Abstract
Clinical guidelines regarding anti-viral prophylaxis for HBV surface antigen (HBsAg) carriers starting anti-TNFα agents are not yet fully established, even in endemic regions of HBV infection. We retrospectively collected the clinical data of 52 HBsAg carriers with rheumatoid arthritis (RA) or ankylosing spondylitis (AS) that had been administered anti-TNFα treatment at seven medical centers in South Korea. Periodic data of liver function tests and serum HBV DNA were both utilized to assess HBV reactivation. The YMDD motif mutation of HBV DNA polymerase was tested in lamivudine-treated patients with elevated HBV DNA. Three of the 52 patients were excluded from the analysis. Of the 49 analyzed patients, 20 patients received anti-viral prophylaxis (15 lamivudine, five entecavir) with anti-TNFα treatment. The remaining 29 patients were treated with anti-viral agents if needed at the discretion of the clinician and did not receive prophylaxis. Of the 29 patients who did not receive primary prophylaxis, two (6.9%) developed viral reactivation within a year of anti-TNFα treatment. In the prophylaxis group, one patient developed viral reactivation at week 64 of anti-TNFα therapy attributed to YMDD mutation caused by lamivudine. Patients with HBV reactivation all responded well to anti-viral therapy. In summary, anti-viral prophylaxis helped preventing HBV reactivation in HBsAg carriers with RA or AS starting anti-TNFα, yet mutation in the YMDD motif of HBV DNA polymerase could be detrimental to some patients under long-term lamivudine prophylaxis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-TNFα agents are widely used to treat inflammatory arthritides, such as rheumatoid arthritis (RA) and ankylosing spondylitis (AS). More than 60% of RA or AS patients achieve a good clinical response to anti-TNFα treatment. However, TNFα is also an important mediator that contributes to the normal host immune response against infectious agents [1]. Opportunistic infections, such as mycobacterial, other bacterial, and fungal infections are of concern when anti-TNFα agents are administered. Furthermore, screening for latent tuberculosis is mandatory prior to anti-TNFα treatment, especially in endemic regions [2].
Hepatitis B virus (HBV) can result in a latent stage of chronic infection, and thus, chronic hepatitis B patients may become inactive carriers following the immune active or clearance phases of the virus. HBV reactivation or flares have been witnessed during the latent stage of HBV infection in the setting of immunosuppressive treatment [3, 4], and in particular, a number of reports have described HBV reactivation after anti-TNFα treatment in chronic hepatitis B and inactive HBsAg carriers or even in hepatitis B core antibody (HBcAb) positive patients [5–12]. Furthermore, it has been reported that the ability of the host to mount an immune response and to clear HBV is impaired by the neutralization of TNFα [13]. It has also been reported that reactivation of HBV infection may occur directly due to lack of TNFα or indirectly via diminishing T cell activation [14, 15]. Nevertheless, clinical guidelines have not been fully established concerning the requirement of anti-viral prophylaxis when initiating anti-TNFα treatment in this subset of patients. Accordingly, our aim was to investigate the clinical characteristics and outcomes in HBV carriers receiving anti-TNFα therapy in Korea.
Materials and methods
Patient selection
We retrospectively reviewed medical records at seven centers nationwide to search for HBsAg carriers with RA or AS who had been treated with an anti-TNFα agent between January 2006 and March 2009. Patients fulfilled the ACR classification criteria for RA [16] and the modified New York criteria for AS [17]. Selected patients were required to have an AST and ALT within twice their normal limits when starting anti-TNFα treatment irrespective of HBeAg or HBV DNA titer. Patients that started an anti-viral agent before or within 6 months of starting anti-TNFα treatment were assigned to the prophylaxis group.
Definition of HBV reactivation
Periodic liver function test and serum HBV DNA were utilized to assess HBV reactivation, which was defined as: (1) a 10-fold rise in HBV DNA compared with baseline resulting in HBV DNA greater than 20,000 IU/ml (HBeAg-positive patients) or 2,000 IU/ml (HBeAg-negative patients), and (2) increase in AST or ALT to above twice the upper normal limit (40 IU/l) [18]. Long-term lamivudine users were tested for mutation in the YMDD motif of HBV polymerase. Anti-viral agent used for prophylaxis or treatment was determined by attending clinicians.
