Abstract
The aim of this study is to assess the recurrence probability and the possible predictors in patients with ankylosing spondylitis from etanercept discontinuation in a 3-year observational cohort (ClinicalTrials.gov: NCT02915354). A cohort of 35 patients who achieved an ASAS 20 response at the end of a randomized controlled trial underwent a 3-year follow-up evaluation. The primary end point was clinical relapse defined as the BASDAI score going back to 80% of its initial level at the beginning of the trial. Prognostic factors of relapse were analyzed using the Cox regression. Median duration of clinical remission was 15.0 months (interquartile range, 3.7–26.3 months). The cumulative probabilities of relapse at 1, 2, and 3 years were 45.7, 57.1, and 60.0%, respectively. The proportion of recurrence was not significantly different between placebo group and etanercept group by Kaplan-Meier analysis (placebo vs. etanercept: 61.11 vs. 58.82%, P = 0.890). Two independent factors associated with increasing risk of relapse were (1) age of patients (25 years or older with risk of 3.07, 95% confidence interval, 1.19–7.97, P = 0.021); (2) onset age (younger than 24 years with risk of 3.12, 95% confidence interval, 1.24–7.83, P = 0.016). No correlation was observed in the present study between the time of relapse and the duration of the treatment with etanercept in AS patients who achieved the ASAS 20 response after receiving the treatment. The older age and younger onset age of patients seems to be important factors associate with an increasing risk of relapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease, which is characterized by inflammatory low back pain, morning stiffness, axial skeleton limited activities, or complicated with peripheral arthritis [1,2,3]. Anti-tumor necrosis factor (TNF)-α agents, such as recombinant human TNF receptor etanercept, are the most frequently used biological agents in patients with AS [4]. Several clinical trials and meta-analyses [5, 6] provide convincing data showing that the benefit from etanercept treatment could lead to the clinical remission in AS patients.
Thus, it is uncertain whether anti-TNF agents withdrawal would be tolerated in AS patients after clinical remission [7]. It has been suggested in a previous study that even after 3 years’ therapy of infliximab, withdrawal from the drug would lead to relapse in all AS patients but one who were in ASAS partial remission [8]. The flare rates of up to 97–100% were found after about 6 months upon discontinuation of TNF-blocker treatment in long-standing AS with more than 10 years’ disease duration. More recently, flare rates of up to 97.6% were found in 40 patients remained in clinical remission without infliximab therapy in 1 year [9]. In another open observational study, the 36-week relapse rate was 100% in the group of 26 patients discontinued on etanercept treatment for at least 48 weeks [10]. In clinical trials previously mentioned, flare rates were up to 100% with a long-term treatment duration between 1 and 3 years. However, the question of whether the risk of anti-TNF agents’ withdrawal is influenced by the duration of treatment has not been resolved. Furthermore, almost nothing was known about the predictors for relapse in AS patients after withdrawal of anti-TNF agent therapy.
Therefore, a follow-up study is conducted here to evaluate the relapse risk upon etanercept withdrawal in all patients in ASAS 20 for 3 years either under 12 weeks’ treatment or 6 weeks’ treatment. The possible predictors for relapse were also analyzed in SA patients with different conditions in this research.
Materials and methods
This study was approved by the Committee on Medical Ethics of the Third Affiliated Hospital of Sun Yat-sen University. All methods were carried out in accordance with the approved guidelines. Approval document no. of clinical trial by SFDA was 2006 L01308. All volunteers provided written informed consent.
