Abstract
The aim of this study is to retrospectively analyze 10-year drug survival of first-line TNF inhibitor (TNFi) in rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), and juvenile idiopathic arthritis (JIA) patients, comparing withdrawal rates and discontinuation pattern between adult- and juvenile-onset populations. RA, AS, PsA, and JIA patients treated with infliximab, etanercept, or adalimumab as first TNFi between 1999 and 2015 were extracted from a local registry. Drug survival up to 10-year follow-up was evaluated by the Kaplan-Meier method and compared according to age (adult vs juvenile onset), TNFi agent, and discontinuation reason by a stratified log-rank test. Three hundred sixty JIA (205 etanercept, 66 adalimumab, and 89 infliximab) and 951 (607 RA, 188 AS, and 156 PsA) adult patients (464 infliximab, 262 adalimumab, and 225 etanercept) were included. After exclusion of systemic-onset JIA (18.5%), overall 10-year retention rate was 31.8%, with no difference between adult- and juvenile-onset patients (32.1 and 30.2%, respectively; HR 0.938 [95% CI 0.782–1.125]). Etanercept showed the highest drug survival in adult-onset population (p < 0.0001 vs both monoclonal antibodies) and infliximab the lowest in juvenile-onset population (p = 0.005 vs adalimumab and p < 0.0001 vs etanercept). Inefficacy was the most frequent reason for TNFi withdrawal in adult population (29.75%) with a significantly higher risk of discontinuation than in juvenile-onset subgroup (HR 1.390 [95% CI 1.060–1.824]). Serious infections and malignancies caused TNFi withdrawal only in adult whereas gastrointestinal, neuropsychiatric, and ocular complications quite only in juvenile patients. Despite a similar 10-year drug survival, adult- and juvenile-onset subpopulations showed a significantly different pattern of TNFi reasons for discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of biotechnological drugs (bDMARDs) at the beginning of the 2000s has dramatically revolutionized the management and the expected outcome of inflammatory arthritides. Tumor necrosis factor inhibitors (TNFis) were the first biotherapies proposed to treat NSAID and synthetic disease-modifying antirheumatic drug (sDMARD) failures in rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), and juvenile idiopathic arthritis (JIA). JIA is an umbrella definition used for indicating a heterogeneous group of seven different childhood-onset arthritides [1]. Although JIA should be considered pathogenically and clinically different from adult-onset arthritis, its management has been often tailored on RA treatment strategies. Nowadays, five bDMARDs have been licensed for JIA by the US Food and Drug Administration and the European Medicines Agency: etanercept, adalimumab, abatacept, tocilizumab, and canakimumab. Even though infliximab failed the primary endpoint in a randomized controlled trial (RCT) in JIA patients [2], it is widely used in daily practice, with convincing evidence of clinical effectiveness [3].
In the last decade, large population-based registries have been increasingly used to evaluate the long-term performance of TNFis in a real-life setting. In this scenario, drug retention may be considered as the most reliable indicator of overall treatment effectiveness, as it is mainly determined by both drug efficacy and safety profile. Thus, several observational studies provided an abundant amount of data about TNFi survival rates in adult-onset arthritides [4,5,6,7,8,9], whereas only few studies have been focused on TNFi retention in pediatric population to date [10, 11]. Moreover, to our best knowledge, no real-life analyses aiming to the comparison between adult- and juvenile-onset TNFi-treated patients have been performed yet.
In order to fill this gap, the aim of this study is to retrospectively analyze the long-term (10 years) drug survival and the pattern of treatment discontinuation in a large cohort of RA, AS, PsA, and JIA patients treated with TNFis, comparing the between-group withdrawal rates for infliximab, adalimumab, and etanercept in adult- and juvenile-onset populations.
