ABSTRACT
To evaluate the impact of anti-TNF-α therapy on the body weight of rheumatoid arthritis (RA) patients following 24 months of treatment. Data were collected on all RA patients included in the Veneto Region’s Registry of Biological Therapy from January 2007 to July 2012. Inclusion criteria were: start of monotherapy with adalimumab, etanercept, or methotrexate, no previous use of biologic therapy, and at least 24 months of treatment. At baseline, 12, and 24 months, each patient completed a questionnaire about physical activity, smoking, alcohol, and food habits. One hundred and thirty-one RA patients in monotherapy with etanercept (n = 47), adalimumab (n = 44), and methotrexate (n = 40) were enrolled for this study. After 24 months of therapy, there was an increase of weight only in patients treated with anti-TNF-α. Patients on etanercept and adalimumab therapy showed a risk to gain weight six times greater compared to those on methotrexate therapy. The results of present study show that the use of anti-TNF-α in RA patients can be associated to a significant increase of body weight. This increase is not shown in patients under treatment with methotrexate. A more careful evaluation of weight changes needs to be considered in RA patients under anti-TNF-α treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor necrosis factor-α (TNF-α) plays a key role in the pathogenesis and progression of rheumatoid arthritis (RA) and represents one of the main therapeutic targets in this disease.
TNF-α is also involved in the development of rheumatoid cachexia, a complex metabolic syndrome associated with underlying illnesses and characterized by loss of muscle with or without loss of fat mass [1, 2]. TNF-α induces muscle loss directly by both stimulating muscle protein breakdown [3] and reducing the sensitivity of skeletal muscle cells to anabolic stimuli. It also induces downregulation of growth factors and anabolic hormones with consequent anorexia and physical inactivity [4].
It has been observed that anti-TNF-α therapy has significant anti-cachectic effects, promoting the increase of body weight [5, 6] especially in patients with lower body mass index (BMI) [7].
Obesity is a condition of abnormal or excessive fat accumulation in adipose tissue as a result of the prevalence of anabolic-orexigenic on catabolic-anorexigenic mechanisms [8].
In general population, BMI is commonly used in both conditions to classify underweight and overweight [9, 10].
On the basis of a higher proportion of fat mass in RA patients compared to healthy individuals, Stavropoulos-Kalinoglou developed and validated RA specific BMI cutoff levels (RA-BMI) and algorithms to calculate body fat from BMI [11].
The aim of this study was to evaluate the effects of treatment with anti-TNF-α and methotrexate (MTX) on the body weight of RA patients following 24 months of therapy.
Patients and methods
We studied all RA patients (n = 542) included in the Veneto Region’s Registry of Biological Therapy from January 2007 to July 2012. These patients participated in a longitudinal observational study aimed at estimating the benefit/risk profile of the biologic agents in real-world practice (MonitorNet) [12]. MonitorNet is a database established by the Italian Society of Rheumatology (SIR) in January 2007 and funded by the Italian Medicines Agency (AIFA) for the active long-term follow-up of patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Details of the variables that are registered have been provided elsewhere [13].
Data were collected on all RA patients, included in the Veneto Region’s Registry, who satisfied the following eligibility criteria: (1) start of monotherapy with adalimumab, etanercept, or methotrexate; (2) no previous use of biologic therapy; and (3) at least 24 months of treatment.
In this study, we enrolled RA patients under treatment with anti-TNF-α only used at fixed dose (adalimumab and etanercept). We did not consider biological therapy that needs dosages varying according to patient’s body weight, in order not to introduce additional bias or confounding factors arising from the eventual changes in weight of each patient at time of the observation.
At baseline, 12, and 24 months, each patient completed a questionnaire about physical activity, smoking, alcohol, and food habits, and body mass index was calculated as part of usual clinical practice [14].
Statistical analysis was performed using SPSS software, version 15 (SPSS inc., Chicago, Ill). Descriptive statistics included mean values and standard deviations (SD) of the continuous variables, and percentages and proportions of the categorical variables. In order to compare continuous and dichotomous variables at baseline, the Mann-Whitney test, and the χ2 test were performed respectively.
