Abstract
The objectives of this study are to assess the frequency of chronic arthritis and compare the clinical and laboratory features in a large population of childhood-onset systemic lupus erythematosus (cSLE) and adult-onset (aSLE) patients. This historical study evaluated 336 cSLE and 1830 aSLE patients. Chronic arthritis was defined as synovitis of at least 6 weeks of duration. Rhupus was characterised as the association of SLE and chronic inflammatory arthritis with erosion and positive rheumatoid factor. Jaccoud’s arthropathy is a non-erosive subluxation leading to severe deformity of the hands and feet. Data were compared using Student’s t test or the Mann-Whitney test for continuous variables. For categorical variables, differences were assessed by Fisher’s exact test and Pearson chi-square. Frequencies of chronic arthritis were similar in cSLE and aSLE (2.4 vs. 3.8 %, p = 0.261). The median time from disease onset to appearance of chronic arthritis was shorter in cSLE (0 vs. 10 years, p < 0.001), and the median of age at chronic arthritis diagnosis was [10.8 (4.2–14.6) vs. 40 (21–67), p < 0.001]. The children presented with more chronic polyarthritis than the adults (75 vs. 32 %, p = 0.024), a higher median number of joints with arthritis [8.5 (1–18) vs. 3 (1–9), p = 0.017] and a higher number of joints with limitation [1.5(0–24) vs. 0(0–4), p = 0.004]. The chronic arthritis diagnosis frequencies of hepatomegaly (25 vs. 0 %, p = 0.009), splenomegaly (25 vs. 0 %, p = 0.009), pericarditis (25 vs. 0 %, p = 0.009), nephritis (37 vs. 3 % , p = 0.006), haematuria (37 vs. 1.4 %, p = 0.002), lupus anticoagulant (40 vs. 1.6 %, p = 0.012), anticardiolipin IgM (40 vs. 1.5 %, p = 0.012) and median Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) [10.5(1–20) vs. 6(4–16), p = 0.029] were higher in cSLE. Frequency of rhupus, (12 vs. 17 %, p = 1.0), Jaccoud’s arthropathy (0 vs. 17 %, p = 0.343) and treatments were similar in cSLE and aSLE. We determined that chronic arthritis in SLE has distinct features in children, with very early onset, polyarticular involvement and association with active disease. We further demonstrated in this series that a proportion of chronic arthritis involvement in SLE is manifested as rhupus and Jaccoud’s arthropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute articular involvement is an important feature of childhood-onset systemic lupus erythematosus (cSLE) and adult-onset (aSLE) patients, and it has been described in up to 70 % of children and 90 % of adults [1].
Despite this, data regarding chronic articular involvement in lupus is hampered because in the majority of the studies, there is no clear definition of chronicity. Erosive arthritis has been restricted to case reports or small series of cSLE and aSLE patients [2, 3], and the association of lupus and juvenile idiopathic arthritis (JIA) or rheumatoid arthritis (RA), called rhupus, has rarely been documented in the literature [2, 4–6].
Moreover, distinct characteristics of chronic arthritis (CA) in these two populations have not been explored, particularly with regard to onset, number of joints and associated clinical and laboratory manifestations.
Therefore, the objective of this study was to assess frequency, demographic data, clinical manifestations, laboratory findings and treatment in a large cSLE and aSLE populations with a homogenous chronic arthritis definition.
Materials and methods
This historical study evaluated 336 cSLE and 1830 aSLE patients followed from 1983 to 2014 at the paediatric and adult lupus outpatient clinics at the same tertiary public hospital in an urban area of São Paulo. All patients fulfilled the American College of Rheumatology criteria for SLE [7].
Chronic arthritis was defined according to the presence of swelling or effusion or two or more of the following: limitation of motion, tenderness or pain on motion and increased heat for at least 6 weeks. Arthritis features were also evaluated as follows: duration, number and type of joints with arthritis and number and type of joints with limitation of range of motion and deformity. Rhupus was characterised as the association of SLE and chronic inflammatory arthritis with erosion and positive rheumatoid factor. Jaccoud’s arthropathy was defined as non-erosive subluxation leading to severe deformity of the hands and feet [8].
