Abstract
The aim of this study is to explore the survival rate and risk factors of mortality in patients with late-onset systemic lupus erythematosus (SLE) in a large cohort. Clinical presentations, disease activity, organ damage scores, autoantibody profile, and mortality data were obtained retrospectively from late-onset SLE patients (onset age ≥50 years) diagnosed between 1995 and 2009. The risk factors of organ damage were evaluated by the chi-square test and logistic regression. The cumulative rate of survival was calculated by Kaplan–Meier method, and factors predictive of mortality were studied by Cox proportion hazard regression model. A total of 158 patients (132 female and 26 male) were studied. The average onset age was 58.66 ± 6.38 years and mean disease duration was 63.85 ± 48.17 months. One hundred and four patients had organ damage at the time of data analysis. Hematological system and kidney involvement were most common. Central nervous system involvement was relatively rare. In univariate logistic analysis, associations were found between SLE disease activity index (SLEDAI) at diagnosis (OR = 1.133, P = 0.001); renal involvement (OR = 2.441, P = 0.009) and edema (OR = 2.812, P = 0.003) were associated with organ damage. And SLEDAI at diagnosis (OR = 1.103, P = 0.034) was independent factor for organ damage in multivariate logistic regression. During the follow-up, 64 patients (51 female and 13 male) died. Five-, 10-, and 15-year survival rates were 80.4, 56.5, and 31.7 %, respectively. Median survival time was 123 months. The analysis of Cox proportion hazard regression model showed that age at disease onset (OR = 1.069, P = 0.002), compliance of medical care (OR = 3.282, P = 0.001), and SLEDAI at diagnosis (OR = 1.091, P = 0.003) were independent risk factors of mortality. Late-onset SLE has a poor long-term prognosis. Infection is the major cause of death in patients with late-onset lupus. Disease activity, medical care, and onset age are strongly related to death of late-onset SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystem, autoimmune disorder. SLE can occur in any age, though it usually affects childbearing women and declines after menopause. It has a broad spectrum of clinical features, laboratory manifestations, and a variable disease course. As a life-long disease, SLE is commonly relapsing, with periods of remission between the flares. Age of onset is an important factor influencing its clinical presentation, course, and prognosis [1, 2]. In the last decades, several studies have carried out in early- and late-onset SLE patients to detect differences of the characteristic clinical and serological features [3–6].
Arguments on whether late-onset SLE has a benign condition persist for a long time. More insidious onset and less severe disease course have been concluded for late-onset SLE in a meta-analysis [7]. In more recent studies, however, poor prognosis and more organ damage were found in late-onset patients, noting that late-onset SLE may be have a comparable worsen course, with different manifestations [4–6].
As a consequence of improvement in treatment, a better prognosis and increased long-time survival rate have been found in all cases of SLE. Significant variation in the prognosis of patients with SLE could be related to different genetic background, disease activity, organ damage, and therapy efficiency [8, 9]. The overall survival analyses have been conducted in different countries and ethnic groups [10–12].
Data on the outcome and predictors of mortality in late-onset SLE patients, however, are limited and confined to small numbers of patients. In this paper, we retrospectively observed a large cohort of late-onset SLE patients and performed survival analysis to examine the independent factors influencing outcome of late-onset SLE.
Methods
Patients
From January 1995 to December 2009, we followed 158 patients with SLE who were diagnosed at or over the age of 50 years. The study had been approved by West China Hospital Ethics Committee and informed consent according to the Declaration of Helsinki was obtained. Four patients, in this cohort of 158 patients, diagnosed before 1995 in other hospitals and follow-up after 1995 in our center were also included. Drug-induced lupus and pure cutaneous lupus with no systemic features were excluded. All of 158 patients have fulfilled at least four of the 1982 American College of Rheumatology (ACR) revised criteria for SLE [13]. The following data were collected retrospectively at first presentation: sex, clinical features, disease activity scores, laboratory tests, comorbidities, and complications. The patients were followed up every 1 to 3 months for active stage and 3 to 6 months for inactive stage. Disease activity scores, organ damage, laboratory tests, mortality data, comorbidities, and complications were recorded during the disease course of patients.
