Abstract
This study aims to perform global gonadal and sexual function assessments in systemic lupus erythematosus-related antiphospholipid syndrome (SLE-APS) patients. A cross-sectional study was conducted in ten SLE-APS male patients and 20 healthy controls. They were assessed by demographic data, clinical features, urological examination, sexual function, testicular ultrasound, seminal parameters, sperm antibodies, and hormone profile. The median of current age was similar in SLE-APS patients and controls with a higher frequency of erectile dysfunction in the former group (30 vs. 0 %, p = 0.029). The median penis circumference was significantly reduced in SLE-APS patients with erectile dysfunction compared to patients without this complication (8.17 vs. 9.14 cm, p = 0.0397). SLE-APS patients with previous arterial thrombosis had a significantly reduced median penis circumference compared to those without this complication (7.5 vs. 9.18 cm, p = 0.039). Comparing SLE-APS patients and controls, the former had a significant lower median of sperm concentration (41.1 vs. 120.06 × 106/mL, p = 0.003), percentages of sperm motility (47.25 vs. 65.42 %, p = 0.047), normal sperm forms by WHO guidelines (11 vs. 23.95 %, p = 0.002), and Kruger criteria (2.65 vs. 7.65 %, p = 0.02). Regarding seminal analysis, the medians of sperm concentration and total sperm count were significantly lower in SLE-APS patients treated with intravenous cyclophosphamide vs. those untreated with this drug (p < 0.05). Therefore, we have observed a novel association of reduced penile size with erectile dysfunction and previous arterial thrombosis in SLE-APS patients. Penis assessment should be routinely done in SLE-APS patients with fertility problems. We also identified that intravenous cyclophosphamide underlies severe sperm alterations in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, we observed normal testicular function in a small group of primary antiphospholipid syndrome (PAPS) patients, in spite of morphofunctional penile abnormalities [1]. This alteration may lead to an issue about fertility or fear of impaired sexual function (impotence) in male patients with rheumatologic diseases. There is, however, no systematic study assessing the overall gonadal and sexual function in systemic lupus erythematosus-related antiphospholipid syndrome (SLE-APS) patients.
Therefore, the aim of this study was to perform global testicular and sexual function assessments in male SLE-APS evaluating its possible association with clinical and laboratorial parameters.
Material and methods
SLE-APS patients and controls
We screened initially 38 male patients with APS without a medical history of hydrocele, hypospadia, cryptorchidism, testicular infection, testicular cancer, orchitis, testicular vasculitis, ureteral stenosis, previous history of any scrotal or inguinal surgery, diabetes mellitus, and tobacco use. None of the participants had history of alcohol consumption or heavy occasional drinking before or at study entry. Of the 38 patients, 28 patients were excluded due to previous vasectomy (n = 3), refusal to collect sperm sample (n = 9), PAPS (n = 12), and APS associated with others rheumatic diseases (n = 4). Therefore, a cross-sectional study was conducted in ten SLE-APS patients regularly followed at the Antiphospholipid Outpatient Clinic of the Rheumatology Division of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. All patients fulfilled the Sapporo and American College of Rheumatology for APS [2] and SLE diagnosis [3], respectively. The control group included 20 healthy subjects followed at the Pre-vasectomy Group of Urology Division of our University Hospital. All SLE-APS patients and controls were at Tanner stage 5. The Local Ethics Committee approved this study, and an informed consent was obtained from all participants
Global reproductive health evaluation
-
1.
Demographic data and body mass index: Current age, age at spermarche (first ejaculation), and race were recorded in SLE-APS patients and controls, while age at disease onset and disease duration in SLE-APS patients. Body mass index (BMI) was defined by the formula, BMI (kg/m2) weight in kilograms/height in meters.
-
2.