Patients and methods
Data source and patients
Eligible study population was extracted from a local registry approved by the local ethics committee including all patients who signed the informed consent for any anonymous analysis of clinical data and have been treated with a bDMARD between October 1999 and June 2015 in our Rheumatology Unit. All analyzed clinical information are reported as anonymous aggregate data, excluding any identifiable individual medical information. Adult-onset arthritis group (disease onset ≥16 years old) included patients diagnosed with RA fulfilling the American College of Rheumatology (ACR) 1987 revised criteria [12], with AS according to the New York modified criteria [13], or with PsA according to the Moll and Wright criteria [14]. Juvenile-onset arthritis group (disease onset <16 years old) comprised patients fulfilling the International League of Associations for Rheumatology (ILAR) criteria for JIA [1]. The analysis was limited to patients who received infliximab, etanercept, or adalimumab as first-line biotherapy. In order to balance the exposure among the considered bDMARDs, the evaluation was limited to the period when all the three TNFis were available in Italy (from January 2003) in a setting of relatively similar access to each drug for all the indications. Exclusion criteria were a previous therapy with a different bDMARD or the enrolment in a RCT. Treatments were administered in routine care in accordance with international recommendations: TNFis were prescribed in almost every case according to licensed regimen and concomitant sDMARDs or corticosteroids were administered if ordered by the referring rheumatologist.
Outcome
Drug survival was retrospectively calculated as the time period until the definitive treatment discontinuation or the first missed dose after initiation of TNFi therapy. Interruptions were considered definitive when indicated in the registry, or when no consecutive re-introduction of treatment was reported. All observations were censored at the last registered visit before 30 June 2015. The reasons for TNFi withdrawal as reported in the registry were evaluated and classified into three major categories: inefficacy (primary or secondary no response, defined according to a cutoff of 6 months since treatment initiation), adverse events (AEs), and others (including remission, desire for pregnancy, and patient preference). The latter was considered as right censored in the drug survival evaluation. The main analysis was conducted by comparing reasons for discontinuation and drug survival in adult- versus juvenile-onset arthritides. Further sub-analyses were performed by stratifying the study population according to TNFi.
Statistical analysis
Descriptive statistics was used to calculate mean and standard deviation of population baseline characteristics. Survival distribution curves were estimated by the Kaplan-Meier method and compared by a stratified log-rank test. Results are presented as hazard ratios (HRs) with 95% confidence intervals (95% CI). Causes of discontinuation in the two subgroups were compared by using Fisher’s exact test. Statistical analyses were performed using SPSS Statistics 20.0 as software package. P values equal to or less than 0.05 were considered statistically significant.
Results
Baseline characteristics
The study population consisted of 1311 patients (360 juvenile- and 951 adult-onset arthritides). In particular, the analysis involved 360 JIA patients (48 with systemic onset, [17 infliximab, 28 etanercept, and 3 adalimumab] and 312 with non-systemic onset [72 infliximab, 177 etanercept, and 63 adalimumab]), 607 RA patients (239 infliximab, 186 etanercept, and 182 adalimumab), 188 AS patients (149 infliximab, 6 etanercept, and 33 adalimumab), and 156 PsA patients (76 infliximab, 33 etanercept, and 47 adalimumab). Baseline patient characteristics in each subgroup are reported in Table 1.
Drug survival analyses
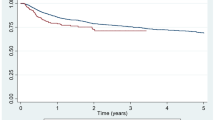
The overall 10-year retention rate of the whole study population was 31% (median time-on-drug 60.8 [95% CI 54–67.5] months), with no difference (p = 0.27) between TNFi monotherapy (30.4%) and patients receiving concomitant MTX (33.1%). The analysis according to each diagnostic subgroup revealed a significantly lower drug survival in systemic-onset JIA (10-year retention rate 18.5%, median time-on-drug 19.5 months). This finding is probably the result of the peculiar pathogenic pathway distinguishing the systemic subset from other ones and suggesting the use of bDMARDs other than TNFis for the treatment of systemic JIA [15]. Thus, we decided to complete all subsequent analyses by separating this subgroup. The recalculated overall 10-year retention rate after exclusion of systemic JIA increased to 30.2% (median time-on-drug of 59.7 months [95% CI 49.2–70.2]) with no difference (HR 1.066 [95% CI 0.888–1.278], p = 0.488) observed in the comparative analysis versus adult subgroup (32.1%; median survival 65.4 months [95% CI 56.2–74.6]) (Fig. 1). Stratifying the population according to TNFi, the estimated proportions of patients maintaining etanercept, adalimumab, or infliximab treatment after 10 years were respectively 56.1, 23.5, and 28.3% in the adult-onset group, and respectively 38.1, 50.7, and 10.6% in the juvenile-onset one. The 10-year drug survival was significantly higher for etanercept compared with both infliximab and adalimumab in adult-onset patients (p < 0.0001), and significantly lower for infliximab versus both adalimumab (p = 0.005) and etanercept (p < 0.0001) in juvenile-onset population (Fig. 2).