Patients
The studied cohort, being composed of the patients included in the etanercept trial between June 2006 and May 2007 in the third Affiliated Hospital of Sun Yat-sen University, were enrolled into this study [11]. The inclusion criteria was patients who aged 18 or older with active ankylosing spondylitis, fulfilled the 1984 modified New York criteria for AS. Inclusion criteria enriched the AS patients with highly active disease, including the following definition: (1) VAS of the duration and severity of morning stiffness ≥ 30; (2) Two out of the three items in VAS ≥ 30: (a) patient’s global assessment (PGA), (b) global back pain and nocturnal back pain, (c) Bath AS functional index (BASFI) [12]. We excluded patients who have ever received biological agents or developed complete spinal fusion. We also excluded patients with kidney disease induced by other conditions, such as pregnancy, suckle, accompany other chronic diseases, various infections in acute stage, and other infectious diseases. Cotherapy with disease modifying anti-rheumatic drugs or non-steroidal anti-inflammatory drugs could be continued if maintained at a stable dose for at least 4 weeks at baseline. The patients were randomized to receive once weekly subcutaneous injections of 50 mg etanercept or placebo in the initial 6-week double-blind period. In the subsequent 6 weeks, patients in both groups would receive etanercept. At the end of the trial, patients would stop etanercept treatment if they reached ASAS 20 response [13], either in the placebo or in the etanercept group.
Data collection
Patients were followed up by telephone every 6 weeks from the time of etanercept withdrawal to September 2010. If symptoms suggestive of relapse or other problems occurred, patients were invited to come back to the center. Relapse after etanercept withdrawal was defined as Bath Spondylitis Disease Activity Index (BASDAI) [14] score going back to 80% of its original at the beginning of the trial [15]. The following data were collected: demographic and disease characteristics, therapeutic modification, clinical values (BASFI, Bath Ankylosing Spondylitis Global Score (BAS-G), Ankylosing Spondylitis Disease Activity Score (ASDAS)) [16, 17] and biologic values at baseline of the trial and the time of relapse. Adverse events and other safety measures were also collected.
Statistical analysis
Demographic and baseline disease characteristics were summarized with descriptive statistics and analyzed with one-way ANOVA for continuous variables and χ2 tests for categorical variables. The Kaplan-Meier method was used to estimate the time to relapse rate after etanercept withdrawal. Time to relapse curves were compared via log-rank test between the group of patients received 12-week and 6-week treatment of etanercept. The influence of the following variables including age, duration of disease, onset age, BASDAI, ASDAS-CRP, C reaction protein(CRP), and ESR was examined using the Cox proportional hazards model to evaluate the effect of etanercept withdrawal on time to relapse. Every continuous variable was divided into three categories at approximately 33 and 67% [18]. If the relative relapse rates were not significantly different in two contiguous categories, they were gathered together. If no clear difference was observed in three categories, the median was used as a cut-off point. Normal value, such as 6 mg/L for CRP level, was tested. The proportional hazards model was used to study the effect of each factor on time to relapse and identify the independent prognostic factors. Relapse rates are presented as estimate with standard error (SE), follow-up times as median (interquartile range [IQR]), and hazard ratio as estimate with 95% confidence interval.
All analyses were performed using SPSS software v16.0 (SPSS, Inc., Chicago, IL).
Results
Study population
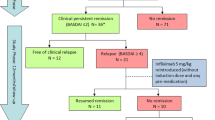
The constitution of the cohort was described in Fig. 1. The 30.0% ASAS 20 responder came from the placebo group, whereas 95.0% of ASAS20 responder came from the etanercept group at week 6 (P = 0.000). After the placebo group switched to etanercept at week 12, the ASAS 20 responder was improved to 95%, similar with 94.7% with the etanercept (P = 1.0). Thirty-five patients who had achieved ASAS 20 were included in the cohort at the inclusion time for withdrawing etanercept, no matter in the etanercept arm or the placebo arm. Characteristics of the study population are listed in Table 1.
Relapse after etanercept withdrawal
The median time of relapse was 15 months (IQR, range 3.7 to 26.3 months). For 3-year follow-up, 21 of the 35 (60.0%) patients fulfilled the criterion of relapse after withdrawal of etanercept. The cumulative probabilities of relapse at 1, 2, and 3 years were 45.7, 57.1, and 60.0%, respectively.
