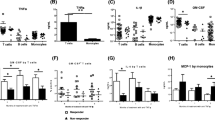
Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by abnormal prevalence of Th1, Th2, Th17, and regulatory (Treg) subsets. Some data suggest that these subsets are influenced by anti-RA agents. Follow-up studies monitoring T cell phenotype in response to therapy are limited. We investigated the alteration of CD4+ T cell subset distribution after the initiation of disease-modifying antirheumatic drug (DMARD) (with glucocorticosteroid (GCS) and methotrexate (MTX)) and anti-TNFα therapy. We enrolled 19 treatment naive (early) RA patients and initiated GCS (in a dose of 16 mg/day for 4 weeks; then 8 mg/day). MTX, 10 mg/week, was started at week 4. We also enrolled 32 RA patients unresponsive to DMARD and initiated anti-TNFα therapy: adalimumab (ADA), 40 mg/2 weeks, n = 12; etanercept (ETA), 50 mg/weeks, n = 12; or infliximab (IFX) on week 0, 2, and 6, 3 mg/kg bw, n = 8. Blood was taken before and 4 and 8 weeks after the initiation of therapy. Ten volunteers served as controls. The T cell phenotype was assessed with flow cytometry. In early RA, Th1, Th2, and Th17 prevalence was higher, while Treg prevalence was lower than normal. GCS alone decreased Th2 prevalence. GCS + MTX decreased Th17 prevalence. Immune phenotype in unresponsive RA before anti-TNF therapy was as in early RA. Four and 8 weeks after initiating anti-TNF therapy, Th1 prevalence was higher than baseline in ETA or IFX, while it was stable in ADA groups. Th2 prevalence was higher than normal in ADA or IFX, while normalized in ETA group. In each group, Treg prevalence increased, while Th17 prevalence was at the baseline. The proinflammatory immune phenotype is normalized only under GCS + MTX combination in early RA. Anti-TNFα therapy exhibit marked effects on all the cell populations investigated (except Th17); some slight differences in this action exist between ADA, ETA, and IFX therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is the most common chronic autoimmune joint disease, affecting approximately 0.5–1 % of the adult population in industrialized countries [1, 2]. Various genes, environmental factors, autoantigens, and still undefined factors play important roles in the pathological events that give rise to the systemic activation of the immune system [3]. The abnormal functioning of CD4+ cells plays a key role in autoimmune process leading to the development of RA. This is reflected by a number of observations indicating that the prevalence of different CD4+ subsets responsible for the harmonized immune response is skewed to a proinflammatory direction. The prevalence of Th1, Th2 helper, and proinflammatory Th17 cells is increased [4, 5], while that of regulatory T cells (Treg) is decreased in the peripheral blood of RA patients [6].
The release of inflammatory cytokines, particularly TNFα, and the activation of proteases triggers an inflammatory cascade culminating in chronic inflammation of the synovium, to synovial hyperplasia, and eventually to the destruction of cartilage and bone [7]. Based on the central role of TNFα and other cytokines in disease's pathogenesis, biologic therapies, respectively, a novel class of antirheumatic agents, were approved for and are used effectively in patients with RA unresponsive to conventional disease-modifying antirheumatic drugs (DMARD) therapies.
Biologic therapies target different elements in autoimmune process. Currently, infliximab, etanercept, and adalimumab, the most commonly used biologic agents in RA are antibodies (infliximab, adalimumab) and soluble receptors (etanercept) that prevent the binding of TNFα to its receptor and inhibit its inflammatory action. These agents are regarded as therapeutically equivalent clinical alternatives in RA. However, there are still some slight differences in their action, individual responses, and side effect profile [8, 9].
Several types of conventional DMARD and biological antirheumatic drugs have been demonstrated to influence the T cell subset distribution, and it is increasingly acknowledged that this action may contribute to the therapeutic effects of such drugs [10]. However, follow-up studies monitoring the changes in T cell subset distribution in response to conventional and biological DMARD therapies are limited at present. Moreover, no data are available as to whether the effects on T cell subset distribution differ between individual anti-TNFα agents. We have, therefore, performed a comprehensive follow-up investigation of T cell phenotype in RA patients before and during the administration of synthetic DMARDs and adalimumab (ADA), etanercept (ETA), and infliximab (IFX), three different anti-TNFα agents.