Reasons for discontinuation
Overall, 367 (27.99%) patients discontinued the first TNFi course because of inefficacy, and 325 (24.79%) because of AEs. In the JIA subgroup, bDMARD withdrawal has been reported in 34 (70.8%) patients with systemic onset (20 [41.6%] because of inefficacy and 14 [29.2%] because of AEs) and in 152 (48.7%) with non-systemic-onset disease (64 [20.5%] because of inefficacy and 88 [28.2%] because of AEs). In the adult-onset subgroup, 506 (55.5%) patients discontinued the TNFi during the 10-year follow-up period (283 [29.75%] because of inefficacy and 223 [23.45%] because of AEs). The risk of stopping the TNFi because of inefficacy was significantly lower in non-systemic juvenile compared with adult-onset patients (HR 0.719 [95% CI 0.548–0.943], p = 0.017), whereas no significant differences were observed in the risk of discontinuation due to intolerance (HR 1.207 [95% CI 0.943–1.546], p = 0.136) (Fig. 3).
The pattern of AEs leading to withdrawal was significantly different in the comparative analysis between the two subgroups (Table 2). Infusion/injection reaction was the most frequent reason for discontinuation in both adult (n = 92 [9.6%]) and juvenile patients (n = 19 [6.1%]). TNFi withdrawal was caused by cutaneous complications (mainly new-onset psoriasis and eczema) in 34 (3.9%) adult and 8 (2.6%) juvenile patients. Of note, most of skin AEs leading to discontinuation were associated with the use of adalimumab in both adult (28 of 34) and juvenile (5 of 8) subgroups. Serious infections, malignancies, and deaths led to discontinuation only in adult-onset population (22 [2.31%], 19 [1.99%], and 15 [1.57%] patients, respectively), whereas gastrointestinal complications (all new-onset inflammatory bowel diseases [IBD]) led to TNFi withdrawal only in juvenile-onset patients (n = 9 [2.88%]; 4 oligoarticular extended, 4 polyarticular rheumatoid factor negative, and 1 PsA-JIA). Moreover, neuropsychiatric (headache, vertigo, fatigue, hyperactivity, nervousness, anxiety, severe unusual aggressiveness, panic attacks, depression, and anorexia nervosa) and ocular (all incident uveitis) complications led to discontinuation more frequently in juvenile (14 [3.89%] and 14 [3.89%] patients, respectively) than in adult-onset subgroup (2 [0.21%] and 2 [0.21%] patients, respectively). Of note, all the patients who stopped bDMARD because of new-onset uveitis were in treatment with etanercept. The occurrence of uveitis in each JIA subtype is detailed in Table 3.
Discontinuation because of persistent remission occurred more frequently in non-systemic juvenile than adult patients (34 [10.8%] and 15 [1.6%], respectively; p < 0.0001), with numerically lower, although not statistically significant, frequency in RA patients (1.1%) within adult subgroup and systemic JIA patients (4.5%) within the juvenile one. In JIA patients, etanercept was associated with the highest rate of TNFi-free remission compared with monoclonal antibodies (14.6 vs 5.9%, respectively; p = 0.022), whereas no statistically significant difference according to the single TNFi was observed in the adult population.
Discussion
To our best knowledge, the current study compares for the first time the long-term retention rate and the pattern of discontinuation of adalimumab, etanercept, and infliximab as first-line TNFi therapy between adult- and juvenile-onset arthritis populations. We demonstrated that after 10 years, about one third of RA, PsA, AS, and JIA patients maintained the first biologic treatment, with no significant survival difference between adult and juvenile subgroups.
The comparative analysis of drug retention according to individual diagnosis showed a significant lower retention rate only in systemic-onset JIA. This finding has been already reported by similar studies [11] and is consistent with the increasing evidence of divergent pathogenic pathways in different JIA categories (especially systemic- vs non-systemic onset). Systemic JIA is an autoinflammatory disease with abnormalities in the innate immune system related to pro-inflammatory cytokines such as IL-1, IL-6, IL-18, and pro-inflammatory S100-proteins, whereas in non-systemic JIA, the adaptive immune system is deregulated with an imbalance between autoreactive Th1/Th17 and Treg cells [15]. The improving knowledge of these different pathogenic aspects may easily explain the recent favorable results observed in the treatment of systemic JIA by using IL-1 and IL-6 blockade, in contrast with poor responses reported with the use of TNFis [16], as confirmed by our findings. Nowadays, international recommendations do not consider TNF blockers as the first choice for treating systemic JIA; thus, we decided to separate this subgroup from overall retention analysis.