Clinical features included disease duration (period from disease diagnosed to last visit or death), diagnosis duration (length of time from disease onset to disease being diagnosed), and disease onset age (age at first symptom). Therapeutic variables included exposure to high-dose glucocorticoid at first diagnosed (pulse therapy or oral prednisone ≥1 mg/kg day), immunosuppressive agents treatment (taken methotrexate, mycophenolate mofetil, cyclophosphamide, azathioprine, or combination), and medication compliance.
Disease activity was evaluated by SLE disease activity index (SLEDAI) [14] at first presentation and subsequent visits. Organs damage were assessed by Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) damage index [15] at the first visit, and then it was scored yearly. The cumulative damage scores were calculated at the end of the study for each patient. Alternatively, the cumulative damage scores were used for analysis before death, for the patients who died during the follow-up period.
Laboratory tests, such as blood and urine routine test, liver and kidney functions, complements (low complements defined as C3 and/or C4 decreased at least 6 months or persistently decreasing until death) were measured regularly at every visit. The features of autoantibodies, such as anti-nuclear antibody (ANA, indirect immunofluorescence using the Hep-2 cells), anti-double-stranded DNA (anti-dsDNA, immunofluorescence against Crithidia luciliae), and extractable nuclear antigens (ENA, anti-Sm, anti-U1RNP, anti-SSA, anti-SSB, and anti-Rib) were determined.
Comorbidities and complications including hypertension, diabetes mellitus (physician-based diagnosis and/or requiring medical intervention), chronic renal failure, infection (has a pathogenic or radiological evidence and be submitted to inpatient because of infection), edema (pitting edema and/or serositis), hypoproteinemia, and hyperglobulinemia were observed.
Statistical analysis
Statistical package for social science (SPSS) version 13.0 was employed to analyze data. Quantitative variables including disease duration, diagnosis duration, disease onset age, disease activity, SLICC score were expressed as mean ± SD. Dichotomous variables, such as sex, laboratory tests, therapeutic and medical care, comorbidities, and complications were showed as number (percentage). Independent t test was performed to compare the means of continuous variable. Mann–Whitney U test was used, when normal distribution or equal variance could not be assumed. Univariate and multivariate analysis of risk factors of organ damage were evaluated by the chi-square test and logistic regression, respectively. The cumulative rate of survival was calculated by Kaplan–Meier method. Variables including prevalence of clinical presentation and laboratory tests, disease duration, diagnosis duration, disease onset age, therapeutic variables, cumulative of organ involvement at last visit, disease activity at diagnosis, as well as medical care were entered into model. Death was the dependent variable. The probability curves of survival were calculated according to the Kaplan–Meier method and compared by the log rank test. Odds ratio (OR) with 95 % confidence interval (CI) was calculated. Variables with significance level of P value < 0.1 in univariate analysis were selected for multivariate analysis. Independent risk factors for mortality and OR with 95 % CI were examined by multivariate analysis using Cox proportional hazard model. A P value of <0.05 (two sides) was considered significance.
Results
From January 1995 to December 2009, 158 patients, 132 female cases (83.5 %) and 26 male cases (16.5 %), were included into this analysis. Overall, SLE was diagnosed at or after 50 years and the average onset age was 58.66 ± 6.38 years. The patients were diagnosed 12.96 ± 19.82 months after first presentation with a range of 0.2–168 months. Mean disease duration was 63.85 ± 48.17 months (range 0.8–311). On average, SLEDAI score at first diagnosis was 12.32 ± 5.56 (range 2–28), 66.6 % patients with active disease nearly twice as common as patients with no activity (33.5 %). SLICC score was 1.16 ± 1.12 (range 0–5) at first visit. The cumulative organ damage score at last visit was 1.65 ± 1.17 and the median score was 2. Fifty-four (34.18 %) patients were without any organ damage during the course of disease.