Patients and methods
Nineteen patients with newly diagnosed (treatment naive) RA and 32 RA patients unresponsive to standard DMARD therapy were enrolled into the study. The detailed clinical data and patient's characteristics are presented in Table 1.
Treatment naive, early RA patients had not received any anti-RA treatment prior to our study. After establishment of the diagnosis, DMARD therapy was initiated according to a fixed protocol as follows: medium-dose oral glucocorticosteroid (GCS, 16 mg/day methylprednisolone) alone for 4 weeks; GCS was subsequently tapered to 8 mg/day, and on week 4, methotrexate (MTX) was started at 10 mg/week. Blood samples were taken before the initiation of DMARD therapy (baseline), then after 4 and 8 weeks of treatment (i.e., after 4 weeks on medium-dose GCS and after a further 4 weeks of combination therapy with low-dose GCS + MTX).
In the group of patients with established RA (n = 32) not responding to standard DMARD combination therapy (MTX at 15 mg/week and leflunomide (LF) at 20 mg/day), anti-TNFα therapy was initiated following the standard-of-care decision of the treating physician: ADA at 40 mg/2 weeks sc, n = 12; ETA at 50 mg/weeks sc, n = 12; or IFX on week 0, 2, and 6 at 3 mg/kg iv, n = 8. Blood samples were taken before the initiation of each anti-TNFα agent, and on week 4 and 8 of therapy. The patients receiving anti-TNF therapy were a homogenous group, including baseline clinical characteristics (age, gender, disease duration, DAS-28 index, CRP, ESR, rheumatoid factor, and anti-MCV status) and medication. Established RA patients were all on long-term methotrexate and leflunomide combination treatment (approximately 3 months), and none of them received glucocorticosteroids or other immunosuppressive agents (Table 1).
Ten age- and gender-matched healthy volunteers served as controls. All of them had a negative history of RA symptoms and a negative status upon detailed physical and laboratory examination. Written informed consent was obtained in advance from all participants. The project was approved by the ethical committee of the University of Szeged (ETT-TUKEB 905/PI/09). This study was conducted in full accordance with the tenets of Declaration of Helsinki (1964).
Five milliliter of lithium–heparin-anticoagulated blood was taken from all participants for the identification of CD4+ cell subsets. PBMCs were separated by gradient centrifugation with Ficoll-Paque (GE Healthcare Life Sciences, Pittsburgh, PA, USA), washed twice with phosphate-buffered saline pH 7.4, and used for cell surface staining with fluorescent antibodies (Becton Dickinson, San Diego, CA, USA) against cell surface markers according to the manufacturer's instructions. Samples were measured within 1 h after staining; at least 300,000 events were recorded for each acquisition.
Cell subtypes were defined as helper T cells (CD4+), Th1 cells (CD4+CXCR3+), Th2 cells (CD4+CCR4+), Th17 cells (CD4+CCR4+CCR6+), regulatory T cells (Tregs; CD4+CD25+CD127-), naive T cells (CD4+CD45RA+), or memory T cells (CD4+CD45RO+). The prevalence values of CD4+ cells expressing early or late activation markers (i.e., CD69 and HLA-DR, respectively) were also determined [11].
All measurements were performed on a BD FACSAria flow cytometer (Becton Dickinson, San Jose, CA, USA). Cell prevalence values were determined with conventional gating, through the use of FACSDiva software (Becton Dickinson, San Jose, CA, USA).
The Mann–Whitney test was applied for the comparison of the data on the controls and the patients, while the paired data in each related patient group were compared by the Friedman test. When the Friedman test demonstrated significant differences, the post hoc Dunn test was used to identify which pairs were significantly different. Levels of p < 0.05 were taken as statistically significant. The clinical data in Table 1 are given as means ± SD, while the results in Tables 2 and 3 are expressed as medians (interquartile range).