Medical charts were assessed for demographic data at time of chronic arthritis diagnosis as follows: age, SLE duration and duration of chronic arthritis. Clinical manifestations of SLE at CA diagnosis included fever (>38 °C), hepatomegaly (>3 cm below the costal arch), splenomegaly (>3 cm below the costal margin), cutaneous lesions (malar or discoid rash, photosensitivity, mucosal ulcers, alopecia or cutaneous vasculitis), articular involvement features [monoarthritis (one-joint involvement), oligoarthritis (two- to four-joints involvement) and polyarthritis (five or more joints affected)], number of joints with arthritis, number of joints with limitation of motion, serositis (pericarditis or pleuritis), neuropsychiatric involvement (central nervous system and peripheral nervous system), nephritis (proteinuria ≥0.5 g/24 h), presence of cellular casts, haematuria, leukocyturia excluding infection and haematologic complications (haemolytic anaemia, leucopoenia with a white blood cell count of <4000/mm3 or lymphopaenia <1500/mm3 on two or more occasions and thrombocytopaenia with a platelet count of <100,000/mm3 in the absence of drugs).
Systemic lupus erythematosus disease activity was measured in all patients at time of chronic arthritis diagnosis using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) [9].
Laboratory evaluation at time of chronic arthritis diagnosis consisted of erythrocyte sedimentation rate (ESR) performed using the Westergren method and C-reactive protein (CRP) by nephelometry. Anti-double-stranded DNA (anti-dsDNA) was detected by indirect immunofluorescence using Crithidia luciliae as a substrate. Anti-Sm was determined using enzyme-linked immunosorbent assay (ELISA).
Statistical analysis
Results are presented as the mean ± standard deviation or median (range) for continuous variables and as the number (%) for categorical variables. Data were compared using Student’s t test or the Mann-Whitney test for continuous variables to evaluate differences between chronic arthritis in childhood and adult SLE patients. For categorical variables, differences were assessed using Fisher’s exact test and Pearson chi-square. P values less than 0.05 were considered significant.
Results
Similar frequencies of chronic arthritis were observed in cSLE vs. aSLE [8/336 (2.4) vs. 69/1830 (3.8 %), p = 0.261], although the median of time from disease onset to appearance of chronic arthritis was shorter in cSLE (0 vs. 10 years, p < 0.001) and the median of age at chronic arthritis diagnosis was [10.8 (4.2–14.6) vs. 40 (21–67), p < 0.001].
Table 1 includes demographic data, clinical manifestations and disease activity/damage scores at time of chronic arthritis diagnosis in the cSLE and aSLE patients. For arthritis distribution, the presence of polyarthritis was significantly higher in the cSLE compared to the aSLE patients (75 vs. 32 %, p = 0.024) and oligoarthritis (12 vs. 55 %, p = 0.028) was lower in children. Ankle involvement was more often observed in cSLE compared to aSLE (100 vs. 16 %, p < 0.001), with no differences for other sites of arthritis in both groups (Table 1).
All cSLE patients had chronic arthritis before or concomitant to lupus diagnosis, and all aSLE patients had chronic arthritis after lupus diagnosis. All but one (87.5 %) cSLE patient had only chronic arthritis as the first lupus manifestation, mimicking JIA. Associated Jaccoud’s arthropathy of the hands was evidenced in 12/69 (17 %) aSLE patients who presented with concomitant chronic arthritis in the wrists, knees and shoulder. Frequencies of Jaccoud’s arthropathy (0 vs. 17 %, p = 0.343) and rhupus syndrome (12 % vs. 17 %, p = 1.0) were similar in cSLE compared to aSLE.