Statistical analysis
SPSS 17.0 was used for comparing mean values of age, ALT levels and gender ratio between the two study groups.
Results
Baseline characteristics
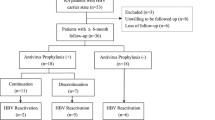
Among the 52 patients enrolled, three were excluded from the analysis (two for a high baseline transaminase level, and one for death due to hepatocellular carcinoma) (Fig. 1). Twenty-nine patients began anti-TNFα treatment without anti-viral prophylaxis (the non-prophylaxis group). Of the 20 patients in the prophylaxis group, 17 began anti-viral prophylaxis (12 lamivudine, five entecavir) with anti-TNFα. In one patient, lamivudine was initiated 6 months after starting etanercept. Two patients had been treated with lamivudine for 15 and 24 months, respectively, before initiating etanercept. Median durations of anti-TNFα treatment in the non-prophylaxis and prophylaxis groups were 52 and 60 weeks, respectively (Table 1). On beginning the anti-TNFα agent, any oral disease modifying anti-rheumatic drug was completely discontinued in patients with AS, and 80% (16/20) in those with RA.
Incidence of HBV reactivation
Of the 29 patients in the non-prophylaxis group, two (6.9%) developed viral reactivation within a year of commencing anti-TNFα treatment (Table 2). Patient 1 was a 43-year-old male with AS who was an inactive HBsAg carrier when etanercept was started. After 24 weeks of etanercept treatment, his ALT rose to 742 IU/l, and his HBV DNA titer was 1.51×107 IU/ml. Etanercept was discontinued and entecavir was initiated. Transaminitis normalized and HBV DNA became undetectable after 36 weeks of anti-viral treatment. Patient 2 was a 31-year-old male with AS who was treated with infliximab. After 14 weeks of treatment, his ALT rose to 1054 IU/l, and his HBV DNA titer was 3.13 × 106 IU/ml. Entecavir was started after discontinuing infliximab, and his laboratory findings returned to baseline 24 weeks after entecavir treatment.
In the prophylaxis group, one patient developed viral reactivation (5.0%) after 64 weeks of anti-TNFα therapy. This patient was a 32-year-old male with a baseline HBV DNA titer above 20,000 IU/ml who had been treated with lamivudine for 15 months prior to starting etanercept. At week 64, transaminitis was detected with a HBV DNA titer of 1.72 × 107 IU/ml. Mutation in the YMDD motif of HBV polymerase was detected. An additional case of YMDD mutation occurred in a patient under lamivudine prophylaxis. The 37-year-old male AS patient had an elevated HBV DNA (2.0 × 107 IU/ml) without combined transaminitis after 48 weeks on etanercept. Lamivudine was switched to entecavir and his HBV DNA titer gradually decreased. No case of reactivation occurred among the five patients who received entecavir as primary prophylaxis during anti-TNFα therapy (median duration 36.7 weeks)
There were five patients (2/29 in the non-prophylaxis group and 3/20 in the prophylaxis group) who had an episode of HBV DNA elevation without combined transaminitis. Table 3 shows the baseline characteristics and the time point of elevation of the HBV DNA level after starting anti-TNFα treatment in these patients. The time points of HBV DNA elevation were all within 1 year of treatment.
Discussion
It has been reported that approximately one third of the world’s population has been infected by HBV, and that about 5% of the infected population develop a chronic HBV infection [13]. Seventy five percent of subjects with chronic HBV infection reside in Southeast Asia and the Western Pacific region. The prevalence of HBsAg carriers in the South Korean population is about 3.7% [19], which is higher than the ~1% of RA. Previous reports regarding the outcome of non-viral prophylaxis in anti-TNFα users with chronic hepatitis B span the disease spectrum from asymptomatic carriers to fulminant hepatitis [20, 21].