Information about food habits gathered from the questionnaires were analyzed by means of a classification algorithm (cluster with k-means method) to identify an adequate number of dietary pattern to be used in the subsequent analyses.
Time-variations of the variables that are the subject of the study were investigated by means of the Wilcoxon matched pairs test.
The ANOVA analysis with Bonferroni multiple comparison test was performed to compare the weight changes between the groups.
We applied binary logistic regression (conditional stepwise models) to determine which variable (physical activity, dietary pattern, disease duration, DAS28, steroid use, anti-TNF-α use) was mostly associated to the weight change. The binary dependent variable was categorized into weight gain of less than 6 % and equal or greater than 6 % from the baseline.
For each method, we set the confidence threshold to p < 0.05.
BMI was calculated as a continuous variable, from the height and the weight measured by the physician, as weight in kilograms divided by the square of height in meters. BMI values were also classified in underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23–27.9 kg/m2) and obese (>28 kg/m2) categories, according to RA specific BMI cutoff points proposed by Stavropoulos-Kalinoglou [11].
The study was carried out according to the principles of the Declaration of Helsinki and all patients gave written informed consent.
Results
One hundred and thirty-one RA patients who started their first monotherapy with etanercept (n = 47), adalimumab (n = 44), and methotrexate (n = 40) were eligible.
Demographic and anthropometric characteristics of the three groups included in the study are reported in Table 1. At baseline the three groups did not differ significantly for age, gender, disease duration, disease activity, RF positivity, smoking habits, BMI, weight, and height. Food clusters obtained by food frequency questionnaire analysis of the patients are shown in Table 2.
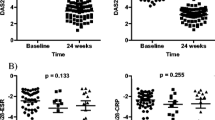
After 24 months of therapy, there were similar disease activity and comparable percentage of disease remission in both groups (58 % in the anti-TNF group and 53 % in the MTX group). Nevertheless an increase of weight 2.1 ± 3.2 kg and 2.7 ± 3.9 kg in patients in treatment with adalimumab and etanercept, respectively, was observed. On the other hand, the weight change in patients in therapy with methotrexate was not significant (0.03 ± 0.93 kg).
The ANOVA comparison of weight changes between the groups was significant (p = 0.002), while the Bonferroni multiple comparison test underlined a significant difference between methotrexate and anti-TNF groups: MTX vs adalimumab p < 0.05 (mean difference 1.97, 95 % CI: 0.16–3.78), MTX vs etanercept p < 0.01 (mean difference 2.64, 95 % CI: 0.86–4.42).
At the end of the study, 10 new cases of obesity (RA-BMI ≥ 28) were recorded, 4 patients on adalimumab therapy and 6 on etanercept therapy.
We searched for possible predictive factors of weight gain and clinical response by binary logistic regression. The final model underlined that among the variables the only predictor of increase of weight was the use of anti-TNF-α (OR = 6.45; 95 % CI: 2.31–18.26; p < 0.001).
Discussion
The results of the present study show that the use of anti-TNF-α (adalimumab and etanercept) in RA patients can be associated to a significant increase in body weight, and this increase is not shown in patients under treatment with methotrexate.
We observed a remarkable (>4 kg) increase of weight in around 30 % of patients in therapy with adalimumab or etanercept. Weight gain in the group under treatment with anti-TNF-α determined 10 new cases of obesity.
Previous studies showed an increase of weight during therapy with anti-TNF-α in patients with psoriasis, spondyloarthropathy, and Crohn’s disease [15–17]. Although the exact causes of this increase are not known, these studies suggest that different type of inflammation/immune response or the genetic background could be important in determining anti-TNF-α effects.
Furthermore, TNF-α could be involved in the homeostasis of the body weight, favoring the catabolism of the muscular cells both under physiological and pathological conditions. Treatment with anti-TNF-α can have an indirect positive effect on lean mass through the improvement of the state of general health of the patient and consequent increase in appetite and can also influence appetite through the modulation of the release of leptin from adipocytes [18].