Urologic evaluation and sexual function: A clinical assessment of the genitalia included evaluation of testicles, epididymis, vas deferens, scrotum, and penis. These assessments were performed blinded to the results of the semen in SLE-APS patients and controls. Penis length was measured as linear distance along the dorsal side of penis extending from the pubopenile skin junction to the tip of the glands in the flaccid state, while penis circumference was measured at the middle of shaft [1]. The evaluation of sexual function in patients and controls was acquired by the male sexual quotient, a self-administered questionnaire validated in Brazilian Portuguese [4].
-
3.
Testicular Doppler ultrasound: Ultrasound was performed blinded to the results of the semen in SLE-APS patients and controls. The largest measurement in each dimension was recorded and used to calculate the testicular volume according to the formula for an ellipsoid (length × width × thickness × 0.52). The normal mean ± SD value in male postpubertal adolescents and adults is 7–23 mL [1].
-
4.
Hormone evaluation: Hormone determinations performed at study entry in SLE-APS patients and controls, included follicle-stimulating hormone (FSH), luteinizing hormone (LH), morning total testosterone, detected by fluoroimmunoassay using DELFIA time-resolved fluoroimmunoassay kits (Wallac, Turku, Finland). Intra- and inter-assay coefficients of variation were limited to 3.5 and 2.1 %, respectively. The normal ranges for FSH, LH, and total morning testosterone were 1–10.5 IU/L, 1–8.4 IU/L, and 271–965 ng/dL, respectively.
-
5.
Sperm evaluation: Sperm analysis was performed according to the guidelines of the World Health Organization (WHO) [5] by two expert medical technologists. All SLE-APS patients and controls collected two semen samples with interval of 15–30 days after 48–72 h of sexual abstinence. The spermatozoa were analyzed by manual hand count as well as by a computer-assisted semen analysis system under × 400 magnification, using an HTM-2030. Sperm morphology included evaluation of sperm head, neck, midpiece, and tail by WHO guidelines [5] and Kruger strict criteria [6]. Azoospermia was defined as no spermatozoa in the ejaculate and oligozoospermia as sperm concentration <20 million/mL [5]. The presence of antisperm antibodies was determined by direct immunobead test using Immunobead® rabbit antihuman Ig (IgA, IgG, and IgM) kits (Irvine Scientific, Santa Ana, CA, USA) in all patients and controls. At least 50 % of the motile spermatozoa (“a” + “b”) must be coated with Immunobeads before the test results are considered to be clinically significant. A negative control should have a score of <10 % bead attachment, and a positive control should have a score of >20 % bead attachment [5].
-
6.
Clinical and treatment evaluations: SLE disease activity and cumulative damage at the time of study entry were measured in all patients by using the SLE Disease Activity Index (SLEDAI) [7] and the Systemic Lupus International Collaborating Clinics/ACR Damage Index (SLICC/ACR-DI), respectively [8]. Data concerning the therapy were evaluated.
-
7.
Serum immunologic analysis: Anticardiolipin antibodies were detected by a commercial kit (Enzyme Immunoassay Kit, BINDAZYME™, Birmingham, UK) in all APS patients. The cutoff values were <11 IgG phospholipid(GPL)/IgM phospholipid (MPL) U/mL negative, ≥11 and <20 GPL/MPL U/mL indeterminate, and ≥20 positive [9]. Lupus anticoagulant was detected according to international guidelines [10]. Serum IgG and IgM anti-beta-2-glycoprotein I were detected by enzyme-linked immunoabsorbent assay technique (ORG 521 Anti-beta-2-Glycoprotein I IgG/IgM; Mainz, Germany) with cutoff values of 8 U/mL for IgM and for IgG with intra-assay variation, 2.1–5.0 %; inter-assay variation, 2.6–7.95 to IgG; and intra-assay variation, 2.1–3.8 %; inter-assay variation, 4.1-6.3 to IgM.
-
8.
Statistical analysis: Data were compared by t test or Mann–Whitney test if numerical data presented normal or abnormal distribution, respectively, to evaluate differences between SLE-APS patients and controls and according to previous arterial thrombosis and erectile dysfunction in SLE-APS patients. For categorical variables, differences were assessed by Fisher’s exact test. P values less than 0.05 were considered significant.