The proportion of relapse was not significantly different between placebo group and etanercept group (placebo/etanercept: 61.11%/58.82%, P = 0.890). The cumulative probabilities of relapse were similar in 17 patients of the etanercept group with previous RCT and in 18 patients of the placebo group (P = 0.708) (Fig. 2a). Furthermore, the mean relapse time in the etanercept group and the placebo group was not statistically different after receiving clinical response (P = 0.488).
a Cumulative probability of remaining in remission after etanercept withdrawal in the etanercept group (n = 17, the solid line) vs. in the placebo group (n = 18, the dotted line). b Cumulative probability of remaining in remission according to age at inclusion of follow-up period as indicated by age ≤ 25-year-old (n = 15, the dotted line) vs. age > 25-year-old (n = 20, the solid line). c Cumulative probability of remaining in remission according to onset age as indicated by an onset age > 24-year-old (n = 9, solid line) vs. an onset age ≤ 24-year-old (n = 26, dotted line)
By the univariate analysis, the patients with age older than 25 and the onset age younger than 24 were associated with higher relapse risk. In the analysis of the age at inclusion, there was also a difference between the patients with younger age (≤25) and older age (>25) age (Fig. 2b; P = 0.015); the mean time to relapse of the patients with old age was 13.6 ± 3.1 months, and the mean TtR of the patients with young age was 26.2 ± 3.3 months. This result was confirmed by a Cox regression analysis (age > 25 vs. ≤25-year-old; hazard ratio, 3.07; 95% CI, 1.19–7.97; P = 0.021). Five of nine patients with onset age ≤ 24 had already experienced a relapse by 2 months, and the remaining two patients, by 6 months (Fig. 2c; P = 0.016). The cumulative probability of relapse was less in patients with younger onset age (55.6% by month 6 and 77.8% by month 24, respectively) than in patients with older onset age (15.4% by month 6 and 50.0% by month 24, respectively). None of the duration of disease, BMI, BASDAI, ASDAS-CRP, CRP, and ESR at inclusion of follow-up period were associated with relapse. Those continuous variables were cut off by the median values in the data analysis, only the CRP level was tested by normal value of 6 mg/L. Those parameters at inclusion of follow-up period were not associated with relapse.
Older age and younger onset age were associated with a shorter time to relapse after etanercept withdrawal. In multivariate analysis, the age older than 25-year-old and the onset age of 24-year-old or younger were independent factors associated with a high relapse risk (Table 2).
Discussion
The efficacy and safety of TNF-α blocker has been clinically proven in treating patients with active AS. The improvement of clinical assessments including BASDAI [19], BASFI, and BASMI has been demonstrated. The results of our previous study also confirmed the efficacy of TNF-α blocker in AS patients [11].
In view of the cost and potential side effects, when could stop the treatment of etanercept in AS is an important question [20]. It was accepted that TNF-α blockers withdrawal in patients with AS, even in patients after long-term treatment of TNF-α blockers, would lead to a clinical relapse of the disease with deterioration of signs and symptoms. High relapse rates from 88.1 to 89.7% were reported after etanercept or infliximab withdrawal, respectively, within 24 weeks in two retrospective studies [8, 10]. In these studies, the durations of continuous TNF-α blockers therapy prior to their interruption were 24 weeks and 3 years, respectively. In our study, the cumulative probabilities of relapse are far lower than the two retrospective studies, only 45.7, 57.1, and 60.0% at 1, 2, and 3 years, respectively. The low probabilities of relapse in our study could explain by the inclusion of the follow-up cohort was strictly limited to the patients with clinical remission (ASAS 20 response) at the end of the trial. Interestingly, the flare rate was not different between the group of 13 patients with early active axial spondyloarthritis treated with etanercept (ETA) and the group of 4 patients with sulfasalazine (SSZ) for 48 weeks, 69% in the ETA group vs. 75% in the SSZ group reported in a recent European study. The flare rate in the second year was much lower than the flare rates mentioned previously, possibly because of the inclusion of the patients during follow-up period who reached study remission (ASAS plus MRI remission) [21]. In the subgroup analysis, the AS patients received infliximab for the preceding 3 years in partial remission at inclusion had a longer duration of response than patients who did not fulfill remission criteria (P = 0.059) [8]. Nearly 30 and 60% of AS patients with flare are showed in another study in patients with ASAS partial remission and ASAS 40 response, respectively [10]. In addition, the difference for defining relapse may be another reason for inconsistency among our study and above studies.