Table 1 shows organ system damage, comorbidity, complications, autoantibody profiles, disease activity, and SLICC score of 158 late-onset SLE patients at first visit and the prevalence of clinical manifestations. In our data, hematological system and kidney involvement were most common in late-onset SLE patients. In patients with hematological involvement, about 48.5 % patients had significant leukopenia, and 25.8 % patients had thrombocytopenia. Nearly 12.4 % of the patients presented as pancytopenia.
There were 90 patients with criteria of lupus nephritis. Approximately, 83.5 % of these patients had urine protein over 0.5 g/24 h and 34 % presented cellular casts. Sixty-three patients developed chronic renal failure during the follow-up. Articular involvement was also frequent, but central nervous system (CNS) affection was relatively rare (four CNS involvement and one psychosis). About 65.82 % (n = 104) patients had organ damage distributing in different organs at the time of data analysis. Average SLICC score for those who had damage was 1.77 ± 0.92, 21 (13.3 %) patients having a score of 3 or more, and the median score was 2. No significance in damage score was found between female and male patients (Mann–Whitney U test, P = 0.918).
ANA and anti-ENA were measured at diagnosis and were not routinely repeated. Complements level for C3 and C4 were determined every 3–6 months. Profiles of autoantibodies and complements at diagnosis and follow-up were shown in Table 1. ANA positive was observed in all of the patients. Fifty four patients were anti-dsDNA positive, anti-Sm antibody was detected in 19.6 %, anti-SSA antibody in 41.1 %, anti-SSB antibody in 19.0 %, anti-Rib antibody in 9.5 %, anti-U1RNP antibody in 26.0 %, and anti-ACA antibody in 2.53 % of the SLE patients, respectively. There were 75.3 % patients presenting with persistent low complement levels.
In order to study the predictive factors for organ damage, univariate and multivariate analysis were performed. As shown in Table 2, SLEDAI at diagnosis, renal disorder and edema were associated with damage. Logistic regression with damage as outcome and clinical features, autoantibodies and treatment as the predictor variables showed that SLEDAI [OR = 1.103 (95 % CI 1.008–1.208), B = 0.117, SE = 0.049, P = 0.034] was independent factors of organ damage.
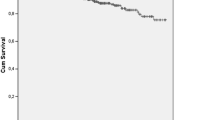
About 25.3 % patients (n = 40) were treated with high-dose prednisone or pulse therapy with methylprednisolone at initial diagnosis. In the follow-up period, 36.1 % patients were treated with at least one immunosuppressive agent, such as azathioprine, cyclophosphamide, or mycophenolate mofetil. Incompliant medical care, which defined as stopping therapy without advices or seeing physicians irregularly, was found in 102 (64.6 %) patients. During the follow-up of 14 years, 64 patients died, of them 51 were female (79.7 %) and 13 were male (20.3 %). The cause of death were infection (22 cases), heart failure (ten cases), renal failure (18 cases), myocardial infarction (four cases), pulmonary disease (six cases), and unknown reasons (four cases). The cumulative survival was analyzed by the Kaplan–Meier method. As shown in Fig. 1, the 5-, 10-, and 15-year survival rates were 80.4, 56.5, and 31.7 %, respectively. Median survival time was 123 months.
Risk factors of mortality were achieved by univariate (log rank test) and multivariate analysis (Cox regression). Demographic data, clinical features, such as onset age, diagnosis duration, and the prevalence of various clinical presentations were involved as variables. Univariate analysis demonstrated that male gender, higher SLEDAI at diagnosis, higher age of onset, incompliant medical care, infections, pulmonary involvement, and chronic renal failure were independent factors for poor survival of late-onset SLE patients, shown in Table 3 and Fig. 2. Hypoproteinemia (P = 0.073) and low complement levels (P = 0.091) may also be related to mortality of late-onset SLE, but no significance were found in univariate analysis. Cox proportion hazard regression model was performed as “enter” method to determine the independent predictors of mortality in multivariate model. The results showed that higher age at disease onset, incompliant medical care, and higher SLEDAI at diagnosis were independent risk factors of death, shown in Table 4.