The present nationwide retrospective study of 49 chronic hepatitis B patients who started anti-TNFα agents had a viral reactivation rate of 6.9% in patients not provided with anti-viral prophylaxis. Furthermore, viral reactivation was discovered in one of 20 patients (5.0%) concomitantly taking lamivudine or entecavir. This single case was in fact owing to lamivudine-induced mutation of the YMDD motif of HBV polymerase, in other words resistance to the anti-viral agent, not by concomitant anti-TNFα therapy. YMDD mutation develops in 15–30% per year of HBV patients on lamivudine prophylaxis [22]. A recent prospective study demonstrated that the YMDD mutation is a major cause of HBV reactivation in patients treated with anti-TNFα agents and lamivudine [23]. This prospective study included a cohort of 14 chronic hepatitis B patients that were treated with anti-TNFα agents under anti-viral prophylaxis. One case of viral reactivation due to the YMDD mutation after the long-term use of lamivudine occurred, as in the present study. It appears that anti-TNFα treatment does not shorten the onset of YMDD mutation by lamivudine based on our findings.
None of the five patients who received entecavir as primary prophylaxis in the present study developed viral reactivation during anti-TNFα treatment (median duration 36.7 weeks). In a recent study, it was noted that entecavir resistance is seldom discovered in nucleoside analogue-naïve chronic hepatitis B patients within 2 years of treatment [24]. Thus, as anti-TNFα agents are normally used for prolonged periods in RA and AS patients, entecavir or adefovir may be a better choice for anti-viral prophylaxis in chronic hepatitis B patients starting an anti-TNFα agent [25].
The definition of viral reactivation used in the present study included a significant HBV DNA increase and hepatocellular damage, whereas in other studies only one of these criteria was used to define viral reactivation during anti-TNFα treatment [6, 7]. However, the presence of transaminitis alone is insufficient to claim viral reactivation. Furthermore, an increased viral DNA titer alone may be associated with the immune tolerance phase of the HBV DNA life cycle, especially during the earlier stage of HBV infection. The relative risks by individual anti-TNFα agents to HBV reactivation are unknown. Infliximab has been cited in more case reports than other agents [5, 7, 20]. The present study shows that 5.3% (1/19) of etanercept, 0% (0/4) of adalimumab, and 16.7% (1/6) of infliximab treated patients that did not receive anti-viral prophylaxis developed viral reactivation. However, the three anti-TNFα agents were not evenly distributed among the patients included in this study. Further studies are required to determine the risks of viral reactivation posed by anti-TNFα agents.
The present study has several limitations that warrant mention. First, missing baseline HBV DNA levels or transaminase levels at certain time points in this retrospective analysis could cause underestimations of the true level of viral reactivation in our patient population. Second, clinicians treating patients without anti-viral prophylaxis are likely to respond readily to laboratory changes. For example, two cases in the non-prophylaxis group were treated with lamivudine when an increase in HBV DNA level was noted. Overall, elevated HBV DNA level, regardless of liver enzyme levels, was found in 13.8% (4/29) and 20.0% (4/20) of the patients in the non-prophylaxis and prophylaxis groups, respectively, and these percentages are higher than the cumulative incidence of 10.6% mentioned in a national report, which also showed that inactive HBsAg carriers can develop high HBV DNA titers within 18 months of follow up [26]. All patients in the prophylaxis group with a high HBV DNA titer received lamivudine long-term (median duration of 69 weeks). Third, the median duration of anti-TNFα agents (52 weeks in the non-prophylaxis group and 60 weeks in the prophylaxis group) may have underestimated the true incidence of viral reactivation in this group of patients that usually needs longer term of anti-TNFα treatment. Lastly, this study did not include HBsAg negative, anti-HBcAb positive patients, which population could also be in risk for HBV reactivation with anti-TNFα treatment [10–12].
Time points of HBV reactivation may not be confined to the period of anti-TNFα treatment. It has been reported that HBV reactivation is prone to develop after discontinuing immunosuppression [27]. Thus, future prospective studies should include additional laboratory monitoring in these patients even after discontinuing the anti-TNFα agent. A guideline proposed by Nathan et al. recommends initiating anti-viral prophylaxis 1–2 weeks prior to starting an anti-TNFα agent and that the prophylaxis be continued until 3 months after discontinuation [28]. They also proposed a preemptive strategy of initiating anti-viral therapy when an increase in ALT or in HBV DNA levels occurs during anti-TNFα therapy. Without specific guidelines, it is prudent that rheumatologists consult specialists to assess liver and virological conditions in HBV carriers before starting anti-TNFα treatment.
