Abstract
The aim of this study is to assess the diagnostic value of interferon-γ release assays (IGRAs) for latent tuberculosis infection (LTBI) in patients with rheumatic disease before receiving biologic agents. MEDLINE and EMBASE databases were used for searching studies concerning the evaluation on the performance of IGRAs [QuantiFERON-TB Gold (QFT-G), QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB] in rheumatic patients before biological therapy. After assessing the quality of all studies included in the review, we summarized the results in subgroups using forest plots and calculated pooled estimates if applicable. The search identified 11 studies with a total sample size of 1940 individuals. Compared with the tuberculin skin test (TST), the pooled agreements in QFT-G/GIT and T-SPOT.TB were 72 % (95 % confidence interval (CI) 65, 78 %) and 75 % (95 % CI 67, 83 %), respectively. BCG vaccination was positively correlated with positive rates of TST (pooled odds ratio (OR) 1.64, 95 % CI 1.06, 2.53). Compared with TST, IGRAs were better associated with the presentence of one or more tuberculosis (TB) risk factors. Neither steroid nor disease-modifying anti-rheumatic drugs (DMARDs) significantly affect positive IGRA results. In contrast, TST positivity was significantly impacted by the use of steroid (pooled OR 0.45, 95 % CI 0.30, 0.69), but less significantly by the use of DMARDs (pooled OR 0.78, 95 % CI 0.50, 1.21). In conclusion, in rheumatic patients with previous BCG vaccination or currently on steroid therapy, IGRAs would be the better choice to identify LTBI by decreasing the false-positivity and false-negativity rate compared with conventional TST.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biologic agents, such as tumor necrosis factor alpha (TNF-α) inhibitors, are approved for the treatment of several rheumatic diseases including rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriasis arthritis (PsA), and juvenile idiopathic arthritis (JIA). Although biologic agents provide profound clinical benefits, an increased risk of tuberculosis infection was reported to be associated with TNF-α inhibitors [1–5].
Tuberculosis is a granulomatous disease caused by infection with Mycobacterium tuberculosis. Most of the individuals latently infected with M. tuberculosis will never progress to active disease. In those latently infected cases, the host’s immune system controls the bacilli in a state of non-replicating persistence [6]. The immunological studies found that the cytokine TNF-α plays a key role in granuloma formation and maintenance [7], and TNF-α inhibitors result in the disintegration of the granuloma and dissemination of M. tuberculosis [2, 8]. For the patients on anti-TNF-α therapy, the risk of latent tuberculosis infection (LTBI) progression to active disease would accordingly increase. Current clinical guidelines mandate the screening for LTBI prior to anti-TNF-α therapy [9–13]. However, no agreement was reached on the best methodology for LTBI screening.
The tuberculin skin test (TST) is the long-established method to identify LTBI due to its simplicity and efficiency; although it has several inherent drawbacks, such as cross-reaction with BCG vaccination or other environmental mycobacteria infection, phenomena of boosting, operator bias and variability in result interpretation [14, 15]. Recently, interferon-γ release assays (IGRAs) have emerged as an alternative for TST [16, 17]. IGRAs measure the immune response to TB-specific antigens either from peripheral blood lymphocytes (T-SPOT.TB; Oxford Immunotec Limited, Abingdon, UK) or whole blood (QuantiFERON-TB Gold [QFT-G] and QuantiFERON-TB Gold In-Tube [QFT-GIT]; Cellestis Limited, Carnegie). Moreover, many rheumatic patient candidates for anti-TNF-α therapy also accept other immunosuppressive therapy (IST), such as steroid and disease-modifying anti-rheumatic drugs (DMARDs). In that both TST and IGRAs measure the magnitude of an adaptive immune response, the testing results may be affected by immunosuppressive drugs or by the autoimmune disease itself. With so much confounding factors associated with both IGRAs and TST, more sufficient data are needed to draw a reliable conclusion on which test is better. To date, limited data from published studies investigated the performance of IGRA for detecting LTBI in rheumatic patients. Most of them had small sample sizes and appeared with controversial conclusions. Besides, many studies included rheumatic patients who had already received biological therapy. While in clinical practice, screening of LTBI in rheumatic patients before initiation of biologic agents is more useful.
