Abstract
The objectives of the study were to compare antibody response in immunosuppressed patients with rheumatoid arthritis (RA) after vaccination with heptavalent pneumococcal conjugate vaccine (PCV7) to that of RA patients and healthy controls vaccinated with 23-valent polysaccharide vaccine (PPV23) and to study the impact of disease and/or treatment characteristics and type of vaccine on antibody response following pneumococcal vaccination in patients with RA. In total, 253 RA patients treated with methotrexate (MTX), anti-TNF blockers as monotherapy or anti-TNF + MTX were vaccinated with a single dose (0.5 ml) of PCV7. In addition, 149 RA patients receiving corresponding treatments and 47 healthy controls were vaccinated with a single dose (0.5 ml) of PPV23. Serotype-specific IgG to 23F and 6B were measured at vaccination and 4–6 weeks after vaccination using ELISA. Antibody response ratio (ARR), i.e. ratio between post-/prevaccination antibody levels, was compared between corresponding treatment groups. Differences in ARR were analysed using analysis of variance. Positive antibody response (posAR) was defined as equal to or greater than twofold increase in prevaccination antibody levels. Possible predictors of posAR were analysed using logistic regression model. Corresponding RA treatment groups showed similar ARR and posAR for both serotypes regardless of vaccine type. Higher age at vaccination and concomitant MTX were identified as predictors of impaired posAR for both serotypes tested, whereas type of vaccine did not influence posAR significantly. PCV7 elicits similar antibody response as PPV23 in patients with RA receiving immunosuppressive treatment. In RA patients, higher age and MTX treatment but not type of vaccine predicted impaired posAR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pneumococcal vaccination using 23-valent polysaccharide vaccine (PPV23) is currently recommended to all individuals age 65 years or older and those receiving immunosuppressive drugs regardless of age [1, 2]. Bacterial infections, including those caused by pneumococci, are major causes of morbidity and mortality in patients with rheumatic diseases. Pneumococcal vaccination should therefore be considered in the majority of patients with inflammatory rheumatic diseases [3, 4]. In Sweden, 23-valent pneumococcal conjugate vaccine (Pneumovax®) is licensed for use in adults. However, clinical trials and meta-analysis of controlled clinical trials studying the efficacy of this unconjugated pneumococcal polysaccharide vaccine have shown conflicting results. The results from Cochrane meta-analysis of randomised clinical trials support the use of PPV23 for protection against invasive pneumococcal diseases in otherwise healthy adults, but the evidence of effectiveness in patients with chronic disease was less strong [5]. Another recently published meta-analysis of controlled clinical trials found little evidence for the effectiveness of PPV23 in reducing risk of all-cause pneumonia in older, chronically ill patients [6]. Furthermore, the immunogenicity of PPV23 is debated [7, 8]. Primary vaccination with PPV23 in generally healthy individuals has been shown to induce significant increase in antibody levels remaining higher compared with vaccine-naïve persons over a period of 5 years [7]. However, decreased antibody response has been reported in some target groups such as older or immunocompromised individuals [8, 9]. Our group previously studied antibody response following vaccination with PPV23 in patients with rheumatoid arthritis (RA) treated with different anti-inflammatory remedies. Antibody response was significantly reduced in RA patients receiving methotrexate (MTX) compared to those receiving anti-TNF remedies or healthy controls. We also found that less than 50% of patients developed positive immune response defined as a double increase in antibody levels [9]. Since PPV23 contains only free polysaccharide antigens, this vaccination mainly induces T cell-independent antibody response. It has been shown that in children, conjugation of these poorly immunogenic polysaccharide antigens to carrier protein gives rise to B cell stimulation resulting in antibody production or development into memory cells [10, 11]. Heptavalent pneumococcal conjugate vaccine (PCV7) was licensed for use in Sweden in 2009 and is included in the national vaccination programme for children but is not approved for immunization of adults. PCV7 has been shown to reduce not only the incidence of invasive pneumococcal disease by >90% in vaccinated children but also decrease the incidence of these infections in non-vaccinated children and adults due to “herd effect” [11, 12]. Data on potential advantage of PCV7 over the 23-valent polysaccharide vaccine in adults and particularly in patients with inflammatory rheumatic disease are sparse and inconclusive. Recently, our group reported antibody response following pneumococcal vaccination using heptavalent conjugate vaccine in patients with established RA and spondylarthropathy receiving different immunosuppressive treatments [13].