Discussion
This is a retrospective study of the long-term survival and prognostic factors of Chinese patients with late-onset SLE. Although the exact definition of late-onset SLE has not been given yet, cut-off age of 50 years at disease onset is commonly accepted [3]. One hundred and fifty-eight late-onset patients (onset over 50 years of age) were included into this study.
Several previous studies have suggested that late-onset SLE patients differ from early-onset patients in clinical presentation, organ damage, severity of disease, and prognosis [1, 3–6, 16, 17]. In this study, we focused on the survival and prognostic indicators in a rather large population of late-onset SLE.
Long-term survival rates of SLE have been improved in the last decades. The overall cumulative probability of survival at 5, 10, and 15 years after diagnosis of SLE were 80–94, 77–83, and 75–80 %, respectively [12, 18]. Compared to early-onset lupus, with survival rates of 84 % at 5 years, 71 % at 10 years, and 59 % at 15 years, worse rates of 66, 44, and 44 %, were found in western and oriental populations in the late-onset SLE group [3, 18]. In accordance with observation of southern Chinese patients [18], we demonstrated that, in our study, the 5-, 10-, and 15-year survival rates were about 80.4, 56.5, and 31.7 %. For univariate analysis, we found that sex, disease activity, age of onset of SLE, infection, medical care, and pulmonary and renal damage were related to the outcome of late-onset of SLE. However, in multivariate model, age of onset, incompliant medical care, and disease activity, but not organ damage, were independent predictors of mortality.
Disease activity and organ damage are important determinants of survival in patients with SLE [19–21]. Our data showed that disease activity at diagnosis is an independent risk factor of death in patients with late-onset SLE, no matter if univariate or multivariate analysis were used. Studies comparing disease activity and patterns of organ damage in early- and late-onset SLE have well been documented. Late-onset SLE was so far considered as a benign disease due to relatively lower SLEDAI score in contrast to patients with SLE beginning in the younger age [22–24]. But Lalani and colleagues [6] recently reported that disease activity was significantly higher in late-onset lupus patients. The differences between individual studies can be explained by the small size of patients, varying definitions of age for late-onset lupus, ethnic differences, and methods for collecting data [22]. Although the arguments on whether SLEDAI score is lower in late-onset patients with SLE will be continued, it is generally accepted that disease activity is closely related to prognosis of SLE.
Major organ damage, particularly CNS and renal involvement, have long been identified as markers of poor prognosis [25]. CNS disease or renal involvement contributed to survival of SLE patients in prospective and retrospective studies, respectively. Unexpectedly, however, in our results organ damage was not a risk factor for poor outcomes in late-onset SLE patients. More frequent neurological complications and lower occurrence of nephritis was observed in late-onset SLE by Bertoli and colleagues [4]. In contrast, Mok and colleagues [18] showed that renal and hematological system involvement was common in late-onset SLE patients and no significant differences in the prevalence of major organ disease were found among young- and late-onset patients. A cross-sectional study in a large cohort suggested that the prevalence of renal and neurologic disease were less frequent in late-onset than young-onset SLE patients [6]. In our study, renal involvement was common in late-onset lupus and was shown to be a predictor for damage in a logistic regression analysis. Interestingly, chronic renal failure is a univariate predictor of mortality in our study, but not renal disorder. One interpretation is that before end-stage renal disease, most patients with renal disorders will respond well to treatment, otherwise the efficiency would attenuate in the case of chronic renal failure.