Results
Treatment naive, early RA patients exhibited higher than normal Th1, Th2, and Th17 cell and lower than normal Treg cell prevalence values (Table 2, Fig. 1). The prevalence of T cells expressing early or late activation markers (CD69 and HLA-DR, respectively) and memory cells were also higher than normal (Table 2). On week 4 of GCS treatment, the Th2 prevalence decreased significantly, and, therefore, Th1/Th2 ratio shifted to the Th1 direction, while the prevalence of activated T cells expressing CD69 and HLA-DR markers normalized. At this point, Th1, Th17, Tregs, memory, or naive cells prevalence values were comparable to the baseline. By week 8 (4 weeks after the initiation of MTX therapy and start of the tapering of GCS), Th17 prevalence decreased but was still higher than normal. It was noteworthy that the Th2 cell prevalence returned to that on week 4, whereas the other cell prevalence values remained unaltered.
In DMARD, non-responders Th1, Th2, and Th17 prevalence values were higher, while Treg prevalence was lower than normal at baseline (i.e., before the initiation of anti-TNFα therapy). For details, see Table 3, Fig. 2. Activated (i.e., CD69 and HLA-DR positive) CD4+ cell prevalence was normal. Par hazard, baseline naive/memory cell ratios markedly differed in patient subgroups to be treated with the different anti-TNFα agents. T cell subset distribution markedly altered under anti-TNFα therapy with some differences between ETA, ADA, and IFX subpopulations. By weeks 4 and 8, Th1 prevalence increased further in ETA or IFX patients, while it was constant in ADA patients. Th2 prevalence was constantly higher than normal under ADA or IFX therapy, while normalized in patients treated with ETA. Anti-TNFα therapy did not affect Th17 prevalence, while it increased (but still not normalized) Treg prevalence, irrespectively of the anti-TNFα agent used. (However, ADA patients exhibited a further increase in Tregs by week 8, while Treg values were comparable to those at week 4 in ETA and IFX patients). Activated CD4+ prevalence values showed great variation according to anti-TNFα agent used. Compared to the baseline, HLA-DR+ prevalence increased in ADA patients on week 4 and on week 8 in ETA patients, while remaining unaltered in IFX patients. Of note, naive/memory cell ratio (also indicating immunoactivation) also increased in ADA and ETA but remained stable in IFX patients.
Discussion
This is the first study providing a longitudinal follow-up of the prevalence of CD4+ cell subsets during different RA treatment protocols and comparing the effects of commonly used anti-TNFα agents on CD4+ phenotypes.
In treatment naive early RA patients, the distribution of the major T lymphocyte subsets responsible for the regulation of the immune response are shifted to a proinflammatory status. We demonstrated increased prevalence of circulating Th1 and Th2 lymphocytes, indicating an overall activation of the immune system. This is in line with previous data pointing to RA being a Th1-dominant disease [12] even in the presence of high Th2 numbers [5].
A dynamic balance between proinflammatory and antiinflammatory forces is crucial in healthy immune homeostasis. As major suppressors of the immune system, Tregs have been at the center of attention for more than a decade, but in recent years, IL-17-producing proinflammatory Th17 cells have also emerged as playing an important role in this balance [13]. Their predominance may contribute to inflammation, cartilage destruction, and bone erosion in RA [14]. Similarly to other studies [15, 16], we observed an increase in the prevalence of Th17 cells, while that of Tregs was decreased in RA. The net effect of the shift in the Th17/Treg ratio in a proinflammatory direction is the increased activation of T lymphocytes. Our findings concerning the immune phenotype support this notion, as the prevalence of cells expressing any of the activation markers tested proved to be increased in RA.
CD69, an early activation marker, is known to trigger local inflammatory responses in RA [17]. A high number of CD69-expressing T cells were earlier detected in the RA synovium [18]. We have now demonstrated that the prevalence of CD69+ T cells in the peripheral blood of RA patients was higher than normal. Another change indicating a systemic immune activation in RA was that, in addition to the increased CD69 level, the prevalence of HLA-DR+ cells was also increased in our patients. This is in line with the finding of Afeltra et al. that the HLA-DR expression changes together with that of CD69 on RA T lymphocytes [19]. A further sign of an activated immune status in RA is the transition from naive to memory cells [20], as reflected by the decreased ratio of naive/memory T cells that we observed in the peripheral blood of the RA patients.
