Abstract
Evidence-based recommendations support the use of multimodal therapy and hydrotherapy for fibromyalgia syndrome; however, there is little standardisation of such programmes. The aim of the study was to assess the effectiveness of a pool-based exercise using deep water running (DWR) as part of a multimodal physiotherapy programme for patients with fibromyalgia syndrome. For a non-randomised clinical study, 44 patients diagnosed with fibromyalgia were recruited from primary care. Patients in the experimental group received a multimodal programme incorporating pool-based exercise using DWR three times a week for an 8-week period. The control group received a leaflet containing advice and continued with normal activities. Patients were evaluated for physical function (Fibromyalgia Impact Questionnaire, FIQ), pain, general health (Short Form-12 Health Survey) and quality of life (European Quality of Life Scale-5D) pre- and post intervention. Statistically significant results were found for the experimental group for FIQ total score, incorporating physical function, pain, fatigue, stiffness and psychological variables (p < 0.05). Statistically significant differences between the experimental group and control were also found for general health (p < 0.05) and quality of life (p < 0.05). The results of this pilot study and the high level of compliance and adherence and low level of attrition suggest that this multimodal programme incorporating DWR is a safe and effective intervention for fibromyalgia syndrome that is acceptable to patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibromyalgia syndrome (FMS) is a chronic non-articular rheumatological chronic widespread pain (CWP) condition characterised by widespread musculoskeletal pain, reduced pain threshold and characterised by other symptoms such as fatigue, non-restorative sleep, stiffness and psychological symptoms such as depression and anxiety [1]. FMS has an estimated prevalence of 2% affecting women six times more commonly than men. Treatment is often symptomatic and consequently a wide range of interventions are used. Promising results have been found for multimodal management with non-pharmacological components constituting exercise and education based on cognitive–behavioural principles, though strong evidence has not been found to emerge for any single intervention [2–6]. Further, the heterogeneity of multimodal programmes and lack of specific information in extant guidelines [7, 8] regarding exercise prescription in terms of dosage and frequency for fibromyalgia/CWP make the comparison of cognate studies difficult. Thus, there is little specific standardisation of programmes.
Hydrotherapy is often used in the management of FMS, with some evidence of its effectiveness [9, 10]. The European League Against Rheumatism (EULAR) evidence-based recommendations [7] support the use of heated pool-based treatment with or without exercise. However, the range of water-based therapies which fall under the aegis of hydrotherapy vary from spa-based treatments to water-based exercise and thus due to considerable heterogeneity of studies; comparison of cognate studies is difficult. A recent systematic review of n = 10 randomised controlled trials (RCTs) of hydrotherapy with mean methodological quality of 4.5/9 on the van Tulder scale [11] reported positive outcomes for pain, health status and tender point count [9]. However, n = 6 of these studies were of balneotherapy or spa-based treatments and only n = 4 of water-based exercise. Only one study was a pragmatic study, the others were a no-treatment control and, in some cases, a combination of approaches in the intervention group. Of the four RCTs that evaluated the effect of pool-based exercise [12–15], it was concluded that pool exercise improves the symptoms of FMS and this was maintained at follow-up. Jentoft et al. [15] recognised that pool exercise may offer additional benefits but found no significant differences between a land- or pool-based exercise programme. A recent RCT of n = 166 participants allocated to either a 20-session pool exercise and six-session education programme or to the education programme only (control) found significant improvements in the intervention group on Fibromyalgia Impact Questionnaire (FIQ) total with an effect size of 0.32 [16]. Similar positive improvements were reported by Munguia-Izquierdo and Legaz-Arrese [17]. Adherence with aquatic therapy was found to be high.
There is thus some evidence to suggest that therapeutic aquatic exercise is beneficial to patients with FMS despite heterogeneity of the exercise programmes. However, it is unclear regarding the nature and dosage of aquatic exercise for therapeutic effect. In an RCT of n = 60 patients randomly allocated to either a 15-week programme of deep water running (DWR) or land-based exercise, DWR, a recognised form of aerobic water-based exercise, was shown to be as effective as land-based exercise regarding pain in terms of functional improvements and patient global assessment of response to therapy [18]. The authors concluded that DWR is as effective as land-based therapy and may be a useful adjuvant therapy with a high level of acceptability to patients with fibromyalgia.