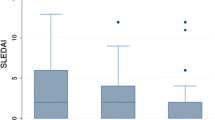
The frequencies of hepatomegaly (25 vs. 0 %, p = 0.009), splenomegaly (25 vs. 0 %, p = 0.009), alopecia (37 vs. 1,4 %, p = 0.002), pericarditis (25 vs. 0 %, p = 0.009) and nephritis (37 vs. 3 %, p = 0.006) were significantly higher in the children, as well as the median SLEDAI-2K at time of chronic arthritis diagnosis [10.5 (1–20) vs. 6 (4–16), p = 0.029].
Laboratory data and treatment at time of chronic arthritis diagnosis in cSLE and aSLE patients are shown in Table 2. Frequencies of haematuria (37 vs. 1.4 %, p = 0.002), lupus anticoagulant (40 vs. 1.6 %, p = 0.012) and anticardiolipin IgM (40 vs. 1.5 %, p = 0.012) were higher in the cSLE compared to the aSLE patients. The frequencies of glucocorticoid, antimalarials and methotrexate were similar in both groups (p > 0.05). No patients received belimumab.
Discussion
This was the first study comparing adults and children to identify a low frequency of chronic arthritis in SLE patients with distinct features. The children had a predominance of earlier onset, polyarticular involvement and high overall disease activity.
The great advantage of the present study is the large cohort analysed, allowing for a more significant representation of this rare manifestation of lupus. Previous reports evaluating children or adults with this inflammatory musculoskeletal manifestation are limited to case reports and series [3, 5, 10]. The inclusion of patients from the same tertiary university hospital provided a more homogeneous population with specific definitions for the diagnosis of chronic arthritis in both groups. By contrast, previous reports do not have a clear definition of chronic arthritis, precluding an accurate comparison with their findings [8, 11, 12]. The main limitation of the present study is possible missing data, and we did not evaluate anti-RA33 antibodies, which were previously associated with chronic arthritis in aSLE patients [3].
Acute arthritis is usually defined as mild, self-limited and without deformity, and it is a common manifestation at time of disease onset in children and adult lupus patients [13]. The frequency of chronic arthritis observed in the present study was very low in both groups, at less than 5 %. Here, in cSLE, chronic arthritis manifestation was the initial presentation involving small joints of the hands and feet and large joints mimicking JIA involvement, whereas in aSLE, chronic arthritis usually occurred later during the course of disease.
Rhupus occurred in a minority of the chronic arthritis cases, suggesting that this syndrome may characterise a subgroup of patients with an overlap of JIA/RA and SLE. There are however no established definitions of whether this overlap condition exists or whether rhupus is, in fact, a rare spectrum of SLE [8, 10, 12–14]. Furthermore, non-erosive deforming arthropathy was not evidenced in cSLE and occurred in almost 20 % of our aSLE patients concomitant with chronic arthritis in other joints. This periarticular and capsular condition was not reported in children and was described in 3.5–35 % of aSLE patients [8, 11, 15].
We confirmed and extended previous observations of variability in diseases phenotypes of cSLE compared to aSLE. cSLE was reported to have a higher frequency of severe nephritis, alveolar haemorrhage and infections than aSLE [1, 16–18]. We further demonstrated that cSLE patients with chronic arthritis often had more active disease than aSLE, possibly because this manifestation occurred predominantly at the time of disease onset in the former group.
Almost half of our patients in both groups received non-steroidal anti-inflammatory drugs for the treatment of chronic arthritis, and those with refractory disease used methotrexate or other immunosuppressive agents [12, 14].
In conclusion, we determined that chronic arthritis in SLE has distinct features in children with very early onset, polyarticular involvement and it is often associated with active disease. We further demonstrated in this series that a proportion of chronic arthritis involvement in SLE is manifested as rhupus and Jaccoud’s arthropathy.