With these uncertainties, we conducted a systemic review and meta-analysis to evaluate the performance of IGRAs and TST in diagnosing LTBI in patients with rheumatic diseases before initiation of biological therapy.
Materials and methods
Search strategy and study selection
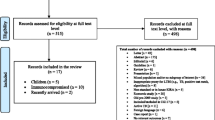
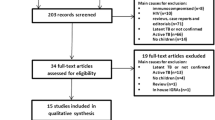
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18] compliant literature search strategy was performed. The electronic databases MEDLINE (1966 to February 2014) and EMBASE (1974 to February 2014) were searched by two reviewers (Q.R. and S.Z.). All titles and abstracts generated from the search strategy were independently reviewed. After the initial screening process, the full text of eligible articles was reviewed against the predefined inclusion criteria by two reviewers (Q.R. and S.Z.). In addition, we scrutinized the reference lists of each eligible paper for any omitted studies.
From all citations of published relevant articles and bibliographies of relevant reviews and guidelines for inclusion, studies were eligible for inclusion if they were original researches published in English and assessed the performance of commercial IGRAs (including QFT-G, QFT-GIT and T-SPOT.TB), and TST in rheumatic patients, and studies were excluded when evaluating an in-house or older-generation IGRAs, lack of data specific to patients with rheumatic disease, lack of sufficient data on desired outcomes, or including patients who had already received biological therapy, such as anti-TNF-α therapy.
Data extraction
Two reviewers (Q.R. and S.Z.) conducted the data extraction using a pre-constructed data table. The data collection included published year, country, number of patients, type of rheumatic disease, demographic characteristics (such as gender and BCG vaccination), IGRA methods (assay used, test vision, cut-off point used), TST methods (dose of purified protein derivative (PPD) used, cut-off point used), positivity of these tests, agreement between IGRA and TST, indeterminate results of IGRAs, and outcomes assessing the impact of IST on IGRA and TST results.
Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) quality assessment tool is a validated tool to evaluate the diagnostic accuracy [19]. However, since there is currently no “gold standard” for the diagnosis of LTBI, specific modifications were made [20]. The following aspects were evaluated including: (1) whether patients were ambulatory or inpatients diagnosed with rheumatic diseases; (2) whether the selection criteria was clearly described; (3) whether the IGRAs was performed before TST; (4) Whether commercial IGRAs were used according to the manuscript; (5) whether the same clinical data were available as in practice; (6) whether indeterminate results were reported; and (7) whether exclusions after enrollment were explained. We scored each of the QUADAS items as Yes (2 points), Unclear (1 point), or No (0 point).
Statistical analysis
Due to no gold standard in assessing the performance of IGRAs and TST, meta-analysis methods in two recent meta-analyses that evaluated IGRAs in HIV-infected individuals and in inflammatory bowel disease patients were used as references [20, 21]. The agreement between two IGRAs and the TST, the proportion of indeterminate IGRAs results, the association between risk factors of LTBI and test positivity, and the impact of IST on IGRAs and TST results were meta-analyzed. The odds ratio (OR) was used to measure the association between test positivity and risk factors of TB. The impact of IST on IGRAs and TST was also measured by pooled OR. Most of the analysis was conducted by groups according to the IGRA used. Continuity corrections were performed when zero events exist in one or both arms of an outcome. The level of statistical significance was established as P < 0.05. Ninety-five percent CIs were presented for each outcome. Heterogeneity was assessed using the I 2 statistics [22]. If I 2 > 25 %, the pooled estimate was obtained using a random effects model. Otherwise, fixed effects model was used. The pooled estimate was not calculated if only three or less studies were available. All analyses were conducted with R (v.3.0) software.