Although it has been reported that increasing age strongly predicts poor outcome in SLE patients [26, 27], little data support this notion in late-onset SLE. On the basis of our results, age of onset is confirmed as a predictor of poor prognosis in multivariate analysis. As discussed above, studies on the differences of disease activity and organ damage between early- and late-onset SLE have shown inconsistent results. Factors not directly related to SLE have been described being associated with the outcome of SLE [28]. Thus, with increasing age, the prevalence of complications and comorbidities, such as cardiovascular disease, diabetes mellitus, and infections, which maybe not related to SLE itself, gradually increase.
It is known that treatment is a critical factor for survival of patients with SLE. The aim of treatment is controlling symptoms by glucocorticoid and immunosuppressive agents and obtaining long-term remission. The treatment strategies often depend on the severity of disease and organ involvement. Unfortunately, comorbidities and concomitant therapies often limit the option for late-onset SLE treatment. As a result of this, high-dose corticosteroid treatment, contributing to organ damage in both adult- and childhood-onset SLE patients previously [29, 30], may not be suitable for late-onset SLE. Differing from adult-onset SLE, our study demonstrated that incompliant medical care, instead of high dose of glucocorticoid and immunosuppressive agents therapy, was an independent predictor of mortality. It has been emphasized that proper education about lifestyle is an important element in the treatment of SLE. For acquiring better treatment response and improved survival rate, correct patient education and medical instruction may be indispensable.
This study concentrated on survival analysis and predictors to prognosis of late-onset SLE patients, so, there are no data on early-onset SLE. As many publications reported the differences between early- and late-onset lupus, it seems that determining contributors to prognosis of late-onset SLE only may be attractive. Another limitation of our study is that clinical features were included in analyses independent of their cause. Though it is unavoidable, non-lupus-related comorbid conditions and complications may influence the analysis. At last, the findings may not be applicable to other races and populations, in particular non-Asian, due to several differences between SLE prognosis and social culture.
In conclusion, this is the first study on survival analysis and outcome prediction of late-onset SLE patients. Our results strongly suggest that late-onset SLE has a poor prognosis. Hematological system and renal involvement are very common in our cohort. Disease activity, edema, and renal disease contribute to organ damage. Infection is the primary cause of death in late-onset SLE. Age of onset, disease activity, and incompliant medical care are independent factors for mortality. Strategies to reduce disease activity and better medical care may be effective in improving outcome of late-onset lupus.
References
Ho CT, Mok CC, Lau CS, Wong RW (1998) Late onset systemic lupus erythematosus in southern Chinese. Ann Rheum Dis 57:437–440
Costallat LT, Coimbra AM (1994) Systemic lupus erythematosus: clinical and laboratory aspects related to age at disease onset. Clin Exp Rheumatol 12:603–607
Boddaert J, Huong DL, Amoura Z, Wechsler B, Godeau P, Piette JC (2004) Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine 83:348–359
Bertoli AM, Alarcón GS, Calvo-Alén J, Fernández M, Vilá LM, Reveille JD, Study Group LUMINA (2006) Systemic lupus erythematosus in a multiethnic US cohort. XXXIII. Clinical (corrected) features, course and outcome in patients with late-onset disease. Arthritis Rheum 54:1580–1587
Maddison P, Farewell V, Isenberg D et al (2002) The rate and pattern of organ damage in late onset systemic lupus erythematosus. J Rheumatol 29:913–917
Lalani S, Pope J, de Leon F, Peschken C, Members of CaNIOS/1000 Faces of Lupus (2010) Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 Faces of Lupus study. J Rheumatol 37:38–44