The objective of this study was to compare antibody response after vaccination with PCV7 vaccine among RA patients treated with immunomodulating drugs including MTX and anti-TNF remedies participating in our recent study [13] to that of RA patients receiving corresponding immunosuppressive treatment and healthy controls vaccinated with PPV23 [9]. In addition, we aimed to investigate whether disease or treatment characteristics and type of vaccine had an impact on antibody response following pneumococcal vaccination in patients with established RA.
Patients and methods
In total, 449 individuals participated in the study. Of these, 253 were patients with RA vaccinated with a single dose (0.5 ml) of 7-valent pneumococcal conjugate vaccine (Prevenar® intramuscularly) as previously described [13]. Additional 149 patients with RA and 47 healthy controls received a single dose of PPV23 (0.5 ml Pneumovax® intramuscularly) as previously reported [9]. Both PPV23 and PCV7 vaccinated RA patients were divided to three predefined corresponding treatment groups: RA on MTX alone and in some cases in combination with other disease modifying antirheumatic drugs (DMARDs) (n = 122), RA on anti-TNF treatment as monotherapy (n = 141) and RA on anti-TNF treatment combined with MTX (n = 139). The number of individuals in different treatment groups and for each vaccine separately is shown in Table 1. Ongoing anti-TNF treatments at vaccination were etanercept (n = 156), infliximab (n = 102) and adalimumab (n = 22). Levels of serotype specific IgG against pneumococcal polysaccharide serotypes 23F and 6B were measured at vaccination and 4–6 weeks after vaccination using standard ELISA according to the WHO protocol [14]. Antibody response ratio (ARR) was defined as ratio between post- and prevaccination antibody levels. Differences in immune responses between vaccines were compared for each serotype and between corresponding treatment groups. Positive antibody response (posAR) was defined as at least twofold increase in prevaccination antibody levels.
The ethical approval for this study was obtained from the Ethical Review Board at Lund University (file number 97/2007). Informed written consent was signed by each participant before the vaccination.
Statistical calculations
Differences in disease and treatment characteristics at vaccination between the groups were analysed using the χ 2 test and the Mann–Whitney U test when appropriate. Differences between post- and prevaccination antibody levels were compared using paired samples t test. Univariate analysis of variance (ANOVA) adjusted for age, gender, prevaccination antibody levels for each serotype, concomitant MTX and prednisolone treatment was used to compare ARR between the corresponding treatment groups. The impact of disease and treatment characteristics at vaccination and type of vaccine on posAR for both serotypes was performed using univariate binary logistic regression analysis. In order to study the possible predictors of posAR, a multivariate regression model with adjustment for baseline characteristics was created. A p value of <0.05 was considered significant.
Results
There were some differences in demographic and disease characteristics between corresponding treatment groups. At vaccination, RA patients treated with TNF blockers as monotherapy or those treated with TNF blockers + MTX vaccinated with PCV7 were older compared to equivalent treatment groups receiving PPV23. Healthy controls were significantly younger than RA patients regardless of treatment or type of vaccine. Disease and treatment characteristics at vaccination are shown in Table 1.
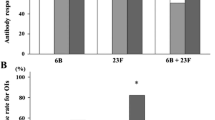
Both vaccinations resulted in significant increase in antibody levels for both serotypes compared to prevaccination antibody levels in each treatment group (p value between <0.001 and 0.035). ARR (median, range) in different groups and percentage of patients with posAR for each serotype separately and both serotypes are summarized in Table 2. Figures 1 and 2 show box plots of ARR between standard 23-valent polysaccharide vaccine and 7-valent conjugate vaccine. There were no statistical significant differences in ARR between corresponding treatment groups for neither 23F nor 6B serotype (p value between 0.079 and 0.946; ANOVA, adjusted for differences in age, gender and prevaccination antibody levels). No significant differences between corresponding treatment groups were found when posAR was used as outcome measure in the analysis.