Results
SLE-APS patients versus controls
The demographic data, BMI, penile anthropometry, testicular volume by ultrasound, sperm analysis, antisperm antibodies, and hormone evaluation in SLE-APS patients and controls are shown in Table 1. The median of current age was similar in both groups [36.9 (21–57) vs. 32.4 (18–54) years, p = 0.310]. In contrast, the median age of spermarche and BMI were significantly higher in SLE-APS patients vs. controls [13.9 (12–16) vs. 12.85 (11–15) years, p = 0.016; and 26.9 (22–35.2) vs. 22.2 (18–27) kg/m2, p = 0.003, respectively].
The frequency of erectile dysfunction was significantly higher in SLE-APS patients compared to that in controls (30 vs. 0 %, p = 0.029). The median right testicular volume by ultrasound was significantly lower in SLE-APS patients [10.38 (3.9–16.7) vs. 13.4 (8.5–20.6) mL, p = 0.03, respectively]. No differences were observed in penis anthropometry and left testicular volume in both groups (Table 1).
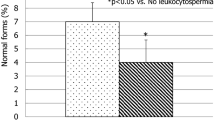
The median values of sperm concentration and sperm motility were significantly lower in SLE-APS patients compared to that in controls [41.1 (0–145) vs. 120.06 (34.5–329) × 106/mL, p = 0.003; 47.25 (0–87.5) vs. 65.42 % (43–82), p = 0.047, respectively]. Normal sperm forms evaluated by WHO guidelines and Kruger strict criteria were significantly lower in SLE-APS patients [11 (0–27) vs. 23.95 % (10–45), p = 0.002; 2.65 (0–8) vs. 7.65 (3–18) %, p = 0.02, respectively]. The frequency of oligo/azoospermia was significantly higher in SLE-APS patients (40 vs. 0 %, p = 0.007). The median of FSH levels and frequencies of elevated FSH were significantly higher in SLE-APS group [9.73 (2.4–25) vs. 3.32 (1–5.9) IU/L, p = 0.025; 40 vs. 0 %, p = 0.007, respectively] (Table 1).
SLE-APS patients
The median penis circumference was significantly lower in SLE-APS patients with (n = 3) erectile dysfunction vs. those without (n = 7) this alteration [8.17 (8–8.5) vs. 9.14 (7–10.5) cm, p = 0.0397]. SLE-APS patients with previous arterial thrombosis (n = 2) had a significantly reduced median penis circumference than those without arterial events (n = 8) [7.5 (7–8) vs. 9.18 (8–10.5) cm, p = 0.039], whereas the median of length in both groups was comparable. Testicular volumes, sperm analysis, antisperm antibodies, hormone profile, thrombocytopenia, SLEDAI, SLICC/ACR-DI, and antiphospholipid antibodies were alike in patients treated with intravenous cyclophosphamide (IVCYC) and those never under this treatment (p > 0.05).
IVCYC was used for lupus nephritis treatment in four patients. The median duration of time from the last dose of IVCYC to study entry was 6 years (2–9). The median of disease duration was significantly higher in those treated with (n = 4) IVCYC compared with those who were never under this drug (n = 6) [9.92 (5–13) vs. 3.38 (0.3–7) years, p = 0.028]. The median values of sperm concentration and total sperm count were significantly lower in SLE-APS patients treated with IVCYC vs. untreated with this drug [6.87 (0–23.5) vs. 63.9 (7.5–145) × 106/mL, p = 0.04; and 16.12 (0–55.5) vs. 226.25 (8.5–471) × 106/mL, p = 0.035, respectively] while no difference was observed for the other sperm parameters (p > 0.05). The rest of the assessment was alike in patients treated with IVCYC and those never under this treatment (p > 0.05).
Discussion
Herein we have observed for the first time an association of reduced penile size with erectile dysfunction and previous arterial thrombosis in SLE-APS patients. We also demonstrated that IVCYC underlies severe sperm alterations in these patients.