Ward MM, Polisson RP (1989) A meta-analysis of the clinical manifestations of older-onset systemic lupus erythematosus. Arthritis Rheum 32:1226–1232
Kasitanon N, Magder LS, Petri M (2006) Predictors of survival in systemic lupus erythematosus. Med (Baltimore) 85:147–156
Cervera R, Khamashta MA, Font J et al (2003) Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Med (Baltimore) 82:299–308
Blanco FJ, Gómez-Reino JJ, de la Mata J et al (1998) Survival analysis of 306 European Spanish patients with systemic lupus erythematosus. Lupus 7:159–163
Contreras G, Lenz O, Pardo V et al (2006) Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 69:1846–1851
Rabbani MA, Habib HB, Islam M et al (2009) Survival analysis and prognostic indicators of systemic lupus erythematosus in Pakistani patients. Lupus 18:848–855
Tan EM, Cohen AS, Fries JF et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Gladman DD, Goldsmith CH, Urowitz MB et al (1994) Sensitivity to change of 3 systemic lupus erythematosus disease activity indices: international validation. J Rheumatol 21:1468–1471
Gladman D, Ginzler E, Goldsmith C et al (1996) The development and initial validation of the systemic lupus international collaborating clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 39:363–369
Appenzeller S, Pereira DA, Costallat LT (2008) Greater accrual damage in late-onset systemic lupus erythematosus: a long-term follow-up study. Lupus 17:1023–1028
Koh ET, Boey ML (1994) Late onset lupus: a clinical and immunological study in a predominantly Chinese population. J Rheumatol 21:1463–1467
Mok CC, Mak A, Chu WP, To CH, Wong SN (2005) Long-term survival of southern Chinese patients with systemic lupus erythematosus: a prospective study of all age-groups. Med (Baltimore) 84:218–224
Zonana-Nacach A, Yañez P, Jiménez-Balderas FJ, Camargo-Coronel A (2007) Disease activity, damage and survival in Mexican patients with acute severe systemic lupus erythematosus. Lupus 6:997–1000
Nossent J, Cikes N, Kiss E et al (2007) Current causes of death in systemic lupus erythematosus in Europe, 2000–2004: relation to disease activity and damage accrual. Lupus 16:309–317
Cook RJ, Gladman DD, Pericak D, Urowitz MB (2000) Prediction of short term mortality in systemic lupus erythematosus with time dependent measures of disease activity. J Rheumatol 27:1892–1895
Rovenský J, Tuchynová A (2008) Systemic lupus erythematosus in the elderly. Autoimmun Rev 7:235–239
Pu SJ, Luo SF, Wu YJ, Cheng HS, Ho HH (2000) The clinical features and prognosis of lupus with disease onset at age 65 and older. Lupus 9:96–100
Lazaro D (2007) Elderly-onset systemic lupus erythematosus: prevalence, clinical course and treatment. Drugs Aging 24:701–715
Ward MM, Pyun E, Studenski S (1996) Mortality risks associated with specific clinical manifestations of systemic lupus erythematosus. Arch Intern Med 156:1337–1344
Abu-Shakra M, Urowitz MB, Gladman DD, Gough J (1995) Mortality studies in systemic lupus erythematosus. Results from a single center. II. Predictor variables for mortality. J Rheumatol 22:1265–1270
Ward MM, Pyun E, Studenski S (1995) Long-term survival in systemic lupus erythematosus. Patient characteristics associated with poorer outcomes. Arthritis Rheum 38:274–283
Gladman DD (1996) Prognosis and treatment of systemic lupus erythematosus. Curr Opin Rheumatol 8:430–437
Brunner HI, Silverman ED, To T, Bombardier C, Feldman BM (2002) Risk factors for damage in childhood-onset systemic lupus erythematosus: cumulative disease activity and medication use predict disease damage. Arthritis Rheum 46:436–444
Zonana-Nacach A, Barr SG, Magder LS, Petri M (2000) Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum 43:1801–1808
Acknowledgments
All of the authors would like to express their thanks to Prof. med. Georg Schett for his expert assistance in preparation of the manuscript.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, H., Wei, J.CC., Tan, Cy. et al. Survival analysis of late-onset systemic lupus erythematosus: a cohort study in China. Clin Rheumatol 31, 1683–1689 (2012). https://doi.org/10.1007/s10067-012-2073-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-012-2073-6