan R (2008) Joint aspirate facilitating device. US Patent 7,468,048, issued December 23, 2008, US Patent and Trademark Office, USA
Band PA, Heeter J, Wisniewski HG, Liublinska V, Pattanayak CW, Karia RJ, Stabler T, Balazs EA, Kraus VB (2015) Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthr Cartil 23:70–76
Zhang Z, Christopher GF (2015) The nonlinear viscoelasticity of hyaluronic acid and its role in joint lubrication. Soft Matter 11:2596–2603
Tamer TM (2013) Hyaluronan and synovial joint: function, distribution and healing. Interdiscip Toxicol 6:111–125
Chaudhry H, Bukiet B, Roman M, Stecco A, Findley T (2013) Squeeze film lubrication for non-Newtonian fluids with application to manual medicine. Biorheology 50:191–202
Band PA (2015) Using Medicare data to understand health care value: measures of incremental cost and effectiveness are both needed to estimate value. JAMA Intern Med 175:462
Acknowledgements
The authors would like to thank Jackie Cremar for logistic assistance in preparation of this manuscript. We would also like to thank the staff of our local IRB (the Human Research Committee) for guiding us in the preparation of this study, data, and manuscript.
Funding
There was no internal or external support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to any procedures and prior to the inclusion in the study.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Bhavsar, T.B., Sibbitt, W.L., Band, P.A. et al. Improvement in diagnostic and therapeutic arthrocentesis via constant compression. Clin Rheumatol 37, 2251–2259 (2018). https://doi.org/10.1007/s10067-017-3836-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3836-x