Results
Search results and study description
Totally, 476 articles were identified from the databases and 7 articles were identified from the reference lists of eligible articles. Forty-one studies were reviewed by full text and 11 studies met our inclusion criteria and the data were extracted (Online Resource 1). Of the 11 included articles, a total of 1940 cases from 10 countries were assessed (Table 1). Among them, the majority of the patients suffered from RA, AS, PsA, and other spondyloarthropathies. All these studies used both TST and QuantiFERON test (four QFT-G and seven QFT-GIT) prior to anti-TNF-α therapy. Four of these studies evaluated both QFT and T-SPOT.TB. Most articles satisfied the modified QUADAS items and the scores ranged from 8 to 14, with a mean score of 12.4 (Table 1). The major quality problems of these studies were the unclearly described selection criteria and the uncertain sequence of TST and IGRAs testing.
Agreement between IGRAs and TST
Both QFT and T-SPOT.TB in these studies were performed according to the manufacturer’s instructions. Eight studies performed TST by Mantoux methods using two tuberculin units [23–26, 28, 30, 31, 33], two studies used five tuberculin units [27, 32], and one used about three tuberculin units [29]. The cut-offs for positivity were selected differently according to their national guidelines (Table 1). The percentage of agreement between TST and QFT was available in nine studies (Fig. 1). And four of these studies also provide sufficient data to calculate the agreement between and TST and T-SPOT.TB. Overall, the pooled agreement was 72 % (95 % confidence interval (CI) 65–78 %) between TST and QFT, and 75 % (95 % CI 67–83 %) between TST and T-SPOT.TB. Random effects model was used for these pooled estimate because of the heterogeneity between studies (I 2 = 85.8 and 75.8 %, respectively). Three studies [25, 26, 31] also reported the concordance between two IGRA tests, which were 98.2, 81, and 88.9 %, respectively. Of the nine studies with sufficient data of discordant results [24–26, 28, 30, 32, 33], seven reported higher proportion of TST+/IGRA− results (ranging from 6.1 to 32.2 %) compared to TST−/IGRA+ results (1.4 to 32.4 %) in individuals with both tests available.
Proportion of indeterminate results
Ten studies [23–27, 29, 31, 32, 34] reported the proportion of indeterminate results of QFT (Online Resource 2). The pooled percentage of indeterminate results was 3 % (95 % CI 2, 4 %). Three studies which measured the indeterminate results of T-SPOT.TB reported that the proportion of indeterminate results ranged from 0 to 6 % [26, 31, 32].
BCG vaccination and TB risk factors
The pooled data showed that BCG vaccination was associated with TST positivity (pooled OR 1.64, 95 % CI 1.06, 2.53) (Fig. 2). In the analysis between TB risk factors and test results, we found that the IGRA positivity was associated with the presence of one or more risk factors for TB (pooled OR 4.49, 95 % CI 2.73, 7.39), including previous close contact with TB patients, birth or extended living in TB-prevalent area, and abnormal chest radiograph (Fig. 3). However, the TB risk factors analyzed in different studies were not consistent. No pooled OR was available between TST positivity and one or more risk factors for TB. Among all risk factors, abnormal chest radiograph was the most frequently investigated; however, no significant association was found between abnormal chest radiograph and IGRA test positivity (Supplementary Figure S2).
Association between IST and test performance
Most patients were treated with steroid and/or DMARDs at the time of screening for LTBI prior to anti-TNF-α therapy. There were no studies assessing the impact of IST on agreement between IGRA and TST. Two studies [24, 32] reported that odds of concordance of QFT and TST were higher in individuals who were on steroid compared to those who were not (OR 3.57, 95 % CI 1.03, 12.5 and OR 1.15, 95 % CI 0.71, 1.85). In addition, individuals with indeterminate results were often excluded from analysis, resulting in the failure to assess the impact of IST on indeterminate results. Only two studies reported that administration of steroid was significantly associated with occurrence of indeterminate results of QFT [24, 28].