These observations support the view that the immune system exhibits a systemic dysregulation in RA. As treatment naive RA patients in this study apparently did not suffer from extra-articular complications, our findings indicate that abnormalities in the immune phenotype in the peripheral blood may precede the progression of RA from a local to a systemic disease.
Interestingly, the therapy of early RA with oral GCS did not induce major changes in the tested CD4+ subgroup prevalence. After 4 weeks of treatment with GCS, the only marked change in T cell subgroup prevalence was the decrease in the prevalence of Th2 cells (It should be noted that an analogous effect of GCS on the peripheral blood immune phenotype was reported in patients with bronchial asthma [21]). In turn, the prevalence of Tregs, Th17, and Th1 remained stable at this point. This results in a skewing towards Th1 in the Th1/Th2 ratio. In light of the Th1 cytokine actions, therefore, this effect can be regarded as a proinflammatory stimulus. This constellation is present in spite of the fact that the inflammatory signs and symptoms associated with RA still improved in the patients (decreases in the T cells expressing early or late activation parameters). This contradiction may indicate a chronic inflammatory stimulus which under successful treatment does not reach the threshold of clinical symptoms. This indicates that the therapeutic effects of GCS in RA are not reflected in systemic distribution of Th1, Th2, Treg, and Th17 cells.
Later, with the gradual tapering of the GCS therapy and the introduction of MTX, at week 8, we observed an increase in the prevalence of Th1 and Th2 cells, while that of Th17 cells decreased. Our data do not allow a decision as to whether the tapering of the GCS dose and/or the administration of MTX is responsible for the altered immune phenotype. Both options appear reasonable. While it is known that GCS therapy may decrease the Th2 prevalence, it seems logical to postulate that its withdrawal may lead to an increase in this cell population, and this is supported by our results. Data have been published, suggesting that MTX monotherapy may increase the Th2 prevalence [22] (albeit the prevalence of Th1 cells was also decreased, which was not observed in our study).
It is difficult to compare our results on the prevalence of Tregs with those of other studies, as the markers used for the identification of Tregs have gone through a major evolution in the past decade. These cells were first described as CD4+CD25+ [23], but later as CD4+CD25high [24], and they were subsequently identified according to their FoxP3 expression (CD4+CD25+FoxP3+) [25]. More recently, it has been shown that the absence of CD127 expression can be used as an alternative to the transcription factor FoxP3 [26], and it is also generally accepted that CD127 inversely correlates with FoxP3 in CD4+ cells [27]. For technical reasons, we defined Tregs as cells exhibiting the CD4+CD25+CD127-phenotype. We found no changes in the prevalence of Tregs during MTX therapy, which confirms previous results on CD4+CD25high Tregs [28] and CD4+CD25+FoxP3+ Tregs [29], showing that MTX has no direct action on this feature in RA patients.
The situation revealed by the available literature as concerns the effect of MTX on Th17 cells is also confusing. During in vitro experiments, Li et al. did not find any changes in the percentage of Th17 cells in response to MTX treatment [30], and other authors too reported that MTX monotherapy left this parameter unchanged after 12 weeks [29] and after 30 weeks [31] in patients with RA. However, the study by Yue et al. indicated that after 12 weeks of MTX treatment, the prevalence of Th17 cells was decreased in patients with RA [32]. Our results are the first to indicate a decrease in the prevalence of Th17 cells in the peripheral blood of RA patients following only 4 weeks of MTX treatment. It is important to note, however, that in our study, we could not differentiate between the effects of MTX alone and those of the gradual withdrawal of GCS. It is also a matter of debate whether this observed decrease in Th17 prevalence is transient or is maintained for a longer period.