Therefore, the aim of the current study was to assess the effectiveness of pool-based exercise using DWR as part of the multimodal physiotherapy programme (MMPP) for patients with fibromyalgia compared with patients receiving education and advice only.
Methods
Design
The design was a non-randomised pilot clinical trial. The experimental group received 3 × 60-min sessions of MMPP plus DWR per week over 8 weeks versus a control group who received an educational leaflet containing advice and continued with usual activities. This group were on a waiting list for entry to the programme. The study was authorised by the Ethics and Research Committee of the Faculty of Medicine at Malaga University. All participants gave written informed consent, confidentiality and anonymity were preserved at all times and the principles of the Declaration of Helsinki were respected.
Subjects
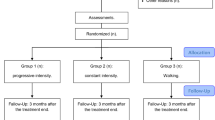
Forty-five sedentary women who fulfilled the American College of Rheumatology classification criteria for FMS [19] were recruited from primary care, of which 44 fulfilled the inclusion criteria and started the programme (Fig. 1). Two participants were unable to complete the programme due to factors unrelated to the treatment and one due to attending a pain clinic. These patients were non-randomly assigned to one of two groups using the order of arrival. The patients aged 18–60 years old and kept in an unchanged drug regimen for at least 4 weeks before starting the study. Patients with symptomatic cardiac failure, uncontrolled thyroid disturbances, body mass index of 40, infectious contagious skin diseases, coronary disease, pulmonary disease, neurologic disease, and rheumatic disease limiting or hindering their ability to exercise and those who had performed regular physical activity in the 6 weeks before the study were not included. Fear of aquatic exercise but not inability to swim was an exclusion criterion.
Outcome measures
The primary outcome was FIQ, a brief 10-item instrument that measures physical functioning and symptom severity, developed and validated for a FMS population [20]. The secondary outcomes were: general health (physical and mental component), determined with the Short Form-12 Health Survey (SF-12) [21] and quality of life, determined with the EuroQoL-5D [22]. A sociodemographic questionnaire collected information about the following variables: gender, date of birth, marital status and education level. The presence of comorbid medical conditions, defined by the World Health Organization [23] as ‘health problems that require ongoing management over a period of years or decades’, was assessed using a yes-or-no checklist. It included questions about a wide range of chronic physical conditions including arthritis, rheumatism, cervical pain, back pain, bronchitis or emphysema, asthma, diabetes, hypertension, heart arrhythmias, heart attack, stroke, gastric or duodenal ulcer, migraines or other chronic headaches, varicose veins, cancer, eyesight problems and hearing problems. Respondents were asked whether they experienced in the last year each of the symptom-based conditions in the checklist. Subjects were assessed on the outcome measures at baseline and at 8-week follow-up by an independent blinded assessor at the Sports and Physical Medical Centre in Torremolinos, Spain.
Interventions
The experimental group performed supervised training at their anaerobic threshold determined by a graded treadmill exercise test and DWR test with lactate and heart rate analyses. This group exercised for 60 min three times a week for 8 weeks, following the MMPP programme plus DWR. Each session was composed of a 0.5 h land-based exercises and 0.5 h water-based exercises. The sessions were delivered in groups of 10–12 subjects. All sessions were supervised by physiotherapists who were not involved in the clinical and fitness assessments.
