Abstract
The purpose of this study is to determine the diagnostic properties of the clinical gout diagnosis (CGD) proposal in patients with gout and other rheumatic diseases. We investigated the presence of current or past history of the previously published CGD criteria: (1) >1 attack of acute arthritis, (2) mono/oligoarthritis attacks, (3) rapid progression of pain and swelling (<24 h), (4) podagra, (5) erythema, (6) unilateral tarsitis, (7) probable tophi, and (8) hyperuricemia. CGD was established in patients with greater than or equal to four out of eight of these criteria. Demographic data and comorbidities were also considered. Statistical analysis included diagnostic test evaluation (sensitivity, specificity, likelihood ratios, positive predictive values and receiving operating characteristic curves). One hundred and sixty-seven patients with the following diagnoses were included: gout (most in intercritical period, n = 75), rheumatoid arthritis (RA, n = 30), osteoarthritis (OA, n = 31) and spondyloarthritis (SpA, n = 31). All gout patients had MSU crystal demonstration and constituted the gold standard for diagnostic test evaluation. There were significant differences across diagnostic groups in most demographic variables and comorbidity. The presence of greater than or equal to four out of eight of the CGD criteria were found in 97% patients with gout, in two patients with SpA, and one each with RA and OA. The sensitivity, specificity, and LR+ of greater than or equal to four out of eight of the CGD criteria were 97.3%, 95.6%, and 22.14, respectively. The presence of more than or equal to four out of eight items from the CGD proposal is highly suggestive of gout.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Gout is a rheumatic disease characterized by episodes of acute arthritis and chronic symptoms including tophi, which results from the deposition of monosodium urate (MSU) crystals in the synovial membrane and in other tissues. The diagnosis of gout may be established independently if the patient in that moment is in the acute or the intercritical phase of the disease; the “gold standard” for such diagnosis is the identification of MSU in the joints or tophi [1].
MSU crystals are usually identified in the synovial fluid of inflamed joints. However, they may also be found in the synovial fluid of non-inflamed asymptomatic joints from patients with gout, including both those with and those without previous inflammation [2] and even in individuals with asymptomatic hyperuricemia [2, 3]. MSU have been identified in extra-articular sites such as the subcutaneous tissue, skin, kidneys, eyes, gastric mucosa, and colon [4–6].
There are enough data to consider that MSU crystal deposits may sometimes occur years before the first acute attack [2–6], but gout diagnosis should be considered in patients with at least one acute attack.
Despite its diagnostic value, the search for MSU is not regularly performed in daily outpatient clinics. Therefore, probable diagnosis of gout relies on clinical findings, particularly in those listed in the American College of Rheumatology (ACR, formerly ARA) diagnostic criteria for acute gout [7], which are widely used for the diagnosis of acute and chronic gout. Interestingly, ACR criteria were issued as “preliminary,” and include clinical and radiographic data of chronic disease [8]. On the other hand, in 2006, a group of experts representing the European League Against Rheumatism Standing Committee for International Clinical Studies Including Therapeutics proposed evidence-based recommendations for diagnosis of gout [9]. Several reports have referred to the poor performance of the ACR criteria for the diagnosis of gout [8] and have therefore issued further definitions for acute gout [10–12].
In this sense, we have previously [13] looked for items from ACR and The European League Against Rheumatism (EULAR) proposals and determined their frequency in a multicentric group of patients with gout. We identified eight criteria and a cutoff of four for establishing clinical gout diagnosis (CGD). Regardless of MSU identification, the diagnostic usefulness of our proposal seemed better than that of the ACR criteria.
In the present study, we aimed to determine the diagnostic value of CGD criteria in patients with gout compared to other rheumatic diseases.
Patients and methods
This is a diagnostic test study evaluating the diagnostic properties of CGD criteria in consecutive outpatients, referred by general physicians because of any type of arthritis, including gout, to two rheumatology departments in México City. The protocol of this investigation was approved by the local ethic and research institutional review committee. All study participants were informed about the nature of the study and accepted their collaboration by signing an informed consent.
Diagnostic groups
The study included consecutive patients with the diagnoses of rheumatoid arthritis (RA) [14], osteoarthritis (OA) [15, 16], spondyloarthritis (SpA) [17], and gout [13]. To be diagnosed as gout, all patients must have MSU crystal demonstration. This group of patients constituted the gold standard for the diagnostic test evaluation. Patients with disease onset before the age of 25 years were classified as young-onset gout; the diagnosis of secondary gout was considered in patients with any disease or condition associated with gout (as chronic renal failure or hematologic conditions) and diagnosed before the first acute attack.
