Abstract
The prevalence of chronic hepatitis B virus (HBV) infection in China is high. Four percent of patients with HBV infection can present with polyarthritis and positive rheumatic factor similar to rheumatoid arthritis (RA). Here, we investigated the association between HBV infection and serological, radiological, or histological disease status in RA. According to HBV infection status, 223 consecutive hospitalized Chinese patients with RA were divided into the groups of chronic HBV infection, past HBV infection, and no HBV infection. Clinical data and hand radiographs were collected. Synovium was obtained by closed-needle biopsy, and serial tissue sections were stained immunohistochemically for HBV surface antigen (HBsAg) and cluster of differentiation (CD) markers. (1) The prevalence of HBsAg positivity and chronic hepatitis B in RA was consistent with the age-matched general Chinese population (11.2 vs. 8.7 %, 1.7 vs. 1.0 %, respectively, P > 0.05). (2) Clinical parameters, disease activity score in 28 joints, or Sharp scores showed no significant difference among the three groups in 206 RA or 140 treatment-naïve patients, both with active disease (all P > 0.05). (3) Synovial immunohistochemical staining showed negative HBsAg in ten RA patients with HBV carrier status and ten RA patients with past HBV infection. Except for higher subintimal CD3+ cell density in the past HBV infection group, Krenn’s synovitis score, mean densities of subintima positive-staining cells (CD20, CD38, CD79a, and CD68), and CD34+ microvessel counts showed no significant difference among RA patients with HBV carrier status, past HBV infection, or no HBV infection (all P > 0.05). Chronic HBV infection may have no significant effect on disease activity, synovitis, or joint destruction in RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a common chronic systemic disease, primarily of the joints, marked by inflammatory changes in the synovial membranes and articular structures which can lead to joint destruction and disability. The etiology of RA is unknown, but autoimmune mechanisms have been implicated [1].
RA involves a complex interaction among genotype, environmental triggers, and chance. Environmental triggers such as infectious agents (e.g., Epstein–Barr virus, cytomegalovirus, Escherichia coli, and Proteus species) and their products (e.g., heat-shock proteins) have been linked with RA, and although any unifying mechanisms remain elusive, some forms of molecular mimicry are postulated and considered as a possible factor that initiates RA [2, 3]. The formation of immune complexes during infection may trigger the induction of rheumatoid factor (RF), a high-affinity autoantibody against the Fc portion of immunoglobulin, which has long served as a diagnostic marker of RA and is implicated in its pathogenesis. Porphyromonas gingivalis (one of the bacteria that are known to cause periodontal disease) expresses peptidylarginine deiminase 4, which is capable of promoting citrullination of mammalian proteins [4]. What is more, the gastrointestinal microbiome is now recognized to influence the development of autoimmunity in articular models, and specific (and potentially tractable) clinical bacterial signatures that are associated with autoantibody-positive RA are emerging. Infectious agents (including bacteria and viruses) may be involved in the pathogenesis of RA.
Hepatitis B virus (HBV), a DNA virus transmitted percutaneously, sexually, and perinatally, affects 350 to 400 million persons worldwide. HBV infection accounts annually for one million deaths worldwide from cirrhosis, liver failure, and hepatocellular carcinoma [5, 6]. The prevalence of chronic HBV infection is high in China, and the weighted prevalence of hepatitis B surface antigen (HBsAg) for the Chinese population aged 1–59 years was 7.18 % in the 2006 national epidemiological serosurvey [7]. Chronic HBV infection is associated with various extra-hepatic manifestations including skin rash, arthritis, and glomerular manifestations [8]. The administration of recombinant hepatitis B surface vaccine may also induce the onset of symptoms of rheumatic diseases and conditions including RA [9]. Furthermore, to date, three patients with HBV-linked chronic polyarthritis who fulfilled the diagnostic criteria of the American College of Rheumatology (ACR) have benefited from antiviral treatment [10, 11]. These observations suggested a possible link between HBV and RA. Recent studies showed that nearly 4 % of patients with HBV infection can present with polyarthritis similar to RA [12] and be RF seropositive [13], which implied similar pathogenic mechanism in these two ailments [14]. In addition, some studies have suggested a possible association between HBV infection and systemic autoimmune diseases such as RA, polymyalgia rheumatica, antiphospholipid syndrome, and systemic lupus erythematosus [15]. A recent survey suggested a lower frequency of HBV infection in patients with autoimmune diseases and implied a putative protective role of HBV inflection from autoimmune disorders [16]. However, there are no data suggesting a causal role for HBV in these diseases [12], and the acute, immune complex-mediated polyarthritis that occurs during the course of acute hepatitis B infections represents the main immunological evidence that connects HBV with arthritis [17, 18]. Here, we investigated the prevalence of chronic HBV infection in Chinese RA patients and the association between HBV infection and serological, radiographical, or histological disease status in RA.