We assessed the impact of steroid and DMARDs on IGRA positivity and TST positivity separately. Nine studies [23–28, 31–33] provided the sufficient data to assess the impact of steroid on the IGRA positivity rate and pooled estimate was calculated. The negative QFT results were not significantly associated with the use of steroid (pooled OR 0.90, 95 % CI 0.63, 1.28) or DMARDs (pooled OR 0.96, 95 % CI 0.69, 1.33) (Fig. 4). Four studies [25, 26, 31, 32] provided sufficient data to access the impact of IST on the T-SPOT.TB results. Similarly, pooled estimates revealed that neither steroid (pooled OR 0.69, 95 % CI 0.38, 1.27) nor DMARDs (pooled OR 1.53, 95 % CI 0.98, 2.39) significantly affects positive T-SPOT.TB results (Fig. 5). In contrast, the pooled estimates from nine studies [24, 26–29, 31–33] indicated that TST positivity was significantly impacted by the use of steroid (pooled OR 0.45, 95 % CI 0.30, 0.69), but less impacted by the use of DMARDs (pooled OR 0.78, 95 % CI 0.50, 1.21) (Fig. 5). However, considerable heterogeneity between studies were seen in assessing the impact of steroid on TST (I 2 = 54.7 %).
Discussion
Lack of a gold standard in diagnosing LTBI, assessment on diagnostic performance of IGRAs and TST becomes knotty and contentious. Especially for the patients with autoimmune disease before biological therapy, prevention from developing active TB necessitates reliable diagnostic approaches to detect LTBI. We reviewed 11 original studies and meta-analyzed the performance of IGRAs versus TST among rheumatic patients prior to anti-TNF-α therapy and evaluated the impact of immunosuppressive therapy on both IGRAs and TST. We found that the concordances between IGRAs and TST were 72 and 75 % for QFT and T-SPOT.TB, respectively. The proportion of indeterminate results of QFT was 5 %. Positivity rate of TST, but not IGRA, was significantly higher in the patients with BCG vaccination history. Compared with IGRAs, TST positivity was more significantly influenced by the use of steroid.
The development of active disease from LTBI in patients treated with TNF-α inhibitors might be an important outcome to evaluate the test performance. However, only four of our included studies [25, 27, 30, 33] reported the follow-up data and nine active cases were reported from one study [30]. Data that focus on the predictive values of IGRAs in developing active disease among rheumatic patients were very limited. In addition, almost all patients with suspected LTBI received preventive therapy according to the current guidelines. Therefore, the capacity of IGRAs of predicting subsequent tuberculosis could not be well assessed.
The concordances between IGRAs and TST were 72 and 75 % for QFT and T-SPOT.TB, respectively, which indicates differences do exist between these two tests. TST is traditionally used to identify the LTBI. As expected, TST results were significantly associated with BCG vaccination in rheumatic patients just as in immune-complete subjects. For IGRAs, confounding factors related to BCG vaccination were avoided. False-positive TST will result in unnecessary anti-tuberculosis prophylaxis bringing about drug adverse events. In addition, when compared with TST, positive IGRA results were more closely related with having one or more TB risk factors.
The existence of indeterminate results is a considerable problem for IGRAs in the clinical practice. Indeterminate results was defined as: (1) the negative control tests positive regardless of the response to TB-specific antigens or (2) the positive control tests negative as does the response to TB-specific antigens. Actually, most indeterminate results in immunocompromised patients resulted from the negative response against mitogen which was used as positive control [35]. For rheumatic patients, the proportion of indeterminate result of QFT was lower compared with patients with inflammatory bowel disease and HIV infection [20, 21]. However, a study found that over 75 % initial indeterminate results of IGRA performed under routine conditions gave clear positive or negative results upon retesting [36].
An important concern for patients with rheumatic disease is that the immunosuppression due to disease itself and IST will impact on the performance of both tests. It has been shown that TST is more likely to produce false negative result in rheumatic patients than the general population due to the weakened cellular immune response. An early study explored the size of the PPD induration in patients with RA and found that the median size was significantly less than that in healthy control (4.5 vs. 11.5 mm, P < 0.01) [37]. In this study, the pooled estimates revealed that only TST was significantly suppressed by the introduction of steroid, which was related to the higher false negative rate of TST. The impact of steroid on IGRAs was less and did not reach statistically significance. In addition, negative effects of steroid varied considerably with respect to different kinds and doses of steroid used. One study revealed that oral prednisolone, not long-acting corticosteroids, severely suppressed the QFT-GIT and TST performance [28]. However, most studies did not perform such sub-analysis and therefore, further relevant study is recommended. Moreover, it is remained unclear if and when this immunosuppression reverts after withdrawal of steroid therapy. All analyses related to the effect of DMARDs on IGRAs and TST did not reach statistical significance.