The exercise prescription of DWR was based on the heart rate at the anaerobic threshold (HRAT) determined at the initial assessments. DWR consisted of simulated running in the deep end of a pool aided by a flotation device that maintained the head above the water. Patients were instructed in the DWR technique [24] and were also instructed to keep a regular speed to achieve the prescribed HR. The HR variation in immersion is influenced by water temperature and exercise intensity. This was based on previous studies of submaximum underwater exercise in temperatures from 28°C to 31°C, from which we developed a DWR-specific test [24]. The individual workload of HRAT was estimated during the DWR test. During this test, the participant was fitted with a flotation belt before entering the warm (27.5°C) and deep (2 m) water and was tethered by an elastic band to the edge of the pool. The test consisted of 2-min bouts of exercise starting at a frequency of 30 leg cycles per minute regulated by a previously recorded audio tape. The cadence increased every 2 min by five cycles per minute. At the end of each exercise bout, blood samples were collected from an ear lobe puncture for the determination of capillary lactate levels (LA). Heart rate was continually recorded with a chest monitor (Polar 610i). No breaks were taken between the test stages. Using this information, the relationship between HR and LA for each individual was established [24]. The HR of the patients was registered in 10-min intervals with a pulse watch recorder model A5 Polar (Polar, Helsinki, Finland). An adaptation interval with low-intensity exercises lasted 2 weeks, emphasising the learning of the new movements. Afterwards, the patients were asked to exercise at the HRAT. In case of pain while exercising, the patients were instructed to reduce the intensity for a short time. After that, they were expected to reach the target HR again.
An individualised MMPP of therapeutic exercises combined with education based on cognitive–behavioural principles was then prescribed for three times a week over 8 weeks based on a common structure of aims to improve physical ability according to the initial individual assessment but also addressing individual findings in a group format (maximal 12 subjects per group). Participants were encouraged to adopt an active role in the programme and the importance of compliance. Each 60-min session comprised 15 min of mobility and flexibility of the tonic muscles, 15 min of resistance and muscle strengthening exercises with individual workload according to the previous test of phasic muscles and additional aerobic exercise in the form of a 20-min session of DWR at HRAT with 10 min for preparation and breakdown. From weeks 1 to 4, workload corresponded to the HR at 2 mmol of LA. For weeks 5 to 8, the workload was set at 3 mmol of LA, all of which were based on the pre-test values. The selected levels were based on previous studies concluding that 2–4 mmol of LA is the AT in swimmers [25]. A physiotherapist led and supervised the sessions. They were also given a 10-point leaflet with information on FMS.
The control group received advice in the form of the 10-point leaflet only and continued with usual activities. The 10 points of the leaflet were based on: postural care, physical activity, lifting weights, sedentary activities, sports, pain-free maximal physical activity level, behavioural advice, fear of movement, false beliefs and an active lifestyle.
Sample size
A priori sample size calculation indicated that 20 patients would be required to complete each group to detect a statistically significant difference (alpha of 0.05, beta of 0.80, difference of 23 points in the FIQ total score based on some previous works, a standard error of 12 and [18] EPIDAT 3.1 software was used).
Statistical analysis
Analyses were performed using SPSS version 15.0 (USA). We examined baseline differences in sociodemographic and clinical characteristics between the intervention and control group, applying the Student's t test for continuous variables and the chi-square test with continuity. The treatment effect on functional status was analysed with factorial ANOVAs (when the sphericity could not be assumed, the Huynh–Feldt correction was used). The 2 × 2 repeated measures ANOVAs were carried out with the groups (MMPP plus DWR vs. waiting list) as one factor and test occasion (pre- and post treatment) as the repeated measures factor to examine differences in the FIQ total score, SF-12, EuroQoL-5D and in each of the FIQ domains (physical impairment, days not feeling well, pain, general fatigue, morning fatigue, stiffness, anxiety and depression). Raw data were transformed into normalised scores ranging from 0 to 10, with higher scores indicating a worse condition A per protocol analyses that included all participants was performed.
Results
The trial flowchart is shown in Fig. 1. A total of n = 45 patients were deemed eligible to the trial; n = 1 was excluded due to fear of aquatic exercise. A total of n = 41 participants completed the intervention. n = 3 numbers dropped out of the study, two from the waiting list control and one from the intervention due to choosing to attend a pain clinic. There were no statistically significant differences between study groups at baseline (Table 1). No adverse events were reported.
Statistically significant differences were found for the intervention group post intervention in FIQ total score (F = 21.19, p = 0.001), general health as measured by the SF12 physical component (F = 2.4, p = 0.002) and mental component (F = 2.66, p = 0.02). Quality of life was also improved significantly post intervention (F = 1.41, p = 0.02). Results are shown in Table 2.