Materials and methods
Patients
Two hundred twenty three consecutive hospitalized Chinese patients with RA treated in Sun Yat-Sen Memorial Hospital from June 2006 to December 2011 were enrolled retrospectively. All patients fulfilled the 1987 revised criteria of ACR [19] or the 2010 ACR/European League Against Rheumatism criteria for the classification of RA [20]. Patients overlapping with other autoimmune diseases, co-infections with hepatitis C virus, hepatitis D virus, or human immunodeficiency virus, or with primary or secondary causes of liver disease other than hepatitis B (e.g., alcoholic hepatitis, cholestasis, steatohepatitis, hemochromatosis, schistosomiasis japonica, Wilson’s disease, and hepatocellular carcinoma) were excluded. This study was conducted in compliance with the Helsinki Declaration. The Medical Ethics Committee of Sun Yat-Sen Memorial Hospital approved the protocol.
Clinical assessments
Clinical data of all RA patients were collected at baseline, including the 28-joint tender and swollen joint count; erythrocyte sedimentation rate; C-reactive protein (CRP); serum RF (determined by nephelometry, Siemens Healthcare Diagnostics, Munich, Germany, normal range < 20 IU/ml); RF IgA, IgG, and IgM (measured by ELISA, Aesku Diagnostics, Wendelsheim, Germany, normal range < 18 U/ml); and anti-cyclic citrullinated peptide antibody (ACPA, measured by ELISA, Aesku Diagnostics, Wendelsheim, Germany, normal range < 18 U/ml). Disease activity was assessed with the disease activity score in 28 joints (DAS28) with three variables including CRP (DAS28(3)-CRP) [21], a continuous index ranging from 0 to 9.4, in which low disease activity (LDA) is defined as ≤3.2; moderate disease activity (MDA) is defined as >3.2 to ≤5.1; high disease activity (HDA) is defined as >5.1 [22]. A commonly used cutoff point for remission in DAS28(3)-CRP is <2.6 [23]. Joint damage was scored according to hand/wrist radiographs (anteroposterior view) and the modified Sharp score [24]. Seventeen areas for erosion and 18 for joint space narrowing were assessed in each hand/wrist. The maximum score per single joint for erosions is 5 and for joint space narrowing is 4, with the sum of the erosion and joint space narrowing subscores constituting the total Sharp score.
The serology and virology of HBV
All RA patients were tested for HBsAg, antibody to surface antigen (anti-HBs), e antigen (HBeAg), antibody to e antigen (anti-HBe), and antibody to core antigen (anti-HBc) by ELISA (Alpha Biotechnology CO.,LTD, Shanghai, China). HBV DNA was detected by quantitative real-time PCR with a fluorogenic probe (Da An Gene Co., Ltd. Of Sun Yat-Sen University, Guangdong, China, normal range < 103 copies per milliliter). According to HBV infection status, all patients were divided into three groups [25, 26]. Chronic HBV infection group was defined as positive HBsAg, positive HBeAg, or serum HBV DNA > 103 copies per milliliter. Patients with persistent or intermittent elevation of alanine transaminase (ALT) levels (normal range 5–40 U/l) were defined as chronic hepatitis, while patients with normal liver function were defined as HBV carriers. Past HBV infection (resolved hepatitis B) group was defined as negative HBsAg, negative HBeAg, and HBV DNA < 103 copies per milliliter but positive anti-HBe and/or anti-HBc. No HBV infection group was defined as negative HBsAg, negative HBeAg, negative anti-HBe, negative anti-HBc, HBV DNA < 103 copies per milliliter, and positive/negative anti-HBs.