Recommendations in screening LTBI in current guidelines vary on the subject of replacing TST with IGRAs or utilizing both tests [38]. The US Centers for Disease Control (CDC) do not explicitly address the choice of test for screening [39]. The European CDC recommends using IGRA and TST in combination for detecting LTBI [40]. The TBNET supported the use of IGRA or, as an alternative in individuals without a history of BCG vaccination, TST in screening adult candidates for TNF inhibitor [41]. In general, IGRA is a more preferred choice in most published national guidelines [38]. However, one major disadvantage of IGRAs is the relatively high cost, and the cost-effectiveness studies remain unclear among patients receiving anti-TNF-α therapy. Based on the available data in the present study, in rheumatic patients with previous BCG vaccination or currently on steroid therapy, IGRAs would be the better choice to identify LTBI by decreasing the risk of false-positivity and false-negativity. The negative results of tests, especially negative TST result should be interpreted with caution in patients treated with any steroid. More information is needed if the test results are negative, including chest radiograph, history of TB exposure, and other risk factors for TB.
There are some limitations in our study. First, without sufficient data, we were not able to assess the capacity of IGRAs to predict the active tuberculosis development. Second, there was considerable heterogeneity between studies, especially in assessing the pooled performance of TST. The heterogeneity could be related to different cut-offs and BCG vaccination status. Third, for study populations on varied regimens of DMARDs and steroid, we could not assess the effect of specific kinds of DMARDs or steroid.
References
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM (2001) Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345(15):1098–1104. doi:10.1056/NEJMoa011110
Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, Vinh DC (2003) Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis 3(3):148–155. doi:10.1016/S1473-3099(03)00545-0
Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD, Group B (2003) Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 48(8):2122–2127. doi:10.1002/art.11137
Wolfe F, Michaud K, Anderson J, Urbansky K (2004) Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 50(2):372–379. doi:10.1002/art.20009
Souto A, Maneiro JR, Salgado E, Carmona L, Gomez-Reino JJ (2014) Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatology (Oxford). doi:10.1093/rheumatology/keu172
Stewart GR, Robertson BD, Young DB (2003) Tuberculosis: a problem with persistence. Nat Rev Microbiol 1(2):97–105. doi:10.1038/nrmicro749
Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ (2002) TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol (Baltimore, Md : 1950) 168(9):4620–4627. doi:10.4049/jimmunol.168.9.4620
Tufariello JM, Chan J, Flynn JL (2003) Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 3(9):578–590. doi:10.1016/S1473-3099(03)00741-2
Lalvani A, Millington KA (2008) Screening for tuberculosis infection prior to initiation of anti-TNF therapy. Autoimmun Rev 8(2):147–152. doi:10.1016/j.autrev.2008.07.011
British Thoracic Society Standards of Care C (2005) BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 60(10):800–805. doi:10.1136/thx.2005.046797
Centers for Disease C, Prevention (2004) Tuberculosis associated with blocking agents against tumor necrosis factor-alpha–California, 2002–2003. MMWR Morb Mortal Wkly Rep 53(30):683–686
Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, Montero D, Pascual-Gomez E, Mola EM, Carreno L, Figueroa M, Group B (2005) Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 52(6):1766–1772. doi:10.1002/art.21043