Rheumatoid arthritis is accompanied by an increase of resting energy expenditure, from a loss of lean mass and from an accumulation of body fat in comparison to healthy subjects: this metabolic alteration is known as rheumatoid cachexia [19].
Rheumatoid cachexia has been attributed in part to increased production of inflammatory cytokines, particularly TNF-α. Anti-TNF-α treatment could slow down the processes involved in the determination of rheumatoid cachexia, such as systemic inflammation, release of cytokines, and physical inactivity.
Obesity is an important cardiovascular risk factor, promoting different physiopathological mechanisms, such as insulin-resistance, type 2 diabetes, hypertension, and dyslipidemia [20].
Assessments for overweight or obesity include the calculation of BMI [9] or more accurate valuations of body fat percentage through different techniques (for example, skin fold thickness, hydrostatic weighing, and bioelectrical impedance) [21]. Body fat estimations need sophisticated equipment and trained personnel, whereas BMI is easy to obtain and is widely used in routine clinical setting. The weakness of BMI is that it does not distinguish between lean body mass and fat mass. Consequently, people of similar stature and weight, but with different muscle content, will have the same BMI but different body fat levels.
In this study, we used RA-BMI proposed by Stavropoulos-Kalinoglou, because as previously described, RA patients could also have a different proportion of fat mass than healthy individuals [11].
It is known that anti-TNF-α therapy can improve some metabolic parameters associated to cardiovascular risk, such as insulin-resistance, C reactive protein levels, and carotid intima-media thickness [22–24]. The weight gain observed in our study cannot be considered a cardiovascular risk factor by itself but a warning sign to better prevent comorbidities.
This work has potential limitations mainly related to the observational design of the study. There was no control over the treatment assignment of MTX versus anti-TNF agents, which could result in selection bias or confounding by indication. In fact, patients taking anti-TNF agents had higher DAS28 levels at baseline and had failed DMARDs before switching. While we have adjusted for these differences using multivariate regression models, we cannot exclude some degree of residual confounding. Nevertheless, the comparison with a MTX monotherapy group could be considered a strength of this study. By controlling the inflammatory activity, a weight gain could be expected also in the MTX group. The fact that this was not observed, favors the conclusion that the increase of body weight might be a direct mechanism of the anti-TNF therapy.
In conclusion, our study suggests that body weight changes in RA patients under treatment with anti-TNF-α should be carefully evaluated. In this context, nutrition consultation should be taken into account in the management and in the long-term follow-up of the patients.
References
Summers GD, Metsios GS, Stavropoulos-Kalinoglou A, Kitas GD (2010) Rheumatoid cachexia and cardiovascular disease. Nat Rev Rheumatol 6:445–451
Roubenoff R, Roubenoff RA, Cannon JG et al (1994) Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 93:2379–2386
Reid MB, Li YP (2001) Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res 2:269–272
Lang CH, Frost RA (2002) Role of growth hormone, insulin-like growth factor-I, and insulin-like growth factor binding proteins in the catabolic response to injury and infection. Curr Opin Clin Nutr Metab Care 5:271–279
Alcorn N, Tierney A, Wu O, Gilmour H, Madhok R (2010) Impact of anti-tumour necrosis factor therapy on the weight of patients with rheumatoid arthritis. Ann Rheum Dis 69:1571
Chen CY, Tsai CY, Lee PC, Lee SD (2013) Long-term etanercept therapy favors weight gain and ameliorates cachexia in rheumatoid arthritis patients: roles of gut hormones and leptin. Curr Pharm Des 19:1956–1964