Synovial tissues
Closed Parker–Pearson needle biopsy [27] was performed on one inflamed knee joint in each of the 133 patients with active RA. At least six pieces of synovial tissue were obtained per patient to minimize sampling error [28]. All samples were fixed in 10 % neutral formalin and embedded in paraffin. Sections (5 μm) were cut serially and mounted on adhesive glass slides. Sealed slides were stored at −20 °C until staining.
Immunohistochemistry
Serial sections of synovial tissues were stained with hematoxylin and eosin (HE) and immunohistochemically stained by a three-step immunoperoxidase method. Sections were deparaffinized with xylol, ethanol, and demineralized water. Antigens were then retrieved by boiling in 1 mM EDTA (pH 8.0) for 15–20 min. After the sections had been washed in demineralized water and phosphate buffered saline (PBS), the primary antibody was added and incubated for 45 min at 37 °C. After washing with PBS, the sections were incubated with EnVision Mouse or Rabbit conjugate for 15 min at 37 °C. The color reaction was completed with the diaminobenzidine (DAB)-positive substrate. Sections were counterstained with hematoxylin. Serial sections were stained with the following commercial antibody preparations (Novocastra Laboratories Ltd, UK and Invitrogen, Carlsbad, CA, USA): antibody to HBsAg, anti-CD20 (clone L26, B cells), anti-CD38 (clone SPC32, plasma cells), anti-CD79a (clone SP18, B lineage cells from pro-B cell to plasma cell stage), anti-CD3 (clone PS1, T cells), anti-CD68 (clone KP1, macrophage-like synoviocytes and macrophages), and anti-CD34 (clone QBEnd/10, vascular endothelial cells), according to standard staining protocols. All antibodies were mouse monoclonal antibodies except anti-CD79a, which was a rabbit monoclonal antibody. PV-6000 Polymer Detection System for immunohistological staining (Zymed Laboratories Inc., South San Francisco, CA, USA) was used. Parallel sections were incubated with irrelevant, isotype, and concentration-matched monoclonal antibodies as negative control. Absence of staining due to technical failure was excluded by including appropriate positive control tissues in each staining run.
Synovitis assessments
Only tissue pieces containing synovial intima and vascularized subintima were included. At least three of such pieces were evaluated in each specimen. Histologic changes in HE-stained sections were graded according to Krenn’s synovitis score [29] with the modification that the average of all fields containing synovial lining was recorded per specimen. Synovitis assessments were performed by two observers who were blinded to the identity of the specimens, using an Olympus BX41-32PO2 microscope (Olympus Corp., Tokyo, Japan). Differences between the observers were resolved by mutual agreement. The densities of CD20, CD38, CD3, CD79a, and CD68 positive-staining cells and microvessel count (MVC, confirmed by CD34 positive endothelial cell) [30] were determined by manual counting. A selection of 16 hpfs (×400) in the superficial subintima was examined for each specimen [31]. A 1-mm graticule was used in each hpf with its edge placed just below the lining. The measured value per hpf was converted to the value per millimeter square by using ×0.0625−1 as the conversion factor [32]. The percentage of intimal CD68-positive macrophage-like synoviocytes was scored semiquantitatively (0: absent, 1: 1–25 %, 2: 26–50 %, 3: 51–75 %, 4: 76–100 %).
Statistical analysis
“SPSS 13.0 for Windows” (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. For continuous variables, descriptive statistics [mean, median, standard deviation (SD), interquartile range (IQR)] were calculated. For comparisons of categorized variables, the Chi-square test was used, and for continuous variables, Kruskal–Wallis analysis of variance on ranks among the three groups was used. P < 0.05 was considered statistically significant.