Mariette X, Salmon D (2003) French guidelines for diagnosis and treating latent and active tuberculosis in patients with RA treated with TNF blockers. Ann Rheum Dis 62(8):791. doi:10.1136/ard.62.8.791
Pouchot J, Grasland A, Collet C, Coste J, Esdaile JM, Vinceneux P (1997) Reliability of tuberculin skin test measurement. Ann Intern Med 126(3):210–214. doi:10.7326/0003-4819-126-3199702010-00005
Menzies D (1999) Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med 159(1):15–21. doi:10.1164/ajrccm.159.1.9801120
Pai M, Zwerling A, Menzies D (2008) Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 149(3):177–184. doi:10.7326/0003-4819-149-3-200808050-00241
Mori T (2009) Usefulness of interferon-gamma release assays for diagnosing TB infection and problems with these assays. J Infect Chemother Off J Jpn Soc Chemother 15(3):143–155. doi:10.1007/s10156-009-0686-8
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed.1000097
Schueler S, Schuetz GM, Dewey M (2012) The revised QUADAS-2 tool. Ann Intern Med 156(4):323. doi:10.7326/0003-4819-156-4-201202210-00018, author reply 323–324
Shahidi N, Fu YT, Qian H, Bressler B (2012) Performance of interferon-gamma release assays in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 18(11):2034–2042. doi:10.1002/ibd.22901
Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, Marston BJ, Huang L, Hopewell PC, Pai M (2011) Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 56(3):230–238. doi:10.1097/QAI.0b013e31820b07ab
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Ponce de Leon D, Acevedo-Vasquez E, Alvizuri S, Gutierrez C, Cucho M, Alfaro J, Perich R, Sanchez-Torres A, Pastor C, Sanchez-Schwartz C, Medina M, Gamboa R, Ugarte M (2008) Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J Rheumatol 35(5):776–781
Soborg B, Ruhwald M, Hetland ML, Jacobsen S, Andersen AB, Milman N, Thomsen VO, Jensen DV, Koch A, Wohlfahrt J, Ravn P (2009) Comparison of screening procedures for Mycobacterium tuberculosis infection among patients with inflammatory diseases. J Rheumatol 36(9):1876–1884. doi:10.3899/jrheum.081292
Martin J, Walsh C, Gibbs A, McDonnell T, Fearon U, Keane J, Codd MB, Dodd J, Veale D, Fitzgerald O, Bresnihan B (2010) Comparison of interferon {gamma} release assays and conventional screening tests before tumour necrosis factor {alpha} blockade in patients with inflammatory arthritis. Ann Rheum Dis 69(1):181–185. doi:10.1136/ard.2008.101857
Vassilopoulos D, Tsikrika S, Hatzara C, Podia V, Kandili A, Stamoulis N, Hadziyannis E (2011) Comparison of two gamma interferon release assays and tuberculin skin testing for tuberculosis screening in a cohort of patients with rheumatic diseases starting anti-tumor necrosis factor therapy. Clin Vaccine Immunol CVI 18(12):2102–2108. doi:10.1128/CVI. 05299-11
Chang B, Park HY, Jeon K, Ahn JK, Cha HS, Koh EM, Kang ES, Koh WJ (2011) Interferon-gamma release assay in the diagnosis of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. Clin Rheumatol 30(12):1535–1541. doi:10.1007/s10067-011-1771-9
Belard E, Semb S, Ruhwald M, Werlinrud AM, Soborg B, Jensen FK, Thomsen H, Brylov A, Hetland ML, Nordgaard-Lassen I, Ravn P (2011) Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflamm Bowel Dis 17(11):2340–2349. doi:10.1002/ibd.21605
Maeda T, Banno S, Maeda S, Naniwa T, Hayami Y, Watanabe M, Sato S, Ueda R (2011) Comparison of QuantiFERON-TB Gold and the tuberculin skin test for detecting previous tuberculosis infection evaluated by chest CT findings in Japanese rheumatoid arthritis patients. J Infect Chemother Off J Jpn Soc Chemother 17(6):842–848. doi:10.1007/s10156-011-0250-1
Chen DY, Shen GH, Chen YM, Chen HH, Hsieh CW, Lan JL (2012) Biphasic emergence of active tuberculosis in rheumatoid arthritis patients receiving TNFalpha inhibitors: the utility of IFN gamma assay. Ann Rheum Dis 71(2):231–237. doi:10.1136/annrheumdis-2011-200489