TNFi drug survival has been extensively evaluated in adult population by several observational studies conducted in RA, PsA, and AS cohorts. However, most of previous similar studies have limited the observation to a short follow-up period, with the only exception of recent data from our group showing TNFi survival rate of 23.4% at 12 years in RA patients [9] and 55.5% at 8 years in SpA population [17]. The short-term drug discontinuation rates in our sample are comparable to those previously described in main international registries [4, 18, 19] and become progressively lower in patients maintaining the bDMARD over 5 years, as a probable consequence of the positive selection of patients presenting a good clinical response without serious AEs over time. Reports on bDMARD retention rate in JIA patients are numerically fewer with, again, a shorter period of evaluation compared with the current analysis. Similarly, previous observational studies reported a high retention rate of biologic treatment in the first 4 years of therapy in JIA patients [10].
Despite the similar drug survival, in our analysis, the reasons for withdrawal were significantly different between the two subgroups. Intolerance was the cause of TNFi discontinuation in about one third of juvenile patients, whereas the majority of adult subjects (about 30%) stopped the drug because of primary or secondary inefficacy. Our data are consistent with what previously reported by similar analyses conducted in adult populations [20, 21], whereas a Finnish cohort [10] and the Portuguese Reuma.Pt registry found inefficacy to be the most frequent reason for discontinuation (24 and 26.4%, respectively) compared with AEs (14 and 6.1%, respectively) in JIA patients. However, both these studies have been conducted over a shorter follow-up period (60 and 48 months, respectively) and did not provide the detailed analysis of side effects leading to discontinuation, so the comparison with our cohort is not completely feasible.
Moreover, our data reported a pattern of AEs leading to withdrawal significantly different in the comparative analysis between the two subgroups: neuropsychiatric, gastrointestinal, and ocular complications led to TNFi withdrawal quite only in juvenile subgroup, while infections and malignancies only in adult population.
During the past decade, inflammation has been revisited as an important etiologic factor of psychiatric disorders, which may be elicited by pro-inflammatory cytokine levels [22]. As previously described [23], the spectrum of neuropsychiatric AEs in TNFi-treated pediatric population may be extremely wide, ranging from unspecific symptoms to important behavior alterations or definite neuropsychiatric syndromes, usually completely recovered after treatment interruption or dose reduction. On the other hand, these complications seem to be very infrequent in adult patients, as demonstrated in our cohort. However, it should be stressed that neuropsychiatric manifestations are quite heterogeneous in patients suffering from autoimmune disorders and frequently the differentiation between organic and dysfunctional symptom is difficult.
Anterior acute uveitis in either adult- or juvenile-onset AS, chronic anterior uveitis in JIA, and IBD in both diseases have been described as extra-articular manifestations frequently complicating both juvenile [24, 25] and adult diseases [26]. The use of synthetic and biologic DMARDs may be effective for treating and preventing these complications in both juvenile [27] and adult [28] populations, with some notable differences among available TNFis. In particular, compared with anti-TNF monoclonal antibodies, etanercept is associated with a higher risk of recurrent or new-onset uveitis in JIA [29] and adult AS [30] patients. Moreover, etanercept is known to be ineffective in the treatment of IBD [31] and has been related to an increased incidence of flares or new-onset IBD in treated patients with JIA [25] and AS [32]. In our cohort, etanercept accounts for the majority of JIA patients and has been initially prescribed (even in patients carrying high risk factors for uveitis such as ANA positivity and early-onset disease), whereas the use of etanercept in our adult AS population has been very infrequent (only 4.7%) because of the lower efficacy in the management of extra-articular manifestations. Thus, it is not surprising that new occurrence or flare of uveitis and IBD have been more frequently reported as the cause of TNFi discontinuation in juvenile compared with adult subgroup. HLA-B27 positivity is known to be associated with the onset of uveitis in both adult AS and ERA-JIA. Unfortunately, given the retrospective design of our study, data on HLA-B27 were largely incomplete; therefore, a correlation analysis was not performed. Nevertheless, only one ERA-JIA patient discontinued the TNFi treatment because of uveitis, suggesting a minor role of HLA-B27 in predicting this complication.