Clinical data
Data were obtained during the patients’ regular visit to the clinic and by reviewing their clinical records. We investigated the presence of current/past history of CGD criteria, specifically: (1) >1 attack of acute arthritis, (2) mono/oligoarthritis attacks, (3) rapid progression of pain and swelling (<24 h), (4) podagra, (5) erythema, (6) unilateral tarsitis, (7) probable tophi, and (8) hyperuricemia. CGD was established in patients with greater than or equal to four out of eight of these criteria. We also investigated if MSU crystals were sought in synovial fluid or tophi by polarized light microscopy as well as if bacteriologic or radiographic studies were performed.
We also registered demographic data and comorbidities such as obesity (waist circumference ≥102 cm in men and ≥88 cm in women); systemic hypertension (≥130/85 or treatment specific for it); dyslipidemia (high-density lipoprotein ≥40 mg/dL in men or ≥50 mg/dL in women and triglycerides ≥150 mg/dL); hyperglycemia as fasting glucose ≥110 mg/dL or diabetes mellitus [19] diagnosis or specific treatment for it, all of them as defined in the Adult Treatment Panel III criteria for metabolic syndrome [18]. Metabolic syndrome was defined by the presence of at least 3/5 ATP III criteria. Hyperuricemia was considered when serum uric acid values were >7 mg/dL for men and >6 mg/dL for women. Chronic renal failure was considered in previously diagnosed patients or 24-h adjusted urinary creatinine clearance <50 mL/min; ischemic heart disease in patients with a previous diagnosis of ischemia, myocardial infarction, and angina pectoris requiring specific treatment and diagnosed by a physician. The diagnosis of lithiasis was made in patients previously diagnosed. Janssens’ proposed score includes: male gender, previous arthritis, onset within 1 day, joint redness, MTP1 involvement, hypertension, or cardiovascular disease and serum uric acid >5.88 mg/dL; each data has a variable score and the sum of ≥8 is considered the best [12].
Statistical analysis
Continuous variables were expressed as mean ± SD, median, and range. Binomial variables were expressed as frequency and percentages. Chi-square, ANOVA, and t test were used for the comparison of demographic and clinical variables between diagnoses included in Table 1. The correlation of CGD criteria and Janssens’ proposal was calculated with Pearson correlation and kappa values; diagnostic test evaluation (sensitivity, specificity, likelihood ratios [LR], positive predictive values, and receiving operating characteristic (ROC) curves) was calculated with standard procedures.
Results
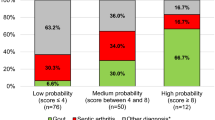
We included 75 patients with gout, all of them with MSU crystal demonstration that were mostly in the intercritical phase of the disease. Eighteen were female, 13 were less than 25 years old at disease onset, eight had gout secondary to chronic renal failure or hematologic conditions. Thirty patients were included in the group of RA, 31 in the OA, and 31 in the group of SpA (including 13 with undifferentiated SpA, eight with ankylosing spondylitis, and ten with psoriatic arthritis) (Table 1). As expected, there were significant differences between the groups in most demographic aspects. Both gout and SpA were more frequently seen in males, whereas the proportion of females was much higher in the groups of RA and OA. The mean age of patients with gout and OA was higher than that of RA and SpA. Metabolic syndrome and related diseases were more frequently found in the group of patients with gout. Regarding the CGD criteria, the mean number of items fulfilled by patients with gout was above 5, whereas the number fulfilled by RA, SpA, and OA patients was 1.3, 2.3, and 0.6, respectively (Table 1). Interestingly, the prevalence of tarsitis in patients with gout was as high as in patients with SpA—most frequently seen in young males. Likewise, the percentage of patients with gout fulfilling the definition of CGD (four of eight positive criteria) was above 90%, whereas the percentage reached by RA and OA was only 3% and 6.5% for SpA. CGD diagnostic criteria properties are shown in Table 2. Sensitivity and specificity values were 97.3% and 95.6%, respectively when four out of eight criteria were fulfilled; the positive likelihood ratio (LR+) was 22.14 and the AUC–ROC was 0.965. As expected, specificity and LR+ were higher when five out of the eight criteria were met, but sensitivity and AU–ROC were slightly lower. Individually, rapid onset of pain and swelling (<24 h) and hyperuricemia were the criteria with the best diagnostic properties (Fig. 1).
To determine the diagnostic value of CGD proposal in patients with gout under special circumstances as women, early onset and secondary gout, we included all patients with such diagnoses seen in that period. As expected, they were different in demographic and associated diseases; the mean CGD criteria found in them was 5.22 ± 1.35 in females, 7.0 ± 1.22 in young onset, and 5.4 ± 1.59 in secondary gout. According to our cutoff points, all the patients with early onset gout, 94.4% of females and in 87.5% of those with secondary gout fulfilled ≥4 CGD criteria. Hyperuricemia and current or past history of mono- or oligoarticular attacks were the items most frequently found in all gout groups (97–100%).