In summary, the rate of viral reactivation in HBV carriers receiving anti-TNFα therapy without anti-viral prophylaxis was 6.9% among our study population. Viral reactivation in anti-TNFα users was well protected with anti-viral prophylaxis, although YMDD mutation should be considered when treating with lamivudine long-term. In general, regular monitoring of liver function and viral titers are important for HBV carriers receiving anti-TNFα therapy, even for those under anti-viral prophylaxis. Prospective controlled studies would be necessary to establish a practical guideline for viral prophylaxis in this subset of patients.
References
Raychaudhuri SP, Nguyen CT, Raychaudhuri SK et al (2009) Incidence and nature of infectious disease in patients treated with anti-TNF agents. Autoimmun Rev 9:67–81
Keane J, Gershon S, Wise RP et al (2001) Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345:1098–1104
Gupta S, Govindarajan S, Fong TL et al (1990) Spontaneous reactivation in chronic hepatitis B: patterns and natural history. J Clin Gastroenterol 12:562–568
Perrillo RP (2001) Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 120:1009–1022
Esteve M, Saro C, Gonzalez-Huix F et al (2004) Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut 53:1363–1365
Sakellariou GT, Chatzigiannis I (2007) Long-term anti-TNFalpha therapy for ankylosing spondylitis in two patients with chronic HBV infection. Clin Rheumatol 26:950–952
Carroll MB, Bond MI (2008) Use of tumor necrosis factor-alpha inhibitors in patients with chronic hepatitis B infection. Semin Arthritis Rheum 38:208–217
Ojiro K, Naganuma M, Ebinuma H et al (2008) Reactivation of hepatitis B in a patient with Crohn’s disease treated using infliximab. J Gastroenterol 43:397–401
Chung SJ, Kim JK, Park MC et al (2009) Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-alpha therapy. J Rheumatol 36:2416–2420
Kim YJ, Bae SC, Sung YK et al (2010) Possible reactivation of potential hepatitis B virus occult infection by tumor necrosis factor-alpha blocker in the treatment of rheumatic diseases. J Rheumatol 37:346–350
Lan JL, Chen YM, Hsieh TY et al (2011) Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis 70:1719–1725
Tamori A, Koike T, Goto H et al (2011) Prospective study of reactivation of hepatitis B virus in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts. J Gastroenterol 46:556–564
Ganem D, Prince AM (2004) Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med 350:1118–1129
Guidotti LG, Ishikawa T, Hobbs MV et al (1996) Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25–36
Guidotti LG, Ando K, Hobbs MV et al (1994) Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci U S A 91:3764–3768
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Hoofnagle JH (2009) Reactivation of hepatitis B. Hepatology 49:S156–S165
Korea Centers for Disease Control and Prevention (2007) The Fourth Korea National Health and Nutrition Examination Survey (KNHANES III), pp 70–71
Kuwabara H, Fukuda A, Tsuda Yet al (2010) Precore mutant hepatitis B virus-associated fulminant hepatitis during infliximab therapy for rheumatoid arthritis. Clin Rheumatol
Michel M, Duvoux C, Hezode C et al (2003) Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset Still’s disease. J Rheumatol 30:1624–1625
Dienstag JL (2008) Hepatitis B virus infection. N Engl J Med 359:1486–1500
Vassilopoulos D, Apostolopoulou A, Hadziyannis E et al (2010) Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Annals Rheum Dis 69:1352–1355
Colonno RJ, Rose R, Baldick CJ et al (2006) Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 44:1656–1665
Lok AS, McMahon BJ (2007) Chronic hepatitis B. Hepatology 45:507–539
Kim ES, Seoh YS, Lee KG et al (2008) Hepatitis B virus DNA level is a significant predictive factor for hepatitis B virus reactivation in inactive HBs antigen carriers. Korean J Hepatol 14:88
Liaw YF (1998) Hepatitis viruses under immunosuppressive agents. J Gastroenterol Hepatol 13:14–20
Nathan DM, Angus PW, Gibson PR (2006) Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol 21:1366–1371
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, South Korea (#A102065). All authors declare that they have no proprietary, commercial, or financial interests that could be construed to have inappropriately influenced this study.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, H.H., Lee, E.Y., Shin, K. et al. Hepatitis B virus reactivation in rheumatoid arthritis and ankylosing spondylitis patients treated with anti-TNFα agents: A retrospective analysis of 49 cases. Clin Rheumatol 31, 931–936 (2012). https://doi.org/10.1007/s10067-012-1960-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-012-1960-1