In view of the cost and potential side effects, whether or not treatment of etanercept can be stopped in AS is an important question [15]. What is more surprising in present study is that no difference was shown between the two time to relapse curves deriving from 6-week or 12-week treatment. These results suggest that longer term TNF-α blockers therapy may not provide a lower risk of recurrence than shorter time of treatments. We speculated that the time to relapse was likely not in correlated with the duration of the treatment with etanercept in AS patients.
In addition, it is the first time reporting in our present study that age and onset age of the disease seem to be important prognostic factors for AS patients. However, it has been previously reported that higher BASDAI, elevated CRP value, older age, and a longer disease duration were associated with a shorter time to relapse [8]. It has been also demonstrated that younger age, male gender, high ASDAS, and BASDAI scores were identified as independent baseline predictors of response and/or continuation of TNF-α blocking therapy [22]. One of the possible reasons to explain this might be due to a more pronounced response and prolonged remission of etanercept in younger patients as compared with older patients [23]. The onset age of disease is the first to be associated with the relapse after the cessation of the biological agents in our study. Moreover, in statistical analysis, the age older than 25 and the onset age of 24 or younger were independent factors associated with a high relapse risk. Due to our relatively small size of study sample, the cut-off points were considered merely as the references. But older age and younger onset age are reliable trends associated with relapse after etanercept withdrawal, and should be considered in treatment strategy.
In the field of the etanercept treatment in AS patients, no extensive consensus has been reached concerning the duration of treatment and the timing of etanercept cessation. There was only an expert advice in ACR 2013 conference, stating that the etanercept treatment should be administrated for at least 12 weeks. Nonetheless, the criterions for etanercept withdrawal were not proposed so far. The data from our study demonstrated that the risk of relapse was not significantly different between the groups receiving 6-week and 12-week treatment. This observation revealed that the clinical remission was possibly the key factor when the rheumatologist makes the decision to withdraw the biological agents in AS patients.
None of BASDAI, CRP, ESR, and disease duration were found to be associated with a longer time of remission in this study. There are two possible explanations. First, the patients in follow-up cohort were in ASAS response, with disease indexes and biological parameters within normal ranges. Second, limited to a small sample size, the trends were not pronounced.
Discontinuation of anti-TNF agent treatment may become necessary for those patients who plan to be pregnant or have to undergo surgery. Several factors should be taken into consideration as further predictive factors: for example, the imaging remission, duration of remission, and ASDAS.
Conclusion
In summary, it was shown that the time to relapse was not in correlation to the duration of the treatment with etanercept in AS patients who achieve ASAS 20 response. It was also suggested that the patients aged and with younger onset age may relapse with higher risks.