Results
Demographic characteristics of the study patients
RA patients enrolled in this study (n = 223) were predominantly women (73.1 %, 163/223), and the ratio of male to female was 1: 2.72, with a mean (SD) age of 52.3 (13.0) years old (range, 12–84). The mean age of disease onset was 46.1 ± 13.4 years, and disease duration ranged from 1 to 480 months. At recruitment, 67.7 % (151/223) of the RA patients had never taken corticosteroids or disease-modifying antirheumatic drugs (DMARDs), including 140 RA patients with active disease and 11 patients in remission. A majority of these patients had taken only traditional Chinese herbal medicine and/or painkillers to relieve arthralgia. Moreover, 19.7 % (44/223) had taken corticosteroids alone. The remaining 12.6 % (28/223) were taking one or more DMARDs, including methotrexate, leflunomide, sulfasalazine, hydroxychloroquine, or etanercept.
HBV status of RA patients
Twenty five of the 223 RA patients had chronic HBV infection, with four patients with chronic hepatitis B and 21 HBV carriers. The prevalence of concurrent chronic HBV infection was 11.2 % in these RA patients, consistent with the prevalence of the general Chinese population aged 40–59 years in the 2006 national epidemiological serosurvey (8.7 %, 1,628/18,762 [7], p > 0.05). 32.3 % (72/223) of the RA patients had past HBV infection, and 56.5 % (126/223) had no HBV infection.
The prevalence of chronic hepatitis B (1.7 %) in this survey did not differ significantly from the morbidity rate of the general population 45 to 59 years of age in the 2005 local survey in Chongqing, China, which was 1.0 % (1,012.89/100,000) [33]. HBsAg were detected in all four patients with chronic hepatitis B infection (including one man and three women). The serum HBV DNA level was elevated in one patient (9.65 × 105 copies per milliliter) but was undetectable (<1.0 × 103 copies per milliliter) in the other three patients. One patient had slightly abnormal liver function (ALT 50 U/l), and the other three showed significantly increased ALT (range 136–446 U/l).
Effects of HBV infection on RA disease activity
Disease activity score was calculated employing DAS28 (3)-CRP. Seventeen patients were in remission, and 206 were RA patients with LDA, MDA, or HDA. As shown in Table 1, no significant difference was found in disease activity among the patient groups with chronic HBV infection, past HBV infection, or no HBV infection. The prevalence of HBsAg was not significantly different among the subgroups of patients who achieved remission or had LDA, MDA, or HDA. Among the RA patients with active disease (n = 206), no significant differences were found in clinical parameters, DAS28, or Sharp scores among the three groups categorized according to HBV infection status. The seropositivity rate of RF, ACPA, and RF IgA, IgG, or IgM showed no significant differences either. Demographic and clinical as well as radiographic RA-related data of different subgroups of the RA patients with active disease are shown in Table 2.
HBV infection status and RA disease activity in treatment-naïve RA patients with active disease
To exclude the influence of medication on disease activity, 140 treatment-naïve RA patients with active disease who had never been treated with DMARDs or glucocorticoids were analyzed. The results showed no significant differences in demographic characteristics, clinical parameters, DAS28, or Sharp scores among the three groups categorized according to HBV infection status. The seropositivity rates of RF, ACPA, and RF IgA, IgG, or IgM did not differ either (Table 3).
Association between HBV infection and RA synovitis
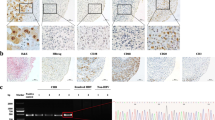
Synovial tissue samples were obtained by closed-needle biopsy from 133 RA patients with active disease, including 10 HBV carriers, 49 patients with past HBV infection, and 74 patients without HBV infection, but no patients with diagnoses overlapping with chronic hepatitis B. All four RA patients with chronic hepatitis B did not undergo the biopsy for financial or personal reasons. Thus, synovial histological parameters were compared in RA patients with HBV carrier status, past HBV infection, and no HBV infection (n = 10 each group) matched for age, gender, and disease duration. Synovial immunohistochemical staining showed negative HBsAg in all these 30 RA patients. Densities of subintimal CD3+ cells in the past HBV infection group were significantly higher than that in the HBV carrier group (1,314.5 (IQR 1,054.6–2,066.4) vs. 493.9 (IQR 165.1–661.2) cells per millimeter square, p = 0.038) and the no HBV infection group (1,314.5 (IQR 1,054.6–2,066.4) vs. 736.8 (IQR 562.5–886.8) cells per millimeter square, p = 0.016) (Fig. 1). Krenn’s synovitis score; mean densities of subintimal positive-staining cells including CD20, CD38, CD79a, and CD68; and CD34+ microvessel counts did not differ among the three groups (all P > 0.05) (Table 4).