As the net result of GCS followed by MTX therapy, the cell surface activation markers (CD69 and HLA-DR) became persistently lower than the baseline after 2 months of immune modulating therapy. We found no published data regarding the expressions of activation markers in the peripheral blood (or in the synovium) of RA patients during GCS or MTX treatment.
In RA patients who were unresponsive to conventional DMARD treatment and who were candidates for anti-TNFα therapy, the prevalence values of Th1, Th2, and Th17 cells were as high as, and that of Treg cells was as low as, the corresponding levels in newly diagnosed RA patients. While this observation raises the possibility that the immune phenotype is comparably shifted to a proinflammatory status as in early RA, the normal prevalence of activated T cells may indicate that, despite the high proinflammatory cell prevalences, the overall activation state of the T cells is comparable to that in the controls. The explanation of this apparent contradiction is not clear. It should be noted, however, that, at this time point, these patients had already been treated with MTX and LF, which probably influenced the prevalences of the activated T cell subtypes.
After 4 weeks of anti-TNFα therapy, the overall condition of the patients was significantly improved in all of the examined groups. Again, the changes in immune phenotype did not fully reflect the clinical status and were somewhat therapy specific. After 4 weeks, there was a tendency to a higher Th1 cell prevalence in the ETA and IFX-treated patients, and it had become significant after 8 weeks of therapy. This finding is in line with the report of Aeberli et al. [4], who demonstrated an increase in Th1 prevalence in patients who received 6 weeks of ETA or IFX therapy. The shift in the Th1/Th2 ratio in the Th1 direction became more significant in our ETA-treated group in consequence of the simultaneous decrease in Th2 prevalence (which was not observed with IFX). Such an effect on Th2 was not documented for ETA in the study of Aeberli et al. The shift in the Th1 direction in the peripheral blood may indicate the impact of the therapy on disease progression. It is increasingly acknowledged that this shift is due to the selective recruitment of Th1 cells from the synovium, which reduces local inflammatory reactions in the joints [33, 34]. Interestingly, ADA left the Th1 and Th2 cell ratios intact in our study. One can speculate whether this finding is due to the different origin of the drug (i.e., ADA is a monoclonal antibody, while IFX and ETA are mouse–human chimeric proteins and TNF receptor-IgG fusion proteins, respectively) [35]. If this is the case, the effect of ETA and IFX on Th1/Th2 ratio can be regarded rather as a consequence of their structure and not as their effect on target molecule. This speculation is seemingly worth to be investigated further.
The effects of anti-TNFα therapy on the prevalence of Th17 cells in RA have previously been investigated in a number of studies. It has been shown that the level of Th17 cells in the periphery of RA patients is reduced as a consequence of IFX [31], ADA [32] or ETA [36] treatment. These observations were made during a much longer follow-up period (12 and 30 weeks of treatment), while our data showed that none of the three anti-TNFα therapies applied had no effect on Th17 prevalence after treatment for 4 or 8 weeks. Although we detected a tendency to a decreased Th17 prevalence in the ADA and IFX-treated groups, the lack of significant alterations may indicate that more time is needed for these drugs to exert a relevant effect on the Th17 cell prevalence.
On the other hand, the prevalence of Tregs was significantly increased after only 4 weeks of anti-TNFα therapy in all of the examined groups. Despite many studies, the impact of anti-TNFα agents on Tregs in RA remains controversial. Valencia et al. reported that IFX increased the expressions of Foxp3 mRNA and protein in CD4+CD25high Tregs in RA patients and restored their suppressive function that had previously been downregulated by TNFα [37]. Ehrenstein et al. demonstrated that IFX increased CD4+CD25+ in the peripheral blood of RA patients, but only in those who responded to IFX therapy; it was ineffective in the non-responders [38]. In accordance with this, Lina et al. observed that the percentages of CD4+CD25highFoxp3+ Tregs in RA patients were increased after combined therapy with MTX and ETA [29]. Vigna-Pérez et al. found an increase in CD4+CD25high Tregs on day 15 of ADA therapy, but this effect was only transient, as it was diminished on day 180 [39]. In contrast, Blache et al. reported that neither ADA nor ETA modified the percentages or absolute numbers of circulating CD4+CD25high Tregs in RA patients after 6 and 12 weeks of treatment [40]. In our study, the ADA, ETA, and IFX groups consistently presented increased Treg prevalence levels as compared with the baseline, although they still remained lower than those in the controls. Treg prevalence tended to increase further by week 8 in each group, though with a significant elevation only in the ADA group. This seems to be a specific effect of ADA (it should be noted, however, that the long-term effects of the anti-TNFα agents used cannot be inferred from our results; in the study by Vigna-Pérez, the effect of ADA on Tregs disappeared by week 26 of therapy [39]).