References
Tarr T, Dérfalvi B, Győri N, Szántó A, Siminszky Z, Malik A, Szabó AJ, Szegedi G, Zeher M (2015) Similarities and differences between pediatric and adult patients with systemic lupus erythematosus. Lupus 24:796–803
Cavalcante EG, Aikawa NE, Lozano RG, Lotito AP, Jesus AA, Silva CA (2011) Chronic polyarthritis as the first manifestation of juvenile systemic lupus erythematosus patients. Lupus 20:960–964
Richter Cohen M, Steiner G, Smolen JS, Isenberg DA (1998) Erosive arthritis in systemic lupus erythematosus: analysis of a distinct clinical and serological subset. Br J Rheumatol 37:421–424
Tani C, D’Aniello D, Delle Sedie A, Carli L, Cagnoni M, Possemato N, Carbone M, Della Rossa A, Riente L, Baldini C, Talarico R, Caramella D, Bombardieri S, Mosca M (2013) Rhupus syndrome: assessment of its prevalence and its clinical and instrumental characteristics in a prospective cohort of 103 SLE patients. Autoimmun Rev 12:537–541
Ziaee V, Moradinejad MH, Bayat R (2013) RHUPUS Syndrome in children: a case series and literature review. Case Rep Rheumatol 819629
Unsal E, Arli AO, Akman H (2007) Rhupus arthropathy as the presenting manifestation in juvenile SLE: a case report. Pediatr Rheumatol Online J 4(5):7
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Pipili C, Sfritzeri A, Cholongitas E (2008) Deforming arthropathy in systemic lupus erythematosus. Eur J Intern Med 19:482–487
Gladman DD, Ibanez D, Urowitz MB (2002) Systemic lupus erythematosus disease activity index 2000. J Rheumatol 29:288–291
Fernández A, Quintana G, Rondón F, Restrepo JF, Sánchez A, Matteson EL, Iglesias A (2006) Lupus arthropathy: a case series of patients with rhupus. Clin Rheumatol 25:164–167
Ostendorf B, Scherer A, Specker C, Mödder U, Schneider M (2003) Jaccoud’s arthropathy in systemic lupus erythematosus: differentiation of deforming and erosive patterns by magnetic resonance imaging. Arthritis Rheum 48:157–65
Ball EM, Bell AL (2012) Lupus arthritis—do we have a clinically useful classification? Rheumatology 51:771–779
Vugt RM, Derksen RH, Kater L, Bijlsma JW (1998) Deforming arthropathy or lupus and rhupus hands in systemic lupus erythematosus. Ann Rheum Dis 57:540–544
Grossman JM (2009) Lupus arthritis. Best Pract Res Clin Rheumatol 23:495–506
Santiago MB, Galvão V (2008) Jaccoud arthropathy in systemic lupus erythematosus: analysis of clinical characteristics and review of the literature. Medicine (Baltimore) 87:37–44
Gormezano NW, Silva CA, Otsuzi CI, Barros DL, da Silva MA, Sallum AM, Pasoto S, Pereira RM, Bonfá E (2015) Higher prevalence and distinct features of herpes zoster infection in children than adults with systemic lupus erythematosus. Pediatr Infect Dis J
Araujo DB, Borba EF, Silva CA, Campos LM, Pereira RM, Bonfa E, Shinjo SK (2012) Alveolar hemorrhage: distinct features of juvenile and adult onset systemic lupus erythematosus. Lupus 21:872–877
Mina R, Brunner HI (2010) Pediatric lupus—are there differences in presentation, genetics, response to therapy, and damage accrual compared with adult lupus? Rheum Dis Clin North Am 36:53–80
Acknowledgments
We are grateful to Ulysses Doria-Filho for the statistical analysis. This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 2009/51897-5 to EB, 301805/2013-0 to RMRP and 302724/2011-7 to CAS), Federico Foundation (to EB, RMRP and CAS) and by Núcleo de Apoio à Pesquisa “Saúde da Criança e do Adolescente” da USP (NAP-CriAd) to CAS.
The brief report was read and approved by all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Gormezano, N.W.S., Silva, C.A., Aikawa, N.E. et al. Chronic arthritis in systemic lupus erythematosus: distinct features in 336 paediatric and 1830 adult patients. Clin Rheumatol 35, 227–231 (2016). https://doi.org/10.1007/s10067-015-3127-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3127-3