Statistically significant differences were found for the symptoms of FMS between the intervention group after the trial period and the control group. There was a significant improvement in physical impairment in the intervention group (F = 21.63, p = 0.001), not feeling good (F = 22.12, p = 0.002) and number of days off work (F = 20.16, p = 0.02). The average number of days off work at baseline was 1.3 (SD 0.66) in the intervention group and 1.25 (SD 1.21) in the control group. At the end of the trial period, this had reduced to 0.6 (SD 0.46) in the intervention group and 1.3 (SD 0.63) in the control group. Pain was significantly reduced in this subscale of the FIQ (F = 23.92, p = 0.001) and also on the EuroQoL VAS in the intervention group (F = 2.55, p = 0.03).
General fatigue was also significantly reduced in the intervention group post intervention (F = 9.83, p = 0.007). Similarly, this was the case for unrefreshing sleep/morning fatigue (F = 19.34, p = 0.001). There were no significant differences in the control group after 8 weeks in any of these variables. There was a significant reduction in stiffness in the intervention group (F = 14.11, p = 0.001).
There was also a significant reduction in the psychological symptoms of anxiety (F = 23.43, p = 0.001) and depression (F = 26.84, p = 0.001) in the intervention group after the 8-week intervention (Table 3).
Discussion
The results of this pilot study showed that a multimodal programme incorporating DWR improves the majority of symptoms of FMS including pain, physical function, sleep, fatigue, morning stiffness and quality of life and psychological symptoms (depression and anxiety). The findings of this study concur with other studies and reviews [e.g. 9, 10, 26] that have concluded that programmes including hydrotherapy are effective. With regard to the form of hydrotherapy, which in this study was confined to the aegis of water-based exercise, specifically DWR, rather than balneotherapy and spa-based treatments, the results concur with Assis [18] who found DWR to be a safe, effective and acceptable form of exercise to patients with fibromyalgia, as there were very few drop-outs compared to other studies of exercise for fibromyalgia. This is the first study that has combined DWR into a multimodal programme and the results suggest that this may be a useful adjunctive method. Despite evidence of effectiveness of aerobic exercise, there are few studies of aquatic exercise, in particular DWR. This study with aquatic exercise differs from previous studies because of its larger sample size, excellent compliance and individualised supervised exercise programmes combined with education. The use of DWR and the precise calculation of the exercise is a further novel feature of the programme which may assist in addressing issues of standardisation and heterogeneity in extant studies.
The significant results are perhaps unsurprising. Evidence has shown that composite treatments are associated with greater improvements [2, 5]; for example, educational programmes when combined with exercise are more effective than either treatment alone [27, 28]. It is difficult to separate the effectiveness of DWR from the multimodal intervention and in conclusion it can be stated that DWR is a safe and effective option for fibromyalgia that may be included as part of the multimodal package. Contrary to the findings of previous studies of exercise where worsening of symptoms and high levels of attrition are reported, there were no adverse effects in the intervention group, confirming that patients with FMS can undergo physical training without damage and increased muscle soreness and that DWR may be a useful adjunctive treatment.
A stepwise approach to the management of FMS has been recommended [29]. The first step includes confirmation of diagnosis and explanation of treatment options and comorbid conditions (e.g. mood and sleep disturbance). The second step includes a trial of low-dose antidepressants combined with education, fitness, exercise and CBT. The third step includes referral to a pain management programme and other relevant specialties and trials of other medication. This particular programme was intended as a step 2 intervention.
In this study, land-based exercise included stretching of tonic muscle and strengthening of phasic muscles combined with advice and education. Aerobic exercise was through DWR, which was found to be acceptable by FMS patients, and concurs with other studies [17]. There is little standardisation of extant multimodal programmes and in the present study standard recommendations of dosage and frequency of this type of intervention were followed according to the ACSM exercise for arthritis guidelines for frequency [30]. However, there are currently no specific guidelines for dosage and though UK National Institute for Health and Clinical Excellence (NICE) guidelines provide recommendations regarding treatment and care of persons with low back pain disorders (there is no extant NICE guidelines for fibromyalgia/chronic widespread pain), there is no specific information regarding exercise dose/prescription [8]. Similarly, EULAR recommendations do not provide this specific information [7]. The current study has provided detailed information regarding dose and frequency of exercise, though further research is warranted.