Distributions of CD3+ T cells in representative synovium from three different RA patients with HBV carrier status (a), past HBV infection (b), and no HBV infection (c), respectively. Densities of subintimal CD3+ cells in the past HBV infection group (b) were significantly higher than that in the HBV carrier group (a) and the no HBV infection group (c). Immunohistochemical stains with DAB as chromogen (brown); original magnification ×400. scale bar = 50 μm
Discussion
The prevalence of HBV infection and chronic hepatitis B in RA patients was identical to the general Chinese population
The prevalence of HBV infection in RA patients varies among countries. Kim DA et al. reported that HBsAg positivity was found in 3.5 % of RA patients (140/3,946) in Korea, which was not significantly different from the results obtained from the Korean general population (4.4 % in men and 3.0 % in women in 2005) [34]. However, Permin H et al. have revealed that 3 out of 74 patients (4 %) with RA were HBsAg positive, which was approximately 20 times more than that found in the Danish population [12]. The prevalence of HBV infection is as high as 7.18 % in China [7]. From this survey, the seroprevalence of HBV in Chinese RA patients was 11.2 %, not significantly different from the age-matched general population (8.7 %) [7]. Meanwhile, the prevalence of chronic hepatitis B in RA patients was 1.7 %, which was also nearly identical to the mean prevalence in the age-matched general Chinese population (1.0 %) [33]. However, RA is a common disease which affects 0.5–1 % of the adult population worldwide [35] and 0.2–0.93 % of Chinese population. There are nearly five million Chinese people suffering from RA, which will lead to progressive disability [36]. According to the HBV prevalence observed in this survey, it is estimated that nearly 600,000 RA patients coexist with HBV infection in China. This calls for intensive attention especially when they are treated with immunosuppressive therapy. Moreover, the occurrence of HBV reactivation has been recently reported in RA patients receiving conventional DMARDs or tumor necrosis factor-α inhibitors [37, 38].
HBV infection had no significant association with RA disease activity or joint destruction
The association between HBV infection and RA remains elusive. Few reports about association between HBV infection and RA disease activity have been published. Pijak M et al. reported that there might be a negative association between RA and hepatitis B infection or recombinant HBsAg vaccination [39]. However, other studies suggested that HBV infection or recombinant HBsAg vaccination might trigger the pathogenesis of “genuine” RA. Pope JE et al. reported that patients with vaccination-linked RA who shared the common HLA-DR haplotypes for RA (DR1*0101, *0301, *0401, *0404) had the predicted binding anchor for peptides 96–104 aa and 161–169 aa within the HBsAg amino acid sequence, providing a possible link between HBV and RA [39]. These recombinant peptides presented by different RA-specific HLA-DR alleles were able to stimulate Th0 or Th2 CD4+ lymphocytes resulting in proliferation and cytokine secretion [40]. This predominant etiopathogenic role of HBV is probably due to its induction of a specific inflammatory reaction and immune complex formation, or the induction of inflammatory cytokines. When a pathogen invades a joint, it may induce an inflammatory reaction via lytic effects on host tissues, immune complex formation, or the induction of inflammatory cytokines such as the well-documented effects of certain alphaviruses that target mononuclear cells within the joint [41]. Alternatively, viruses can induce autoimmunity and inflammation via numerous mechanisms such as molecular mimicry, bystander activation, or epitope spreading [42]. However, in most cases of virally associated arthritis, the mechanisms are poorly understood [17]. In our survey, RA patients were categorized into three groups according to HBV infection status, including chronic HBV infection, past HBV infection (resolved hepatitis B), and no HBV infection groups. No significant difference was found in disease activity among the three groups either. The prevalence of HBsAg positivity was not significantly different among the RA subgroups according to disease activity (remission, LDA, MDA, HDA). To exclude the influence of medication on disease activity, we analyzed 140 treatment-naïve RA patients with active disease who had never received DMARDs or glucocorticoid therapy. There were no significant differences in clinical parameters, DAS28, and Sharp scores among the three groups categorized according to HBV infection status. The seropositivity rate of RF, ACPA, and RF IgA, IgG, or IgM showed no significant differences either. Those results implied that HBV infection might have no significant association with RA disease activity or joint destruction. As only 25 RA patients with chronic HBV infection (including four RA patients with chronic hepatitis B) were enrolled in this study, we speculated that there might be actually a lack of power to detect this difference in a statistically significant manner. More RA patients, especially RA patients with chronic HBV infection or with chronic hepatitis B, are needed for further study in the future.