References
Braun J, Sieper J (1996) The sacroiliac joint in the spondyloarthropathies. Curr Opin Rheumatol 8:275–287
de Vlam K, Mielants H, Cuvelier C et al (2000) Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J Rheumatol 27:2860–2865
Braun J, de Keyser F, Brandt J et al (2001) New treatment options in spondyloarthropathies: increasing evidence for significant efficacy of anti-tumor necrosis factor therapy. Curr Opin Rheumatol 13:245–249
Gorman JD, Sack KE, Davis JC Jr (2002) Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 346:1349–1356
Baji P, Pentek M, Szanto S et al (2014) Comparative efficacy and safety of biosimilar infliximab and other biological treatments in ankylosing spondylitis: systematic literature review and meta-analysis. Eur J Health Econ 15(Suppl 1):S45–S52
Li ZH, Zhang Y, Wang J et al (2013) Etanercept in the treatment of ankylosing spondylitis: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials, and the comparison of the Caucasian and Chinese population. Eur J Orthop Surg Traumatol 23:497–506
Kiltz U, Baraliakos X, Fau Braun J, van der Heijde D et al (2013) Withdrawal of medical therapies in axial spondyloarthritis: what would be the optimal trial design? Clin Exp Rheumatol 31:S47–S50
Baraliakos X, Listing J, Brandt J et al (2005) Clinical response to discontinuation of anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther 7:R439–R444
Baraliakos X, Listing J, Rudwaleit M et al (2007) Safety and efficacy of readministration of infliximab after longterm continuous therapy and withdrawal in patients with ankylosing spondylitis. J Rheumatol 34:510–515
Brandt J, Listing J, Haibel H et al (2005) Long-term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitis. Rheumatology (Oxford) 44:342–348
Lin Q, Lin ZF, Gu J, Gu JF, Huang F et al (2010) Abnormal high-expression of CD154 on T lymphocytes of ankylosing spondylitis patients is down-regulated by etanercept treatment. Rheumatol Int 30:317–323
Calin A, Garrett S, Whitelock H et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 21:2281–2285
van der Heijde D, Dougados M, Davis J et al (2005) Assessment in Ankylosing Spondylitis International Working Group/Spondylitis Association of America recommendations for conducting clinical trials in ankylosing spondylitis. Arthritis Rheum 52:386–394
Garrett S, Jenkinson T, Kennedy LG et al (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath ankylosing spondylitis disease activity index. J Rheumatol 21:2286–2291
Brandt JF, Haibel H, Haibel HF, Sieper J, Sieper JF, Reddig J et al (2001) Infliximab treatment of severe ankylosing spondylitis: one-year followup. Arthritis Rheum 44:2936–2937
Lukas C, Landewe R, Sieper J et al (2009) Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 68:18–24
van der Heijde D, Lie E, Kvien TK et al (2009) ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 68:1811–1818
Peto RF, Pike MC, Pike MF, Armitage P, Armitage PF, Breslow NE et al (1977) Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II: analysis and examples. Br J Cancer 35:1–39
Braun J, Brandt J, Listing J et al (2003) Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum 48:2224–2233
Navarra SV, Tang BF, Lu L, Lu LF, Lin H-Y et al (2014) Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis 17:291–298
Song IH, Althoff CE, Haibel H et al (2012) Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 71:1212–1215
Arends S, Brouwer E, van der Veer E et al (2011) Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 13:R94
Rudwaleit M, Listing J, Brandt J et al (2004) Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis 63:665–670
Acknowledgements
Supported by 5010 Subject (2007) of Sun Yat-sen University (Grant No. 2007023), National Natural Science Foundation for the Youth NSFY of China (Grant No. 81302583), Guangdong Natural Science Funds for Distinguished Young Scholar (Grant No. 2014A030306039), high-level personnel of special support program for Technology Innovative Talents and the Top Young of Guangdong Province (Grant No. 2015TQ01R516), the Fundamental Research Funds for the Central Universities (16ykzd05), Distinguished Young Scholar Candidates Programme for The Third Affiliated Hospital of Sun Yat-sen University, and the Pearl River Nova Program of Guangzhou (Grant No. 201610010005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Zhao, M., Zhang, P., Fang, L. et al. Possible predictors for relapse from etanercept discontinuation in ankylosing spondylitis patients in remission: a three years’ following-up study. Clin Rheumatol 37, 87–92 (2018). https://doi.org/10.1007/s10067-017-3763-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3763-x