The advantage of the present study was the use of a global protocol and the inclusion of only postpubertal subjects, assuring a more homogeneous group regarding gonadal and sexual evaluation. The rigorous exclusion criteria of SLE-APS patients and controls without a medical history of genital diseases and comorbidities are relevant since these alterations may affect gonadal evaluation [11, 12]. The low number of patients and the cross-sectional design are limitations of this study.
The penile development is influenced by race, hypothalamic–pituitary–testicular axis dysfunction, and chronic diseases, as described in our SLE without APS and in PAPS patients [1, 13]. Of note, SLE-APS patients evaluated herein with previous arterial events had reduced circumference of penile size, suggesting the possibility that chronic subclinical endothelial dysfunction involving corpora cavernosa or penis arteries may result in atrophy and sexual alteration [14]. These findings were also observed in our PAPS patients [1], and we are currently carrying out penis pharmacologic ultrasound in lupus patients with APS to evaluate the reduced penis blood flow and intimal proliferation of penile vessels. Interestingly, none of the patients reported a history of penile edema or pain, as also observed in PAPS patients [1].
Erectile dysfunction occurred particularly in SLE-APS patients with reduced penile circumference, as also evidenced in our previous study evaluating PAPS patients [1]. This sexual dysfunction is characterized by the inability of a man to achieve and maintain an erection sufficient for satisfactory sexual performance [4]. Sexual impotence of our SLE-APS patients may be linked to local subclinical endothelial cells dysfunction and may cause psychological problems with a consequent reduction of health-related quality of life [3]. Further studies, including the evaluation of efficacy and safety of phosphodiesterase type 5 inhibitors in this population of autoimmune thrombosis, will be necessary.
Additionally in lupus patients with APS, we have confirmed our previous observation of sperm abnormalities and gonadal hormone alterations in SLE patients, however, without this thrombophilic disorder [13]. Similar to right testicular volume, left testicular size also was lower in SLE-APS patients; nevertheless, the difference did not achieve a statistical significance. The etiology for that is unknown. Indeed, the main limitation of the present study is cohort size. Moreover, discrepancies between right and left testicular volumes were previously evidenced in other studies including SLE patients and healthy controls [13, 16].
Importantly, the use of cyclophosphamide seemed to contribute to severe semen alterations in our SLE-APS patients, leading a persistent or long-lasting damage to primordial sperm cells, as also observed in our male SLE without this condition [13, 15, 16] and in male dermatomyositis [17, 18]. This study emphasizes the importance of sperm cryopreservation for assisted reproductive techniques before alkylating agent use in the future [11].
Of note, no clear etiology for SLE-APS delayed spermarche was identified herein. The possible influence of disease or drug was suggested in our previous study in female juvenile SLE [19–22] and juvenile dermatomyositis [23] patients with late occurrence of menarche in which the disease occurred before first menstruation compared with normal Brazilian adolescents. This was not, however, the case in male SLE-APS since all of our patients had disease onset after spermarche.
In conclusion, we have identified that IVCYC is the major factor for severe and potentially permanent damage to the tests in SLE-APS patients. We also observed for the first time an association of reduced penile size with erectile dysfunction and previous arterial thrombosis in these patients.