HBV infection had no definite association with RA synovitis
Vassilopoulos D et al. pointed out that the possible pathogenesis of HBV-associated arthritis was attributed to the deposition of immune complexes containing viral antigens (HBsAg or HBeAg) and their respective antibodies (anti-HBs and anti-HBe) in synovial tissues [17]. Momohara S et al. reported a patient with knee osteoarthritis who had worsened pain after steroid injection. His plain radiographs at the time of admission showed a large bone defect in the medial tibia and slight narrowing of the articular gap, and immunohistochemical studies revealed massive expression of HBsAg in synovial cells and positive serum HBsAg and HBeAg, which implied rapidly destructive knee arthropathy associated with HBV infection [18]. Schumacher HR et al. described two patients with arthritis in the prodrome of acute viral hepatitis and as an early manifestation of chronic active hepatitis, with evidence of HBsAg in the synovium, which was confirmed by direct immunofluorescence and electron microscopic identification of 200 to 250 A and 400 to 600 A particles in vessel endothelium, synovial lining cells, and other deep synovial cells. Cell injury in synovial cells containing virus-like particles suggested that a direct virus effect on the synovium may be the mechanism for production of arthritis [43].
In our preliminary study, synovial immunohistochemical staining showed the absence of HBsAg in RA patients with HBV carrier status, past HBV infection, or no HBV infection. During closed-needle biopsy, synovial fluid was obtained from one RA patient with HBV carrier complaining of knee pain and swelling. The patient had positive serum HBsAg, anti-HBe, and anti-HBc but normal liver function. Serum viral load was 7.47 × 104 copies per milliliter. Meanwhile, HBsAg, anti-HBe, and anti-HBc were detected in his synovial fluid, and HBV DNA was 2.37 × 103 copies per milliliter, which indicated that synovial fluid from RA patients concurrent with HBV infection could contain virus and HBV-related antigens or antibodies. However, there are no reports suggesting the existence of HBV and HBV-related antibodies/antigens in RA synovial membranes. The deposition of immune complexes containing viral antigens and their respective antibodies in synovial tissues may not exist and be attributed to the synovial injury of RA patients with chronic HBV infection.
The natural course of chronic HBV infection can be divided into different phases including an immune tolerant phase, an immune clearance phase, and a residual inactive phase [44]. The majority of HBV carriers are in the immune tolerant phase, while patients with past HBV infection (resolved hepatitis B) are those who successfully clear the virus and recover from HBV infection after the immune clearance phase. It is generally acknowledged that clearance of HBV is T cell dependent and that specific cytotoxic T lymphocytes play a key role [45]. In our survey, the pathological comparison revealed that except for higher mean subintima CD3+ cell density in the past HBV infection group, Krenn’s synovitis score, mean densities of subintima positive-staining cells (CD20, CD38, CD79a, and CD68), and CD34+ microvessel counts showed no significant difference among RA patients with HBV carriers, past HBV infection, or no HBV infection. Subintima CD3+ cell density in RA patients with past HBV infection was higher than that in RA patients with HBV carrier or no HBV infection, indicating higher distribution of T cells in the synovium of RA patients with past HBV infection, which was probably attributed to the condition that patients with past HBV infection are those who successfully clear the virus and recover from HBV infection after the immune clearance phase. It is worth further investigation whether HBV infection influences the local inflammation in RA synovium via a T lymphocyte-mediated cellular immune response.
A potential limitation of the present study was the relatively small number of RA patients with chronic HBV infection, especially chronic hepatitis B, but this represents the natural distribution of HBV infection status in the community. Another limitation was the absence of synovium from RA patients with chronic active hepatitis B. More patients and long-time follow-up are needed in the future to investigate the association between HBV infection and disease activity as well as local synovitis in RA patients.