The incidence of serious infections has been reported to be increased in both juvenile [33] and adult [34] arthritides independently of the treatment, as an effect of disease itself (which impairs the immune responses), extra-articular manifestations, or comorbidities such as chronic lung disease and diabetes mellitus. The use of immunosuppressant treatments may contribute to further increase this risk, as reported for TNFis in international registries including adult (especially RA) population [35,36,37], but not in juvenile patients [32, 38]. These findings may explain the higher incidence of serious infection leading to TNFi withdrawal recorded in our adult cohort compared with the juvenile one.
Similarly, RA and JIA patients carry an increased risk of malignancies (specifically lymphoma) compared with the general population; this is partly explained by the typical immune deregulation of the diseases [39, 40], whereas data on AS and PsA do not show the same link [41]. Anyway, cancer should be considered as a rare event in childhood compared with adult population [42]. Despite the evidence of a potential role of TNFis in further increasing the incidence of solid and hematological malignancies reported almost immediately after their introduction [43], more recent data from observational registries have definitely clarified that the addition of TNFi to sDMARD does not alter the risk of cancer in both RA [44, 45] and JIA [46] patients. Thus, in our analysis malignancies have been reported as a frequent reason for withdrawal only in adult but not in juvenile cohort as an effect of the different incidence of this complication according to age, independently of TNFi treatment.
Several studies evaluated the impact of TNFi withdrawal in patients achieving a clinical remission [47, 48], but data on the incidence of bDMARD-free remission in a real-life setting are still lacking in both adult and juvenile cohorts. Although it has been excluded from the retention analysis, in our cohort, discontinuation because of a persistent remission was significantly more frequent in juvenile than in adult patients and in etanercept-treated patients compared with anti-TNF monoclonal antibodies in the juvenile subgroup alone.
According to previous studies evaluating bDMARD survival in adult population, etanercept may be considered the most persistent TNFi in RA [4, 9, 49, 50], as the result of a better safety profile and a lower immunogenicity leading to less frequent secondary no response [51]. On the other hand, etanercept is known to be less effective compared with anti-TNF monoclonal antibodies in the treatment of extra-articular manifestations of SpA, resulting in a similar survival on treatment of individual TNFis in both PsA and AS. Thus, the highest etanercept retention rate in our adult cohort may be the consequence of the great proportion of enrolled RA patients (63.8% of the whole population) combined with the favorable etanercept survival for this indication. Being the first to be licensed for the treatment of JIA, in our juvenile cohort, etanercept was largely the most prescribed TNFi (56.7% of non-systemic subset), with a greater retention rate compared with infliximab and a similar drug survival of adalimumab.
Our study has been conducted in a real-life cohort of TNFi users, with the intrinsic limitations of its observational and retrospective design. We are aware that, in the absence of randomization, patients with a different discontinuation risk may have been channeled to a specific drug, producing selection bias and potentially affecting our analysis. Moreover, as usual in long-term drug survival analyses, the number of patients at risk tends to progressively decrease by time, being reduced at the end of the evaluated follow-up period: this trend may partially influence the impact of results. On the other side, the most important strength of the present study, beside the very long evaluation period, is the direct comparison for the first time of adult and juvenile subpopulations in order to identify similarities and differences in the TNFi performance.
Conclusions
In summary, we have presented the first observational data comparing long-term retention rate and reasons for first-line TNFi withdrawal between adult and juvenile patients. Despite a similar overall drug survival, our main finding is a significantly different pattern of discontinuation between the two subgroups. JIA patients had a lower risk of withdrawal because of inefficacy. Neuropsychiatric, gastrointestinal, and ocular complications led to TNFi discontinuation quite only in juvenile subgroup, while infections and malignancies only in adult population. As previously reported, etanercept showed the highest drug survival in adult-onset population and infliximab the lowest in juvenile-onset population.