The lowest proportion of patients with posAR was found in RA patients treated with MTX alone or MTX combined with anti-TNF drugs for both serotypes regardless of vaccination type (Fig. 3). Other differences in the antibody response as measured here between the two vaccination types were small and not statistically significant for any treatment group.
Proportion (in percent) of patients with positive antibody response (posAR) following vaccination with 23-valent pneumococcal polysaccharide vaccine and 7-valent pneumococcal conjugate vaccine for serotype 6B (a), 23F (b) and both 23F and 6B (c) in different groups of patients with RA and healthy controls
Possible predictors of posAR for both serotypes among all RA patients regardless of vaccine type were analysed using logistic regression model. In total, 402 RA of 449 vaccinated individuals were included in the analysis. Using univariate regression model, higher age (p = 0.030) and ongoing MTX treatment (p < 0.001) predicted impaired posAR for both serotypes whereas concomitant prednisolone (p = 0.002) and anti-TNF treatment (p = 0.006) predicted better posAR. Disease duration (p = 0.250), gender (p = 0.651) and RF status (p = 0.887) did not predict significantly posAR for any of the serotypes tested. After adjustment for demographics and baseline disease characteristics and antibody levels for both 23F and 6B in multivariate logistic regression model, higher age and MTX treatment remained predictors of impaired antibody response (Table 3). As shown in the table, patients with ongoing MTX treatment had only 36% chance to achieve positive antibody response compared to those not receiving MTX. Concomitant prednisolone treatment was a predictor of better posAR, while vaccine type (PCV7 compared to PPV23) and ongoing anti-TNF treatment had no significant impact on posAR for any of the serotypes.
Discussion
The present study reports of a comparison between immunogenicity of 7-valent pneumococcal conjugate vaccine and standard 23-valent pneumococcal polysaccharide vaccine in patients with RA treated with immunosuppressive drugs. The main finding in the study is that PCV7 and PPV23 elicits similar antibody response for two serotypes common for both vaccines (6B and 23F) among RA patients treated with MTX, anti-TNF drugs as monotherapy or combination of these remedies as corresponding treatment groups of RA patients vaccinated with PPV23.
The conjugation of polysaccharides antigens to a polypeptide is shown to elicit immune response and vaccination results in better protection against vaccine serotypes that caused invasive pneumococcal disease in children [10, 11, 15–17] but does not seem to induce stronger antibody response in adult patients [18, 19].
Our findings with similar antibody response in treatment stratified RA patients are in accordance with previously reported studies including elderly patients [18, 19]. In contrast, de Roux et al. demonstrated higher antibody levels following the initial dose of PCV7 compared to PPV23-vaccinated elderly vaccine-naïve adults not exposed to immunosuppressive drugs [20]. Superior antibody response to PVC7 versus PPV23 was shown in patients with chronic obstructive pulmonary disease [21]. The vaccinated subjects were treated with glucocorticoids and no other immunosuppressive remedies which could be one possible explanation for these diverging results.
In the present study, we found that the proportion of patients with posAR for both serotypes was lowest in MTX-treated patients for both vaccines (21% and 14%, respectively). Maximal posAR for both serotypes tested (approximately 50% of patients) was found in RA patients on anti-TNF treatment as monotherapy. This is in line with previously reported experimental data showing that exposition to anti-TNF antibodies reverse the T cell activation caused by chronic exposition to TNF in RA [22]. Our analyses including all RA patients did not identify vaccine type or anti-TNF treatment as significant predictors of immune response, while MTX treatment and higher age remained predictors of impaired response for both serotypes also after adjustment for differences in demographic and disease characteristics at vaccination. The underlying mechanisms by which MTX influences antibody response may include both increased T cell apoptosis and impact on humoral response [23].
The association between higher age and diminished immune response previously also reported by others [18, 19] could at least in part be explained by age-related immune disturbance affecting both the innate and adaptive immune systems including decreased ability of B cells to produce antibodies [24].