Improvement in the FIQ total in the intervention group was supported in several components related to health. The FIQ pain showed significant improvement in the intervention compared with the control group. As the drug regimen was unchanged, changes in pain cannot be attributed to pharmacology, a finding supported in other studies [e.g. 16, 31].
A widely accepted model that activation of the endogenous opioid system during exercise plays a key role in the analgesic response mechanism has been purported [31]. Several studies have also suggested a multiple analgesic system including non-opioid mechanisms mediated by growth hormone and corticotrophin [e.g. 32]. An analgesic effect of exercise may also help break the cycle of ‘pain–immobility–pain’. Another positive effect of regular exercise is the improvement of fatigue. Sleep quality improved in the current study in the intervention group. Sleep disorders play an important role in the perpetuation of symptoms of FMS and better sleep may contribute to improvement in other symptoms and overall quality of life. Quality of life and general health also improved in the intervention group in line with previous studies [e.g. 16]. Similar to other studies based on cognitive–behavioural principles and combining exercise and education, psychological symptoms of depression and anxiety were found to be significantly reduced in the intervention group following the intervention. As the control group also received education and advice in the form of a leaflet and did not show improvement after the study period, it can be suggested that it is the multimodal aspect of the programme that is important in mediating psychological effects rather than education alone.
In conclusion, the multimodal programme, incorporating DWR, in comparison to a control group receiving only advice and continuing usual activities led to improvements in the symptoms of FMS, physical function and also in general health and quality of life. The high level of compliance and adherence and lack of reported adverse effects suggests that this is a safe and effective programme for FMS that is acceptable to patients.
There were a number of limitations to this pilot study including the lack of long-term follow-up which is a recommendation for future research. Further, this was a non-randomised study and despite there being no statistically significant differences between groups at baseline, randomisation would have reduced potential bias. The role of non-specific effects such as the level of attention received by each group was also a limitation. Patients in the experimental group received considerably more attention in terms of the intervention compared with the control group. Suggestions for future research include the use of an adequately powered pragmatic trial to compare interventions, such as the control group receiving the same multimodal programme but without the addition of DWR. Recruitment of men into a larger scale randomised study and a comparison of the effects of gender is a suggestion for future research. An economic evaluation of cost-effectiveness of such programmes is also recommended.
Given the limited availability of hydrotherapy, particularly in UK, the development of patient-based education–exercise programmes using cognitive–behavioural principles, perhaps in a community setting, using local leisure facilities and resources, as in Spain, may also be a consideration.
References
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russel AS, Russell IJ, Winfield JB, Yunus MB (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 62(5):600–610
Sim J, Adams N (2002) Systematic review of randomized controlled trials of nonpharmacological interventions for fibromyalgia. Clin J Pain 18:324–336
Burckhardt CS (2006) Multidisciplinary approaches for management of fibromyalgia. Curr Pharm Des 12:59–66
Van Koulil S, Effting M, Kraaimaat FW, van Lankveld W, van Helmond T, Cats H, van riel PLCM, de Jong AJL, Haverman JF, Evers AWM (2007) Cognitive–behavioural therapies and exercise programmes for patients with fibromyalgia: state of the art and future directions. Ann Rheum Dis 66:571–581
Häuser W, Bernardy K, Arnold B, Offenbacher M, Schilternwolf M (2009) Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum 61(2):216–224
Häuser W, Klose P, Langhorst J, Moradi B, Steinbach M, Schilternwolf M, Busch A (2010) Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 12(3):R79