In previous studies, it was hypothesized that there may be possible link between RA and HBV infection. However, due to the lack of large-scale clinical studies, there were no definitive answers regarding the association between HBV infection and clinical or histological disease activity in RA. Here, we performed a wide scale study in Chinese patients with RA, looking for evidence of any association with HBV infection. Meanwhile, our data are accompanied by histochemical data looking at the expression of HBsAg in synovial tissues in RA patients. Our preliminary findings suggested the absence of association between chronic HBV infection and disease activity, synovitis, or joint destruction in RA patients, implying that effects of chronic HBV infection on the clinical course of RA, if any, are relatively minor.
References
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219
Auger I, Roudier J (1997) A function for the QKRAA amino acid motif: mediating binding of DnaJ to DnaK. Implications for the association of rheumatoid arthritis with HLA-DR4. J Clin Invest 99:1818–1822
Kamphuis S, Kuis W, de Jager W, Teklenburg G, Massa M, Gordon G et al (2005) Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet 366:50–56
Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K et al (2010) Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum 62:2662–2672
Ganem D, Prince AM (2004) Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med 350:1118–1129
Lok AS, McMahon BJ (2007) Chronic hepatitis B. Hepatology 45:507–539
Liang X, Bi S, Yang W, Wang L, Cui G, Cui F et al (2009) Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine 27:6550–6557
Han SH (2004) Extrahepatic manifestations of chronic hepatitis B. Clin Liver Dis 8:403–418
Maillefert JF, Sibilia J, Toussirot E, Vignon E, Eschard JP, Lorcerie B et al (1999) Rheumatic disorders developed after hepatitis B vaccination. Rheumatology (Oxford) 38:978–983
Csepregi A, Rojkovich B, Nemesanszky E, Poor G, Hejjas M, Horanyi M (2000) Chronic seropositive polyarthritis associated with hepatitis B virus-induced chronic liver disease: a sequel of virus persistence. Arthritis Rheum 43:232–233
Scully LJ, Karayiannis P, Thomas HC (1992) Interferon therapy is effective in treatment of hepatitis B-induced polyarthritis. Dig Dis Sci 37:1757–1760
Permin H, Aldershvile J, Nielsen JO (1982) Hepatitis B virus infection in patients with rheumatic diseases. Ann Rheum Dis 41:479–482
Lim MK, Sheen DH, Lee YJ, Mun YR, Park M, Shim SC (2009) Anti-cyclic citrullinated peptide antibodies distinguish hepatitis B virus (HBV)-associated arthropathy from concomitant rheumatoid arthritis in patients with chronic HBV infection. J Rheumatol 36:712–716
Csepregi A, Nemesanszky E, Rojkovich B, Poor G (2001) Rheumatoid arthritis and hepatitis B virus: evaluating the pathogenic link. J Rheumatol 28:474–477
Maya R, Gershwin ME, Shoenfeld Y (2008) Hepatitis B virus (HBV) and autoimmune disease. Clin Rev Allergy Immunol 34:85–102
Ram M, Anaya JM, Barzilai O, Izhaky D, Porat KB, Blank M et al (2008) The putative protective role of hepatitis B virus (HBV) infection from autoimmune disorders. Autoimmun Rev 7:621–625
Vassilopoulos D, Calabrese LH (2008) Virally associated arthritis 2008: clinical, epidemiologic, and pathophysiologic considerations. Arthritis Res Ther 10:215
Momohara S, Okamoto H, Tokita N, Tomatsu T, Kamatani N (2006) Rapidly destructive knee arthropathy associated with hepatitis B. Clin Exp Rheumatol 24:111–112
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CR et al (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G et al (2003) A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 42:244–257
Aletaha D, Smolen JS (2006) The definition and measurement of disease modification in inflammatory rheumatic diseases. Rheum Dis Clin N Am 32:9–44
Fransen J, Creemers MC, Van Riel PL (2004) Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford) 43:1252–1255
van der Heijde D (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27:261–263
Dienstag JL (2008) Hepatitis B virus infection. N Engl J Med 359:1486–1500