Brown RA, Spina D, Butt S, Summers GD (2012) Long-term effects of anti-tumour necrosis factor therapy on weight in patients with rheumatoid arthritis. Clin Rheumatol 31:455–461
Inui A, Meguid MM (2003) Cachexia and obesity: two sides of one coin? Curr Opin Clin Nutr Metab Care 6:395–399
Wellens RI, Roche AF, Khamis HJ, Jackson AS, Pollock ML, Siervogel RM (1996) Relationships between the body mass index and body composition. Obes Res 4:35–44
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495
Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y et al (2007) Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis 66:1316–1321
Scirè CA, Caporali R, Sarzi-Puttini P, Frediani B, Di Franco M, Tincani A, Sinigaglia L, Sfriso P, Tirri R, Bellis E, Delsante G, Porru G, Salaffi F, Giuggioli D, Rossini M, Todoerti M, Bazzichi L, Govoni M, Gerli R, Raschetti R, Minisola G, Montecucco C, Todesco S, Monitornet project (2013) Drug survival of the first course of anti-TNF agents in patients with rheumatoid arthritis and seronegative spondyloarthritis: analysis from the MonitorNet database. Clin Exp Rheumatol 31:857–863
Sfriso P, Salaffi F, Montecucco CM, Bombardieri S, Todesco S (2009) MonitorNet: the Italian multi-centre observational study aimed at estimating the risk/benefit profile of biologic agents in real-world rheumatology practice. Reumatismo 61:132–139
Questionario di valutazione degli stili di vita, ISS (Italian National Institute of Health) [http://www.iss.it/binary/ofad/cont/questionario%20giovani%20in%20forma.1225957648.pdf]
Briot K, Garnero P, Le Henanff A, Dougados M, Roux C (2005) Body weight, body composition, and bone turnover changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor alpha treatment. Ann Rheum Dis 64:1137–1140
Franchimont D, Roland S, Gustot T, Quertinmont E, Toubouti Y, Gervy MC, Deviere J, Van Gossum A (2005) Impact of infliximab on serum leptin levels in patients with Crohn’s disease. J Clin Endocrinol Metab 90:3510–3516
Gisondi P, Cotena C, Tessari G, Girolomoni G (2008) Anti-tumor necrosis factor-alpha therapy increases body weight in patients with chronic plaque psoriasis: a retrospective cohort study. J Eur Acad Dermatol Venereol 22:341–344
Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS (1997) Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest 100:2777–2782
Engvall IL, Elkan AC, Tengstrand B, Cederholm T, Brismar K, Hafstrom I (2008) Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand J Rheumatol 37:321–328
Pi-Sunyer FX (2002) The obesity epidemic: pathophysiology and consequences of obesity. Obes Res 10(Suppl 2):97S–104S
Nevill AM, Stewart AD, Olds T, Holder R (2004) Are adult physiques geometrically similar? The dangers of allometric scaling using body mass power laws. Am J Phys Anthropol 124:177–182
Cacciapaglia F, Navarini L, Menna P, Salvatorelli E, Minotti G, Afeltra A (2011) Cardiovascular safety of anti-TNF-alpha therapies: facts and unsettled issues. Autoimmun Rev 10:631–635
Dixon WG, Symmons DP (2007) What effects might anti-TNF alpha treatment be expected to have on cardiovascular morbidity and mortality in rheumatoid arthritis? A review of the role of TNF alpha in cardiovascular pathophysiology. Ann Rheum Dis 66:1132–1136
Costa L, Caso F, Atteno M, Del Puente A, Darda MA, Caso P, Ortolan A, Fiocco U, Ramonda R, Punzi L, Scarpa R (2014) Impact of 24-month treatment with etanercept, adalimumab, or methotrexate on metabolic syndrome components in a cohort of 210 psoriatic arthritis patients. Clin Rheumatol 33:833–839
Acknowledgments
This study was in part supported by the Italian Medicines Agency (AIFA) within the independent drug research program, contract no. FARM5KJ9P5 and by Veneto Region (DGR3256 2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Sfriso, P., Caso, F., Filardo, G.S. et al. Impact of 24 months of anti-TNF therapy versus methotrexate on body weight in patients with rheumatoid arthritis: a prospective observational study. Clin Rheumatol 35, 1615–1618 (2016). https://doi.org/10.1007/s10067-016-3244-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3244-7