Minguez S, Latorre I, Mateo L, Lacoma A, Diaz J, Olive A, Dominguez J (2012) Interferon-gamma release assays in the detection of latent tuberculosis infection in patients with inflammatory arthritis scheduled for anti-tumour necrosis factor treatment. Clin Rheumatol 31(5):785–794. doi:10.1007/s10067-012-1938-z
Mariette X, Baron G, Tubach F, Liote F, Combe B, Miceli-Richard C, Flipo RM, Goupille P, Allez M, Salmon D, Emilie D, Carcelain G, Ravaud P (2012) Influence of replacing tuberculin skin test with ex vivo interferon gamma release assays on decision to administer prophylactic antituberculosis antibiotics before anti-TNF therapy. Ann Rheum Dis 71(11):1783–1790. doi:10.1136/annrheumdis-2011-200408
Klein M, Jarosova K, Forejtova S, Becvar R, Sedova L, Pavelka K, Simkova G, Svobodova R, Hviscova K, Mann H, Putova I, Vencovsky J (2013) Quantiferon TB Gold and tuberculin skin tests for the detection of latent tuberculosis infection in patients treated with tumour necrosis factor alpha blocking agents. Clin Exp Rheumatol 31(1):111–117
Kwakernaak AJ, Houtman PM, Weel JF, Spoorenberg JP, Jansen TL (2011) A comparison of an interferon-gamma release assay and tuberculin skin test in refractory inflammatory disease patients screened for latent tuberculosis prior to the initiation of a first tumor necrosis factor alpha inhibitor. Clin Rheumatol 30(4):505–510. doi:10.1007/s10067-010-1550-z
Richeldi L, Losi M, D’Amico R, Luppi M, Ferrari A, Mussini C, Codeluppi M, Cocchi S, Prati F, Paci V, Meacci M, Meccugni B, Rumpianesi F, Roversi P, Cerri S, Luppi F, Ferrara G, Latorre I, Gerunda GE, Torelli G, Esposito R, Fabbri LM (2009) Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest 136(1):198–204. doi:10.1378/chest. 08-2575
Beffa P, Zellweger A, Janssens JP, Wrighton-Smith P, Zellweger JP (2008) Indeterminate test results of T-SPOT.TB performed under routine field conditions. Eur Respir J 31(4):842–846. doi:10.1183/09031936.00117207
Ponce de Leon D, Acevedo-Vasquez E, Sanchez-Torres A, Cucho M, Alfaro J, Perich R, Pastor C, Harrison J, Sanchez-Schwartz C (2005) Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis 64(9):1360–1361. doi:10.1136/ard.2004.029041
Smith R, Cattamanchi A, Steingart KR, Denkinger C, Dheda K, Winthrop KL, Pai M (2011) Interferon-gamma release assays for diagnosis of latent tuberculosis infection: evidence in immune-mediated inflammatory disorders. Curr Opin Rheumatol 23(4):377–384. doi:10.1097/BOR.0b013e3283474d62
CDC (2010) Updated guidelines for using interferon gamma release assays to detect Mycobacterium-United States, 2010. Morb Mortal Wkly Rep 59
ECDC (2011) Use of interferon-gamma release assays in support of TB diagnosis. 2011
Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, Kampmann B, Hellmich B, Groves R, Schreiber S, Wallis RS, Sotgiu G, Scholvinck EH, Goletti D, Zellweger JP, Diel R, Carmona L, Bartalesi F, Ravn P, Bossink A, Duarte R, Erkens C, Clark J, Migliori GB, Lange C (2010) The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J 36(5):1185–1206. doi:10.1183/09031936.00028510
Acknowledgments
This work was supported by the National Natural Science Foundation of China [81302572]. We express our gratitude to authors who responded to our request for additional data from their studies.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Study selection. (GIF 69 kb)
Online Resource 2
Proportion of indeterminate IGRA results. (GIF 31 kb)
Rights and permissions
About this article
Cite this article
Ruan, Q., Zhang, S., Ai, J. et al. Screening of latent tuberculosis infection by interferon-γ release assays in rheumatic patients: a systemic review and meta-analysis. Clin Rheumatol 35, 417–425 (2016). https://doi.org/10.1007/s10067-014-2817-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2817-6