References
Rabelo-Júnior CN, Carvalho JF, Gallinaro AL, Bonfa E, Cocuzza M, Saito O et al (2012) Primary antiphospholipid syndrome: morphofunctional penile abnormalities with normal sperm analysis. Lupus 21(3):251–256
Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC et al (1999) International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum 42:1309–1311
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Abdo CH (2007) The male sexual quotient: a brief, self-administered questionnaire to assess male sexual satisfaction. J Sex Med 4:382–389
World Health Organization (WHO) (1999) Laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 4th edn. Cambridge University Press, New York, pp 1–128
Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S (1988) Predictive value of abnormal sperm morphology in vitro fertilization. Fertil Steril 49:112–117
Bombardier C, Gladman DD, Urowitz MB, Karon D, Chang CH, and The Committee on Prognosis Studies in SLE (1992) Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum 35:630–640
Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M et al (1996) The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum 39:363–369
Haris EN, Pierangeli S, Birch D (1994) Cardiolipin Wet Workshop report. Am J Clin Path 101:616–624
Wisloff F, Jacobsen EM, Liestol S (2002) Laboratory diagnosis of the antiphospholipid syndrome. Thromb Res 108:263–271
Silva CA, Bonfa E, Ostensen M (2010) Maintenance of fertility in patients with rheumatic diseases needing anti-inflammatory and immunosuppressive drugs. Arthritis Care Res (Hoboken) 62:1682–1690
Nukumizu LA, Saad GC, Ostensen M, Almeida BP, Cocuzza M, Gonçalves C, et al. (2012) Gonadal function in male patients with ankylosing spondylitis. Scand J Rheumatol (in press)
Vecchi A, Borba E, Bonfá E, Cocuzza M, Pieri P, Kim CA et al (2011) Penile anthropometry in systemic lupus erythematosus patients. Lupus 20:512–518
El-Sakka AI, Yassin AA (2010) Amelioration of penile fibrosis: myth or reality. J Androl 31:324–335
Soares PM, Borba EF, Bonfa E, Hallak J, Al C, Silva CA (2007) Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum 56:2352–2361
Suehiro RM, Borba EF, Bonfa E, Okay TS, Cocuzza M, Soares PM et al (2008) Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology (Oxford) 47:1692–1697
Moraes AJ, Pereira RM, Cocuzza M, Casemiro R, Saito O, Silva CA (2008) Minor sperm abnormalities in young male post-pubertal patients with juvenile dermatomyositis. Braz J Med Biol Res 41:1142–1147
Moraes AJ, Pereira RM, Cocuzza M, Casemiro R, Saito O, Silva CA (2010) Gonad evaluation in male dermatomyositis. A pilot study. Clin Exp Rheumatol 28:441–442
Silva CA, Leal MM, Leone C, Simone VP, Takiuti AD, Saito MI et al (2002) Gonadal function in adolescents and young female with systemic lupus erythematosus. Lupus 11:419–425
Febronio MV, Pereira RM, Bonfa E, Takiuti AD, Pereyra EA, Silva CA (2007) Inflammatory cervicovaginal cytology is associated with disease activity in juvenile systemic lupus erythematosus. Lupus 16:430–543
Silva CA, Deen ME, Febronio MV, Oliveira SK, Terreri MT, Sacchetti SB et al (2011) Hormone profile in juvenile systemic lupus erythematosus with previous or current amenorrhea. Rheumatol Int 31:1037–1043
Aikawa NE, Sallum AM, Pereira RM, Suzuki S, Viana VS, Bonfá EETAL (2012) Subclinical impairment of ovarian reserve in juvenile systemic lupus erythematosus after cyclophosphamide therapy. Clin Exp Rheumatol 30(3):445–449
Aikawa NE, Sallum AM, Leal MM, Bonfá E, Pereira RM, Silva CA (2010) Menstrual and hormonal alterations in juvenile dermatomyositis. Clin Exp Rheumatol 28:571–575
Acknowledgments
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (grants 2004/07832-2 and 2005/56482-7 to CAS) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPQ (grants 301411/2009-3 to EB, 3300665/2009-1 to JFC, and 302724/2011-7 to CAS), the Federico Foundation Grants (grants to JFC, EB, and CAS), and by the Núcleo de Apoio à Pesquisa Saúde da Criança e do Adolescente da USP. Our gratitude to Maribê Salan Marcos and Rosa Casemiro for technical support and Francisco Erivelton Aragão for the statistical analysis.
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabelo-Júnior, C.N., Bonfá, E., Carvalho, J.F. et al. Penile alterations with severe sperm abnormalities in antiphospholipid syndrome associated with systemic lupus erythematosus. Clin Rheumatol 32, 109–113 (2013). https://doi.org/10.1007/s10067-012-2083-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-012-2083-4