References
Petty RE, Southwood TR, Manners P et al (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392
Ruperto N, Lovell DJ, Cuttica R et al (2010) Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum dis 69:718–722
Gerloni V, Pontikaki I, Gattinara M et al (2005) Efficacy of repeated intravenous infusions of an anti-tumor necrosis factor alpha monoclonal antibody, infliximab, in persistently active, refractory juvenile idiopathic arthritis: results of an open-label prospective study. Arthritis Rheum 52:548–553
Marchesoni A, Zaccara E, Gorla R et al (2009) TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci 1173:837–846
Glintborg B, Østergaard M, Dreyer L et al (2011) Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 63:382–390
Glintborg B, Østergaard M, Krogh NS et al (2010) Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum dis 69:2002–2008
Lie E, Kristensen LE, Forsblad-d’Elia H et al (2015) The effect of comedication with conventional synthetic disease modifying antirheumatic drugs on TNF inhibitor drug survival in patients with ankylosing spondylitis and undifferentiated spondyloarthritis: results from a nationwide prospective study. Ann Rheum dis 74:970–978
Fagerli KM, Lie E, van der Heijde DM et al (2014) The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum dis 73:132–137
Favalli EG, Pregnolato F, Biggioggero M et al (2016) Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: real-life data from a local registry. Arthritis Care & Research 68:432–439
Tynjala P, Vahasalo P, Honkanen V et al (2009) Drug survival of the first and second course of anti-tumour necrosis factor agents in juvenile idiopathic arthritis. Ann Rheum dis 68:552–557
Mourão AF, Santos MJ, Melo Gomes JA et al (2016) Effectiveness and long-term retention of anti-tumour necrosis factor treatment in juvenile and adult patients with juvenile idiopathic arthritis: data from Reuma.pt. Rheumatology (Oxford) 55:697–703
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Linden SVD, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 27:361–368
Moll JM, Wright V (1973) Psoriatic arthritis. Semin Arthritis Rheum 3:55–78
Lin Y-T, Wang C-T, Gershwin ME et al (2011) The pathogenesis of oligoarticular/polyarticular vs systemic juvenile idiopathic arthritis. Autoimmun rev 10:482–489
Vastert SJ, Kuis W, Grom AA (2009) Systemic JIA: new developments in the understanding of the pathophysiology and therapy. Best Pract res Clin Rheumatol 23:655–664
Favalli EG, Selmi C, Becciolini A, et al. Eight-year retention rate of first-line tumor necrosis factor inhibitors in spondyloarthritis: a multi-center retrospective analysis. Arthritis Care Res 2016; Accepted Author Manuscript. doi:10.1002/acr.23090
Soliman MM, Ashcroft DM, Watson KD et al (2011) Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum dis 70:583–589
Carmona L, Gomez-Reino JJ, BIOBADASER Group (2006) Survival of TNF antagonists in spondylarthritis is better than in rheumatoid arthritis. Data from the Spanish registry BIOBADASER. Arthritis Res Ther 8:R72
Pan Du SM, Dehler S, Ciurea A et al (2009) Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 61:560–568
Kievit W, Fransen J, Adang EMM et al (2011) Long-term effectiveness and safety of TNF-blocking agents in daily clinical practice: results from the Dutch Rheumatoid Arthritis Monitoring register. Rheumatology (Oxford) 50:196–203
Rosenblat JD, Cha DS, Mansur RB et al (2014) Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuro-Psychopharmacol Biol Psychiatry 53:23–34
Gerloni V, Pontikaki I, Gattinara M et al (2008) Focus on adverse events of tumour necrosis factor alpha blockade in juvenile idiopathic arthritis in an open monocentric long-term prospective study of 163 patients. Ann Rheum dis 67:1145–1152
Sen ES, Dick AD, Ramanan AV (2015) Uveitis associated with juvenile idiopathic arthritis. Nat rev Rheumatol 11:338–348
Barthel D, Ganser G, Kuester R-M et al (2015) Inflammatory bowel disease in juvenile idiopathic arthritis patients treated with biologics. J Rheumatol 42:2160–2165
Stolwijk C, van Tubergen A, Castillo-Ortiz JD et al (2015) Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum dis 74:65–73
Tappeiner C, Schenck S, Niewerth M et al (2016) Impact of antiinflammatory treatment on the onset of uveitis in juvenile idiopathic arthritis: longitudinal analysis from a nationwide pediatric rheumatology database. Arthritis Care & Research 68:46–54