We also tested the prevalences of T cell subtypes with a different activation status. As described above, the decreased ratio of naive/memory T cells indicate an overall activation of the immune system in RA [20]. Interestingly, in general, naive/memory T cell ratio was normal in RA (a remarkable exception was the IFX-group that exhibited lower than normal cell ratios. As this parameter was not used when patients were subjected to any of the anti-TNFα agents, this baseline heterogeneity is probably of chance). Of note, CD69 and HLA-DR activation markers were also normal in all groups (including IFX). The normal expression of CD4+ activation markers in the presence of marked inflammation in RA may indicate a mild form of T cell anergy in patients not responding to conventional DMARD therapy.
This phenomenon has also been noted by others. It has been explained by prolonged exposure to high TNFα levels [41], as TNFα impairs the activation of the T cells through the T cell receptor/CD3 complex in RA [42], but, in our patients, the immune modulatory action of MTX (seen in treatment naive patients) may also contribute.
Literature data suggest that T cell anergy can also be reversed by anti-TNFα treatment [41, 42]. Our results may indicate that this reversing effect may depend on the anti-TNFα agent used. In ADA patients, the ratio of naive/memory cells was significantly decreased relative to the baseline after 4 weeks (due to the increase of memory cell prevalence) and remained high at week 8 in patients treated with ADA. In contrast, in ETA patients, this ratio increased further by week 8 as compared with the baseline. IFX had no effect on the ratio of naive/memory cells at any time point in the studied period. Another activation marker, HLA-DR, increased in ADA and ETA but not in IFX patients at week 4, and remained high at week 8. The mechanism behind the heterogeneity of the effect of different anti-TNFα agents on CD4+ activation markers should be explored further. However, our finding may indicate that these biological agents may provide different modulatory actions on CD4+ cells in spite of comparable clinical efficacy.
Conclusions
This study on peripheral blood samples has provided comprehensive information regarding the impact of anti-RA therapy on CD4+ cell distributions.
In early RA, the peripheral T cells are skewed to an activated status (due to low Treg and high Th1, Th2, and Th17 and to a high prevalence of activated T cell subtypes). This skewing was not normalized by GCS administration, while the introduction of MTX resulted in a lower activation status by influencing mainly the prevalence of Th17 cells. The abnormalities in DMARD-refractory RA were comparable to those in early RA. Anti-TNFα therapy exhibited major effects on all the cell populations investigated (except Th17), with some differences between ADA, ETA, and IFX therapy.