Finally, we calculated how many of our patients with gout according to CGD criteria fulfilled the proposed Janssens score [12]. Overall, 90% of our gout patients scored ≥8 in Janssens’ diagnostic rule, the Pearson’s lineal correlation between Janssens score and number of CGD criteria was r = 0.95 (p = <0.001) and kappa value was 0.85.
Discussion
This study demonstrates the usefulness of the criteria for the diagnosis of chronic gout that we have recently proposed [13]. Indeed, 3 items included in CGD had positive LR higher than ten and 2 between seven and ten. It also shows the appropriateness of the four out of eight criteria cutoff level (sensitivity of 97.3, specificity of 95.6, and LR+ of 22.14). The values of the CGD criteria are also supported by the fact that they were initially derived from the ACR criteria [7] and EULAR recommendations [8] and on the other hand, are consistent with five of the seven items of Janssens et al.’s score [12]. Thus, the diagnostic properties of our criteria are probably acceptable for CGD. CGD may be an appropriate alternative for the working diagnosis of gout, in settings without the possibility of searching for MSU crystals from primary health clinics and even some specialized departments worldwide. The possibility to demonstrate MSU crystals in daily outpatient clinics is highly desirable but not very common worldwide.
As done in our original CGD proposal, we did not consider radiographic findings—specifically those included in the ACR proposal—because they are nonspecific data, and the typical radiographic data of gout are seen only in long-term gout patients. In contrast, it is possible that other imaging methods, such as, ultrasound and magnetic resonance should be considered if available.
After the first episode of acute arthritis, the course of gout is characterized by recurrent episodes of arthritis; without proper treatment, the number of episodes of arthritis, their intensity, and the number of affected joints increase in parallel with a progressive shortening of the intercritical periods. In our opinion, the diagnosis of clinical gout should be considered in patients with more than one episode of rapid (<24 h) acute, mono or oligoarticular, pain, erythema, and swelling at any time during the course of the disease. It seems that, although initially not evident at physical examination, MSU crystals are deposited—microscopic tophi—before as well as at the time of the first episode of arthritis [3]; several years later, bigger MSU crystal deposits—or tophi—may be clinically recognized.
Similar to our previous work [13], two recent studies determined the usefulness of demographic, clinical, and auxiliary data for the diagnosis of gout. In the study by Janssens et al. [12], patients with acute monoarthritis seen by general physicians were referred to the rheumatologist for clinical examination and MSU crystal search. Through a very nice and elegant analysis of the data, specific weights were assigned to a number of variables before selecting seven criteria and a cutoff point of eight, which best fitted with the diagnosis of gout. Five of such seven criteria were already included in our CGD proposal and have high concordance with Janssens et al.’s score [12]. Male gender and associated cardiovascular diseases were the two variables in Janssens et al.’s score [12] that were not included in the CGD criteria. While most patients with gout are male and cardiovascular diseases are frequent, they are associated conditions—and not manifestations—of gout itself.
In a recent French study [20], general physicians and rheumatologists collected information on 1,003 patients—around half of them with acute arthritis and the clinical suspicion of gout—and looked for the frequency of both ACR criteria and EULAR recommendations for the diagnosis of gout (MSU crystals were identified in 84 out of 1,003). Eighty-six percent of the patients had six of 11 ACR criteria, 89.1% fulfilled the first EULAR recommendation [9]: “Rapid development of severe pain, swelling and tenderness that reaches its maximum with just 6–12 h, especially with overlying erythema” and 92.5% the same recommendation if erythema was excluded. All characteristics in such recommendation are part of our CGD proposal.
Our study is limited by the fact that our patients were seen in rheumatology departments and therefore, may be different from those seen in primary health clinics. Long-term, chronic tophaceous gout is usually seen in the former and short course, acute gout in the latter. The high concordance of CGD with previous criteria [7], recently published proposals [9], and a score [12] suggest that CGD could be useful in primary health clinics although their diagnostic properties in these scenarios need to be determined, as well as their usefulness in other crystal arthropathies as CPPD.
In patients in whom, long time urate-lowering therapy will be indicated, the reason for it should be clearly documented (UMS crystal proved gout always when possible), although nowadays, this therapy is frequently prescribed by several physicians to patients with hyperuricemia and high cardiovascular risk.