Unexpectedly, concomitant treatment with glucocorticoids elicited superior antibody response after pneumococcal vaccination using conjugate vaccine but had no significant impact on antibody response to polysaccharide vaccine. The reasons for these diverging effects of steroids are not known. An improved antibody response to pneumococcal polysaccharide vaccine in RA patients receiving oral glucocorticoids has previously been observed [25].
There are some limitations to this study. RA patients not taking immunosuppressive drugs were not included in the study and therefore the influence of immunological disturbance as part of the RA disease on antibody response was not possible to investigate. The number of patients with RA not treated with DMARDs at our department is limited which precluded inclusion of such patients in the study. Since PCV7 vaccine is not approved for use in adults in Sweden, recruitment of population-based healthy controls would pose ethical, logistic and administrative problems.
We were not able to study the influence of previous pneumococcal vaccination on antibody response since these data were not collected at vaccination using PPV23. However, pneumococcal vaccination within the previous 5 years was one of the exclusion criteria for both vaccinations. Previous pneumococcal vaccination >5 years prior to vaccination with PCV7 had no significant impact on antibody response [13].
We measured antibody response to only two serotypes (23F and 6B). The reason for choosing these serotypes is that they are commonly associated with invasive pneumoccocal diseases in northern Europe [26]. Overall, we believe that immune response to these serotypes represents the pattern expected for all serotypes regarding the impact of disease and treatment, but individual serotypes may have variable immunogenicity. It must be remembered that antibody response is a surrogate measure of vaccine effectiveness, and a study of the true prevalence of invasive pneumococcal diseases following these vaccinations is needed.
Results from this study contribute to the knowledge about immunogenicity of currently available pneumococcal vaccines in this group of immunosuppressed patients with RA. Although individual serotypes might be more immunogenic and elicit better antibody response, in general, PCV7 does not appear to have convincing advantage over currently available PPV23 in this high-risk group of RA patients.
Key messages
Heptavalent conjugate pneumococcal vaccine elicits similar antibody response as polysaccharide vaccine in immunosuppressed RA patients. In RA, higher age and MTX treatment but not type of vaccine predicted impaired antibody response.
References
Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (2010) Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 59(34):1102–1106
Recommendations of the Swedish National Board of Health and Welfare. Pneumococcal Vaccination(1994). (Socialstyrelsens allmänna råd) SOSFS 1994:26. Vaccination mot pneumokocker. Available at http://www.socialstyrelsen.se/sosfs/1994-26
Vinogradova Y, Hippisley-Cox J, Coupland C (2009) Identification of new risk factors for pneumonia: population-based case–control study. Br J Gen Pract 59(567):e329–e338
Greenberg JD, Reed G, Kremer JM, Tindall E, Kavanaugh A, Zheng C, Bishai W, Hochberg MC, CORRONA Investigators (2010) Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis 69(2):380–386, Epub 2009 Apr 8
Moberley SA, Holden J, Tatham DP, Andrews RM (2008) Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 23(1):CD000422
Huss A, Scott P, Stuck AE, Trotter C, Egger M (2009) Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 180(1):48–58
Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL (2010) Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis 201(4):516–524
Sankilampi U, Isoaho R, Bloigu A, Kivelä SL, Leinonen M (1997) Effect of age, sex and smoking habits on pneumococcal antibody levels in an elderly population. Int J Epidemiol 26(2):420–427
Kapetanovic MC, Saxne T, Sjöholm A, Truedsson L, Jönsson G, Geborek P (2006) Influence of methotrexate, TNF-blocker and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology 45:106–111
Prevenar. Information från Läkemedelsverket (Medical Products Agency, Sweden) 2001;12(4). ISSN 1101–7104. Available at http://www.lakemedelsverket.se
CDC (2005) Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep 54(36):893–897
Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, Hadler J, Cieslak PR, Whitney CG, Active Bacterial Core Surveillance Team (2005) Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294(16):2043–2051
Kapetanovic MC, Roseman C, Saxne T, Jönsson G, Truedsson L, Geborek P (2011) Methotrexate but not TNF-blockers reduces immune response following pneumococcal vaccination using 7-valent conjugate pneumococcal vaccine (Prevenar®) in adult patients with established arthritis. Arthritis Rheum (in press)
WHO. Training manual for Streptococcus pneumoniae serotype specific IgG (PN PS ELISA). Available at: http://www.vaccine.uab.edu/ELISA%20Protocol.pdf. Accessed 30 Sept 2010
Rodenburg GD, de Greeff SC, Jansen AG, de Melker HE, Schouls LM, Hak E, Spanjaard L, Sanders EA, van der Ende A (2010) Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis 16(5):816–823
Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM, Reingold A, Thomas A, Glode MP, Zell ER, Jorgensen JH, Beall B, Schuchat A (2006) Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 368(9546):1495–1502
Foster D, Walker AS, Paul J, Griffiths D, Knox K, Peto TE, Crook DW, Oxford Invasive Pneumococcal Surveillance Group (2011) Reduction in invasive pneumococcal disease following implementation of the conjugate vaccine in the Oxfordshire region, England. J Med Microbiol 60(Pt 1):91–97, Epub 2010 Sep 23
Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, McIntyre PB (2009) Immunological responses to pneumococcal vaccine in frail older people. Vaccine 27(10):1628–1636, Epub 2008 Dec 17
Shelly MA, Jacoby H, Riley GJ, Graves BT, Pichichero M, Treanor JJ (1997) Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun 65(1):242–247
de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, Gruber WC, Lockhart S, Burkhardt O, Welte T, Lode HM (2008) Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 46(7):1015–1023
Dransfield MT, Nahm MH, Han MK, Harnden S, Criner GJ, Martinez FJ, Scanlon PD, Woodruff PG, Washko GR, Connett JE, Anthonisen NR, Bailey WC, COPD Clinical Research Network (2009) Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180(6):499–505, Epub 2009 Jun
Wessels Cope AP, Londei M, Chu NR, Cohen SB, Elliott MJ, Brennan FM, Maini RN, Feldmann M (1994) Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest 94(2):749–760
Wessels JA, Huizinga TW, Guchelaar HJ (2008) Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 47(3):249–255, Epub 2007 Nov 28
Ongrádi J, Kövesdi V (2010) Factors that may impact on immunosenescence: an appraisal. Immun Ageing 7:7
Visvanathan S, Keenan GF, Baker DG, Levinson AI, Wagner CL (2007) Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J Rheumatol 34(5):952–957
Harboe ZB, Benfield TL, Valentiner-Branth P, Hjuler T, Lambertsen L, Kaltoft M, Krogfelt K, Slotved HC, Christensen JJ, Konradsen HB (2010) Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis 50(3):329–337
Acknowledgements
The authors wish to thank the late Lotta Larsson, Elna Haglund, Eva-Karin Kristoffersson, Helén Axelsson and Käthe Nilsson for their help with vaccination, collecting blood samples and carrying through the study; Peter Kapral, Maria Jacobsson, Ingrid Moberg, Ingrid Bondesson, Ingrid Hermansson and Eva Hommerberg, all from the Department for Rheumatology, Skåne Universitetssjukhus Lund and Malmö, for taking care of blood samples and Ingrid Mattsson-Geborek for skilful help with the figures. We also thank all patients for their participation in the study and all colleagues for their cooperation and support during the study. The study was supported by grants from the Swedish Rheumatism Association, the Swedish Research Council, the Medical Faculty of the University of Lund, Alfred Österlund's Foundation, The Crafoord Foundation, Greta and Johan Kock's foundation and The King Gustaf V 80th Birthday Fund, and Lund University Hospital. Prevenar vaccine for this study was provided by Wyeth Pharmaceuticals.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kapetanovic, M.C., Roseman, C., Jönsson, G. et al. Heptavalent pneumococcal conjugate vaccine elicits similar antibody response as standard 23-valent polysaccharide vaccine in adult patients with RA treated with immunomodulating drugs. Clin Rheumatol 30, 1555–1561 (2011). https://doi.org/10.1007/s10067-011-1856-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1856-5