Abbreviations
- ADA:
-
Adalimumab
- CRP:
-
C-reactive protein
- DMARD:
-
Disease-modifying antirheumatic drug
- ESR:
-
Erythrocyte sedimentation rate
- ETA:
-
Etanercept
- GCS:
-
Glucocorticoid
- IFX:
-
Infliximab
- LF:
-
Leflunomide
- MCV:
-
Mutated citrullinated vimentin
- MTX:
-
Methotrexate
- NSAID:
-
Nonsteroidal antiinflammatory drug
- PBMC:
-
Peripheral blood mononuclear cells
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
References
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108
Tobón GJ, Youinou P, Saraux A (2010) The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun 35(1):10–14
Klareskog L, Catrina AI, Paget S (2009) Rheumatoid arthritis. Lancet 373(9664):659–672
Aeberli D, Seitz M, Jüni P, Villiger PM (2005) Increase of peripheral CXCR3 positive T lymphocytes upon treatment of RA patients with TNF-alpha inhibitors. Rheumatology (Oxford) 44(2):172–175
Yang PT, Kasai H, Zhao LJ, Xiao WG, Tanabe F, Ito M (2004) Increased CCR4 expression on circulating CD4(+) T cells in ankylosing spondylitis, rheumatoid arthritis, and systemic lupus erythematosus. Clin Exp Immunol 138(2):342–347
Chavele KM, Ehrenstein MR (2011) Regulatory T cells in systemic lupus erythematosus and rheumatoid arthritis. FEBS Lett 585(23):3603–3610
Appel H, Loddenkemper C, Miossec P (2009) Rheumatoid arthritis and ankylosing spondylitis–pathology of acute inflammation. Clin Exp Rheumatol 27(4 Suppl 55):S15–19
Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ (2003) Infliximab but not etanercept induces apoptosis in lamina propria T lymphocytes from patients with Crohn's disease. Gastroenterology 124(7):1774–1785
Benucci M, Saviola G, Manfredi M, Sarzi-Puttini P, Atzeni F (2012) Tumor necrosis factors blocking agents: analogies and differences. Acta Biomed 83(1):72–80
Vital EM, Emery P (2008) The development of targeted therapies in rheumatoid arthritis. J Autoimmun 31(3):219–227
Szalay B, Mészáros G, Cseh Á, Ács L, Deák M, Kovács L, Vásárhelyi B, Balog A (2012) Adaptive immunity in ankylosing spondylitis: phenotype and functional alterations of T cells before and during infliximab therapy. Clin Dev Immunol 2012:808724
Aarvak T, Chabaud M, Thoen J, Miossec P, Natvig JB (2000) Changes in the Th1 or Th2 cytokine dominance in the synovium of rheumatoid arthritis (RA): a kinetic study of the Th subsets in one unusual RA patient. Rheumatology (Oxford) 39(5):513–522
Selmi C (2011) Autoimmunity in 2010. Autoimmun Rev 10(12):725–732
Peck A, Mellins ED (2009) Breaking old paradigms: Th17 cells in autoimmune arthritis. Clin Immunol 132(3):295–304
Shen H, Goodall JC, Hill Gaston JS (2009) Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum 60(6):1647–1656
Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL (2011) Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res Ther 13(4):R126
Rueda B, Fernandez-Gutierrez B, Balsa A, Pacual-Salcedo D, Lamas JR, Raya E, Gonzalez-Gay MA, Martin J (2008) Investigation of CD69 as a new candidate gene for rheumatoid arthritis. Tissue Antigens 72(3):206–210
Ortiz AM, Laffon A, Gonzalez-Alvaro I (2002) CD69 expression on lymphocytes and interleukin-15 levels in synovial fluids from different inflammatory arthropathies. Rheumatol Int 21(5):182–188
Afeltra A, Galeazzi M, Sebastiani GD, Ferri GM, Caccavo D, Addessi MA, Marcolongo R, Bonomo L (1997) Coexpression of CD69 and HLADR activation markers on synovial fluid T lymphocytes of patients affected by rheumatoid arthritis: a three-color cytometric analysis. Int J Exp Pathol 78(5):331–336
Mamoune A, Durand V, Le Goff P, Pennec YL, Youinou P, Le Corre R (2000) Abnormal distribution of CD45 isoforms expressed by CD4+ and CD8+ T cells in rheumatoid arthritis. Histol Histopathol 15(2):587–591
Kurashima K, Fujimura M, Myou S, Kasahara K, Tachibana H, Amemiya N, Ishiura Y, Onai N, Matsushima K, Nakao S (2001) Effects of oral steroids on blood CXCR3+ and CCR4+ T cells in patients with bronchial asthma. Am J Respir Crit Care Med 164(5):754–758
Xinqiang S, Fei L, Nan L, Yuan L, Fang Y, Hong X, Lixin T, Juan L, Xiao Z, Yuying S, Yongzhi X (2010) Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Biomed Pharmacother 64(7):463–471