Carville SF, Arendt-Nielsen S, Biddal H et al (2008) EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 67:536–541
National Collaborating Centre for Primary Care (2009) Low back pain. Early management of persistent non-specific low back pain. NICE Clinical Guidelines, No. 88. Royal College of General Practitioners (UK), London
McVeigh JG, McGaughey H, Hall M, Kane P (2008) The effectiveness of hydrotherapy in the management of fibromyalgia syndrome. Rheumatol Int 29:119–130
Langhorst J, Musial F, Klose P, Hauser W (2009) Efficacy of hydrotherapy in fibromyalgia syndrome—a meta-analysis of randomized controlled trials. J Rheumatol 48:1155–1159
van Tulder Mw, Assendelft WB, Koes BW, Bouter LM (1997) Method guidelines for systematic review in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine 22:2323–2330
Gusi N, Tomar-Carus P, Hakkinen A, Hakkinen K, Ortega-Alonso A (2006) Exercise in waist-high warm water decreases pain and improves health-related quality of life and strength in the lower extremities in women with fibromyalgia. Arthritis Rheum 55:66–73
Gowans SE, de Hueck A, Voss S, Richardson M (1999) A randomised controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care Res 12:120–128
Mannerkorpi K, Nyberg B, Ahlmen M, Ekdahl C (2000) Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective randomized study. J Rheumatol 27:2473–2481
Jentoft ES, Kvalvik AG, Mengshoel AM (2001) Effects of pool-based and land-based aerobic exercise on women with fibromyalgia/chronic widespread muscle pain. Arthritis Care Res 45:42–47
Mannerkorpi K, Norderman L, Ericsson A, Arndorw M, GAU study group (2009) Pool exercise for patients with fibromyalgia or chronic widespread pain: a randomized controlled trial and subgroup analyses. J Rehabil Med 41:751–760
Munguia-Izquierdo D, Legas-Arrese A (2008) Assessment of the effects of aquatic therapy on global symptomatology in patients with fibromyalgia syndrome: a randomized controlled trial. Arch Phys Med Rehabil 89:2250–2256
Assis MR, Silva LE, Alves AMB, Pessanha AP, Feldman VVD et al (2006) A randomized controlled trial of deep water running: clinical effectiveness of aquatic exercise to treat fibromyalgia. Arthritis Rheum 55:57–65
Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL et al (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum 33:160–172
Burckhardt CS, Clark SR, Bennett RM (1991) The Fibromyalgia Impact Questionnaire: development and validation. J Rheumatol 18:728–733
Vilagut G, Valderas JM, Ferrer M, Garin O, López-García E, Alonso J (2008) Interpretation of SF-36 and SF-12 questionnaires in Spain: physical and mental components. Med Clin 130(19):726–735
Jia H, Lubetkin EI (2008) Estimating. EuroQol EQ-5D scores from Population Healthy Days data. Med Decis Making 28(4):491–499
World Health Organization (2002). Innovative care for chronic conditions: building blocks for action. WHO, Geneva
Cuesta-Vargas A, Garcia-Romero JC, Kuisma R (2009) Maximum and resting heart rate in treadmill and deep-water running in male international volleyball players international. IJARE 3:398–405
Fernández-Pastor VJ, Pérez F, García JC, Diego AM, Guirado F, Noguer N (1997) Maintenance of the threshold/maximum heart rate quotient in swimmers. Rev Esp Fisiol 53(3):327–334
Hammond A, Freeman K (2006) Community patient education and exercise for people with fibromyalgia: a parallel group randomized controlled trial. Clin Rehabil 20:835–846
Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A (1994) A randomised controlled trial of education and physical training for women with fibromyalgia. J Rheumatol 21:714–720
King DJ, Wessel J, Bhambhami Y, Sholter D, Maksymowych W (2002) The effects of exercise and education, individually or combined in women with fibromyalgia. J Rheumatol 29:2620–2627
Goldenberg DL, Burckhardt C, Crofford L (2004) Management of fibromyalgia syndrome. JAMA 292:2388–2395
Haskell WL, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39:1423–1434
Altan L, Bingol U, Aykac M, Koc Z, Yurtkuran M (2004) Investigation of the effects of pool-based exercise on fibromyalgia syndrome. Rheumatol Int 24:272–277
Koltyn KF (2000) Analgesia following exercise. A review. Sports Med 29:85–98
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuesta-Vargas, A.I., Adams, N. A pragmatic community-based intervention of multimodal physiotherapy plus deep water running (DWR) for fibromyalgia syndrome: a pilot study. Clin Rheumatol 30, 1455–1462 (2011). https://doi.org/10.1007/s10067-011-1825-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1825-z