Hoofnagle JH (2009) Reactivation of hepatitis B. Hepatology 49:S156–S165
Schumacher HJ, Kulka JP (1972) Needle biopsy of the synovial membrane—experience with the Parker–Pearson technic. N Engl J Med 286:416–419
Gerlag D, Tak PP (2005) Synovial biopsy. Best Pract Res Clin Rheumatol 19:387–400
Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B et al (2006) Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 49:358–364
Nanashima A, Nakayama T, Sumida Y, Abo T, Takeshita H, Shibata K et al (2008) Relationship between microvessel count and post-hepatectomy survival in patients with hepatocellular carcinoma. World J Gastroenterol 14:4915–4922
Bresnihan B, Cunnane G, Youssef P, Yanni G, Fitzgerald O, Mulherin D (1998) Microscopic measurement of synovial membrane inflammation in rheumatoid arthritis: proposals for the evaluation of tissue samples by quantitative analysis. Br J Rheumatol 37:636–642
Youssef PP, Smeets TJ, Bresnihan B, Cunnane G, Fitzgerald O, Breedveld F et al (1998) Microscopic measurement of cellular infiltration in the rheumatoid arthritis synovial membrane: a comparison of semiquantitative and quantitative analysis. Br J Rheumatol 37:1003–1007
Wang Q, Chen YS, Wang XJ, Wang WJ, Wang YL, Liang XF (2007) Investigation and study of the prevalence of chronic hepatitis B and C. Modern Preventive Medicine 21:4005–4006
Kim DA, Kim TY (2010) Is screening for hepatitis necessary in diagnostic evaluation of rheumatoid arthritis in South Korea? J Rheumatol 37(674–675):675
Gabriel SE (2001) The epidemiology of rheumatoid arthritis. Rheum Dis Clin N Am 27:269–281
Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen SB, Wigley R et al (2008) Rheumatic diseases in China. Arthritis Res Ther 10:R17
Chung SJ, Kim JK, Park MC, Park YB, Lee SK (2009) Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-alpha therapy. J Rheumatol 36:2416–2420
Thong BY, Koh ET, Chng HH, Chow WC (2007) Outcomes of chronic hepatitis B infection in Oriental patients with rheumatic diseases. Ann Acad Med Singap 36:100–105
Pijak M, Gazdik F (2002) Negative association between rheumatoid arthritis and both hepatitis B infection and rHBsAg vaccination. Hepatology 36:2208
Pope JE, Stevens A, Howson W, Bell DA (1998) The development of rheumatoid arthritis after recombinant hepatitis B vaccination. J Rheumatol 25:1687–1693
Rulli NE, Melton J, Wilmes A, Ewart G, Mahalingam S (2007) The molecular and cellular aspects of arthritis due to alphavirus infections: lesson learned from Ross River virus. Ann N Y Acad Sci 1102:96–108
Posnett DN, Yarilin D (2005) Amplification of autoimmune disease by infection. Arthritis Res Ther 7:74–84
Schumacher HR, Gall EP (1974) Arthritis in acute hepatitis and chronic active hepatitis. Pathology of the synovial membrane with evidence for the presence of Australia antigen in synovial membranes. Am J Med 57:655–664
Liaw YF (2009) Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int 29(Suppl 1):100–107
Chisari FV, Isogawa M, Wieland SF (2010) Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 58:258–266
Acknowledgments
We thank all the patients and medical staff who generously contributed to this study. This work was supported by the Chinese National Natural Science Research Grant (grant no. 30972742) and the Natural Science Research Grant of Guangdong Province, China (grant no. 9151008901000130) to L. Dai; Chinese National Natural Science Research Grant (no. 81001334), Guangdong Natural Science Research Grant (no. 10451008901004542), Health Department Grant of Guangdong Province (no. B2010083), Yat-Sen Scholarship for Young Scientist, and the Fundamental Research Funds for the Central Universities (no. 10ykpy19) to L-J Zhu.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chan-Juan Zou and Lang-Jing Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zou, CJ., Zhu, LJ., Li, YH. et al. The association between hepatitis B virus infection and disease activity, synovitis, or joint destruction in rheumatoid arthritis. Clin Rheumatol 32, 787–795 (2013). https://doi.org/10.1007/s10067-013-2170-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-013-2170-1