In conclusion, CGD criteria, specifically the presence of four of eight criteria are highly sensitive and specific for the diagnosis of gout and are easy to use in daily clinical practice, GP practice, rural areas, and even some specialized departments worldwide. We acknowledge that the diagnosis of gout should be confirmed whenever possible by the demonstration of MSU crystals, but CGD seems to represent an accessible way to diagnose clinical gout in centers without MSU crystals identification facilities.
References
McCarthy DJ, Hollander JL (1961) Identification of urate crystals in gouty synovial fluid. Ann Intern Med 54:452–460
Pascual E, Batlle-Gualda E, Martínez A, Rosas J, Vela P (1999) Synovial fluid analysis for diagnosis of intercritical gout. Ann Intern Med 131:756–759
Rouault T, Caldwell DS, Holmes EW (1982) Aspiration of the asymptomatic metatarsophalangeal joint in gout patients and hyperuricemic controls. Arthritis Rheum 25:209–212
Carter JD, Kedar RP, Anderson SR, Osorio AH, Albritton NL, Gnanashanmugam S, Valeriano J, Vasey FB, Ricca LR (2009) An analysis of MRI and ultrasound imaging in patients with gout who have normal plain radiographs. Rheumatology (Oxford) 48:1442–1446
Choi HK, Al-Arfaj AM, Eftekharis A, Munk P, Shojania K, Reid G, Nicolaou S (2009) Dual energy computed tomography in tophaceous gout. Ann Rheum Dis 68:1609–1612
Puig JG, de Miguel E, Castillo MC, Rocha AL, Martínez MA, Torres RJ (2008) Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids 27:592–595
Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF (1997) Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 20:895–900
Malik A, Schumacher HR, Dinnella JE, Cayburne GM (2009) Clinical diagnostic criteria for gout. J Clin Rheumatol 15:22–24
Zhang W, Doherty M, Pascual E, Bardin T, Barskova V, Conaghan P et al (2006) EULAR evidence based recommendations for gout. Part I: diagnosis. Report of a task force of the standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis 65:1301–1311
Taylor WJ, Schumacher HR Jr, Baraf HS, Chapman P, Stamp L, Doherty M, McQueen F, Dalbeth N, Schlesinger N, Furst DE, Vazquez Mellado J, Becker MA, Kavanaugh A, Louthrenoo W, Bardin T, Khanna D, Simon LS, Yamanaka H, Choi HK, Zeng X, Strand V, Grainger R, Clegg D, Singh JA, Diaz-Torne C, Boers M, Gow P, Barskova VG (2008) A modified Delphi exercise to determine the extent of consensus with OMERACT outcome domains for studies of acute and chronic gout. Ann Rheum Dis 67:888–891
Janssens HJ, Janssen M, van de Lisdonk EH, Fransen J, van Riel PLCM, van Weel C (2010) The limited validity of the criteria of the American College of Rheumatology for classifying gout patients in primary care. Ann Rheum Dis 69:1255–1256
Janssens HJ, Fransen J, van de Lisdonk EH, van Riel PL, van Weel C, Janssen M (2010) A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med 170:1120–1126
Peláez Ballestas I, Hernández-Cuevas C, Burgos Vargas R, Hernández Roque L, Terán L, Espinoza J, Esquivel-Valerio JA, Goycochea Robles MV, Aceves FJ, Bernard AG, Ventura L, Shumsky C, Hérnandez Garduño A, Vázquez-Mellado J (2010) Diagnosis of chronic gout: evaluating the American College of Rheumatology proposal and European League against Rheumatism recommendations and clinical judgement. J Rheumatol 37:1743–1748
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Altman R, Alarcón G, Appelrouth D et al (1990) The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 33:1601–1610
Altman R, Asch E, Bloch G et al (1986) Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 29:1039–1049
Dougados M, van der Linden S, Juhlin R et al (1991) The European Spondyloarthropathy Study Group preliminary criteria for the classification of spondyloarthropathy. Arthritis Rheum 34:1218–1227
Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert (2001) Panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285:2486–2497
The Expert Committee on the diagnosis and classification of Diabetes Mellitus (1999) Report of the Expert Committee on the diagnosis and classification of Diabetes Mellitus. Diabetes Care Suppl 2:4–19
Lioté F, Ea HK, Saraux A, Guggenbhul P, Aubert JP, Lantz S, Lambert C, Chiarelli P, Delva C, Lancrenon S (2010) A prospective survey of 1003 patients with gout: comparison between general practitioners and rheumatologists. Concordance of EULAR gout diagnostic criteria. The GOSPEL1000 study. Ann Rheum Dis Suppl 3:OP0197
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vázquez-Mellado, J., Hernández-Cuevas, C.B., Alvarez-Hernández, E. et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol 31, 429–434 (2012). https://doi.org/10.1007/s10067-011-1873-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1873-4