Wu D, Guo Y-Y, Xu N-N et al (2015) Efficacy of anti-tumor necrosis factor therapy for extra-articular manifestations in patients with ankylosing spondylitis: a meta-analysis. BMC Musculoskelet Disord 16:19
Simonini G, Druce K, Cimaz R et al (2014) Current evidence of anti-tumor necrosis factor α treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care & Research 66:1073–1084
Braun J, Baraliakos X, Listing J et al (2005) Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum 52:2447–2451
Sandborn WJ (2003) Optimizing anti-tumor necrosis factor strategies in inflammatory bowel disease. Curr Gastroenterol rep 5:501–505
Braun J, Baraliakos X, Listing J et al (2007) Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor alpha agents. Arthritis Rheum 57:639–647
Beukelman T, Xie F, Chen L et al (2012) Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 64:2773–2780
Doran MF, Crowson CS, Pond GR et al (2002) Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 46:2287–2293
Listing J, Strangfeld A, Kary S et al (2005) Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 52:3403–3412
Greenberg JD, Reed G, Kremer JM et al (2010) Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum dis 69:380–386
Galloway JB, Hyrich KL, Mercer LK et al (2011) Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50:124–131
Becker I, Horneff G. Risk of serious infection in juvenile idiopathic arthritis patients associated with TNF-inhibitors and disease activity in the German BIKER registry. Arthritis care & research Published Online First: 7 July 2016.
Baecklund E, Iliadou A, Askling J et al (2006) Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 54:692–701
Nordstrom BL, Mines D, Gu Y et al (2012) Risk of malignancy in children with juvenile idiopathic arthritis not treated with biologic agents. Arthritis Care & Research 64:1357–1364
Hellgren K, Smedby KE, Backlin C et al (2014) Ankylosing spondylitis, psoriatic arthritis, and risk of malignant lymphoma: a cohort study based on nationwide prospectively recorded data from Sweden. Arthritis Rheumatol 66:1282–1290
Onel KB, Onel K (2012) Tumor necrosis factor inhibitors and cancer in juvenile idiopathic arthritis: disentangling the web. Arthritis Rheum 64:966–969
Bongartz T, Sutton AJ, Sweeting MJ et al (2006) Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. Jama 295:2275–2285
Strangfeld A, Hierse F, Rau R et al (2010) Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis res Ther 12:R5
Mercer LK, Lunt M, Low ALS, Dixon WG, Watson KD, Symmons DPM et al (2015) Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum dis 74(6):1087–1093
Beukelman T, Haynes K, Curtis JR et al (2012) Rates of malignancy associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 64:1263–1271
Tanaka Y, Hirata S, Saleem B, Emery P (2013) Discontinuation of biologics in patients with rheumatoid arthritis. Clin Exp Rheumatol 31(4 Suppl 78):S22–S27
Chang CY, Meyer RML, Reiff AO (2015) Impact of medication withdrawal method on flare-free survival in patients with juvenile idiopathic arthritis on combination therapy. Arthritis Care res 67(5):658–666
Hetland ML, Christensen IJ, Tarp U et al (2010) Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 62:22–32
Iannone F, Gremese E, Atzeni F et al (2012) Longterm retention of tumor necrosis factor-α inhibitor therapy in a large italian cohort of patients with rheumatoid arthritis from the GISEA registry: an appraisal of predictors. J Rheumatol 39:1179–1184. doi:10.3899/jrheum.111125
Schaeverbeke T, Truchetet M-E, Kostine M, Barnetche T, Bannwarth B, Richez C (2016) Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology (Oxford) 55(2):210–220
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure statement
EGF, AM, and PLM have received lecture fees from BMS, Roche, MSD, UCB, Pfizer, and Abbvie. IP, MB, CC, AB, and MR have declared no conflicts of interest.
Funding statement
No specific funding was received from any funding bodies in the public, commercial, or not-for-profit sectors to carry out the work described in this manuscript.
Rights and permissions
About this article
Cite this article
Favalli, E.G., Pontikaki, I., Becciolini, A. et al. Real-life 10-year retention rate of first-line anti-TNF drugs for inflammatory arthritides in adult- and juvenile-onset populations: similarities and differences. Clin Rheumatol 36, 1747–1755 (2017). https://doi.org/10.1007/s10067-017-3712-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3712-8