van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS (2004) CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 50(9):2775–2785
Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, Boylston AW, Emery P, Ponchel F, Isaacs JD (2006) Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford) 45(10):1210–1217
Jiao Z, Wang W, Jia R, Li J, You H, Chen L, Wang Y (2007) Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol 36(6):428–433
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, de St F, Groth B (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203(7):1693–1700
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St F, Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203(7):1701–1711
Boissier MC, Assier E, Biton J, Denys A, Falgarone G, Bessis N (2009) Regulatory T cells (Treg) in rheumatoid arthritis. Joint Bone Spine 76(1):10–14
Lina C, Conghua W, Nan L, Ping Z (2011) Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol 31(4):596–605
Li Y, Jiang L, Zhang S, Yin L, Ma L, He D, Shen J (2011) Methotrexate attenuates the Th17/IL-17 levels in peripheral blood mononuclear cells from healthy individuals and RA patients. Rheumatol Int
Shen H, Xia L, Lu J, Xiao W (2010) Infliximab reduces the frequency of interleukin 17-producing cells and the amounts of interleukin 17 in patients with rheumatoid arthritis. J Investig Med 58(7):905–908
Yue C, You X, Zhao L, Wang H, Tang F, Zhang F, Zhang X, He W (2010) The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int 30(12):1553–1557
Norii M, Yamamura M, Iwahashi M, Ueno A, Yamana J, Makino H (2006) Selective recruitment of CXCR3+ and CCR5+ CCR4+ T cells into synovial tissue in patients with rheumatoid arthritis. Acta Med Okayama 60(3):149–157
Mohan K, Issekutz TB (2007) Blockade of chemokine receptor CXCR3 inhibits T cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol 179(12):8463–8469
Mitoma H, Horiuchi T, Tsukamoto H, Tamimoto Y, Kimoto Y, Uchino A, To K, Harashima S, Hatta N, Harada M (2008) Mechanisms for cytotoxic effects of antitumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum 58(5):1248–1257
Lu TT, Zhu P, Li XY, Fan CM (2008) Functional status of T helper cells in rheumatoid arthritis and effect of etanercept. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 24(5):495–497
Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE (2006) TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108(1):253–261
Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNF-alpha therapy. J Exp Med 200(3):277–285
Vigna-Pérez M, Abud-Mendoza C, Portillo-Salazar H, Alvarado-Sánchez B, Cuevas-Orta E, Moreno-Valdés R, Baranda L, Paredes-Saharopulos O, González-Amaro R (2005) Immune effects of therapy with Adalimumab in patients with rheumatoid arthritis. Clin Exp Immunol 141(2):372–380
Blache C, Lequerré T, Roucheux A, Beutheu S, Dedreux I, Jacquot S, Le Loët X, Boyer O, Vittecoq O (2011) Number and phenotype of rheumatoid arthritis patients' CD4+CD25hi regulatory T cells are not affected by adalimumab or etanercept. Rheumatology (Oxford) 50(10):1814–1822
Berthelot JM, Maugars Y (2004) Role for suppressor T cells in the pathogenesis of autoimmune diseases (including rheumatoid arthritis). Facts and hypotheses. Joint Bone Spine 71(5):374–380
Cope AP, Londei M, Chu NR, Cohen SB, Elliott MJ, Brennan FM, Maini RN, Feldmann M (1994) Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest 94(2):749–760
Acknowledgments
The study was supported by the Hungarian Scientific Research Fund (OTKA) (grant no. 101661), Social Renewal Operational Programme (grant no. TÁMOP-4.2.2.-08/1/KMR-2008-0004), and research grants from Abbott Laboratories and Pfizer. Attila Balog was supported by the Zoltán Magyary Scholarship (grant no. TÁMOP-4.2.4. A/2-11-1-2012-001). László Kovács was supported by the Bolyai János Scholarship from the Hungarian Academy of Sciences.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szalay, B., Vásárhelyi, B., Cseh, Á. et al. The impact of conventional DMARD and biological therapies on CD4+ cell subsets in rheumatoid arthritis: a follow-up study. Clin Rheumatol 33, 175–185 (2014). https://doi.org/10.1007/s10067-013-2352-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-013-2352-x