Abstract
Our objective was to audit the respiratory outcome, toxicity and long-term survival of systemic sclerosis associated interstitial lung disease (SSc-ILD) treated with intravenous (i.v.) cyclophosphamide. We ascertained whether i.v. cyclophosphamide associates with a better outcome in SSc-ILD diagnosed due to a decline in screening lung function than in those diagnosed due to respiratory symptoms. A retrospective case-note audit was carried out for SSc-ILD patients treated with i.v. cyclophosphamide between January 1999 and March 2009 at the Royal Derby, Kings Mill and Nottingham University Hospitals. Forced vital capacity (FVC) and transfer factor at 6, 12 months after starting i.v. cyclophosphamide were the primary end points. Kaplan–Meier curves were plotted to estimate survival. Thirty-seven i.v. cyclophosphamide treatment cycles were administered to 36 patients (27 women). Fourteen cycles associated with side effects and eight were terminated prematurely. SSc-ILD was diagnosed due to respiratory symptoms in 13 and in response to deteriorating screening pulmonary function test (PFT) in 24 instances. Overall, i.v. cyclophosphamide led to stabilisation in lung function. However, the FVC declined by 7% in SSc-ILD presenting with respiratory symptoms over 12 months. These patients had significantly lower FVC at 6 and 12 month than those with SSc-ILD diagnosed due to decline in screening lung function. The 5-year survival was 76.1% (overall), 62.9% (diagnosed due to respiratory symptoms) and 91.5% (diagnosed due to decline in screening lung function, p = 0.05). I.V. cyclophosphamide stabilises lung function in individuals with SSc-ILD and may associate with better respiratory outcome in patients diagnosed on screening PFTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interstitial lung disease (ILD) and pulmonary hypertension are the commonest causes of death in systemic sclerosis (SSc) [1]. Cyclophosphamide is the treatment of choice for alveolitis in SSc associated ILD (SSc-ILD) [2]. This is supported by two randomised controlled trials, where oral cyclophosphamide improved forced vital capacity (FVC) significantly and i.v. cyclophosphamide had similar effects [3, 4]. Of these, i.v. cyclophosphamide is preferred as it has less side effects, lower cumulative dose, and smaller malignancy risk [5].
In clinical practice, SSc-ILD is diagnosed if there is a decline in screening pulmonary function test (PFT)—with or without respiratory symptoms on enquiry; or due to respiratory symptoms like shortness of breath. Although intuitive, it is not established if the former group has a better respiratory outcome with i.v. cyclophosphamide [6].
Previous reports of i.v. cyclophosphamide for SSc-ILD are frequently small, selective, and only one reports long-term survival [4, 7–23]. The side effect profile of i.v. cyclophosphamide, at the doses used for SSc-ILD is not well established. This combined with a lack of information about predictors of favourable clinical response causes difficulty in counselling these patients about treatment options. This difficulty is pronounced in instances where SSc-ILD is diagnosed due to asymptomatic decline in screening PFTs.
We reviewed case notes of all SSc-ILD patients treated with i.v. cyclophosphamide over the past 10 years at three National Health Service (NHS) hospitals in East Midlands, UK, in order to audit their respiratory outcome, toxicity and long-term survival. Outcome comparisons were made between SSc-ILD diagnosed because of declining PFTs and respiratory symptoms.
Methods
All patients treated with i.v. cyclophosphamide for SSc or Mixed Connective Tissue Disease (MCTD)-associated ILD at the Royal Derby, Kings Mill and Nottingham University Hospitals between January 1999 and March 2009 were identified using rheumatology day-case unit databases. In our hospitals, i.v. cyclophosphamide is the first line drug for SSc-ILD. Patients are not required to fail on other drugs prior to i.v. cyclophosphamide. No patient received oral cyclophosphamide or rituximab before i.v. cyclophosphamide. The standard treatment regime includes six, monthly pulses of i.v. cyclophosphamide (15 mg/kg) as in the FAST trial [4]. Four patients received fortnightly i.v. cyclophosphamide between 2000 and 2002 (median dose, duration 4.25 g, 2 months). ILD was diagnosed on the presence of either ground glass or fibrotic changes on HRCT chest. This audit was registered with audit department at each hospital.
Information was collected about disease phenotype; age at onset of Raynaud’s phenomenon, diagnosis of SSc/MCTD, diagnosis of ILD and i.v. cyclophosphamide treatment; smoking status and history of chronic obstructive pulmonary disease (COPD) or asthma. Most recent echocardiogram was used to assess ejection fraction and systolic pulmonary artery pressure.
For each i.v. cyclophosphamide treatment cycle, information about pre-treatment PFT, total cyclophosphamide dose (g), treatment duration (months), adverse events, peak prednisolone dose (mg/day) and sequential immunosuppressive treatment was recorded. We ascertained whether the request for HRCT chest that led to diagnosis of ILD was triggered by the patient presenting with respiratory symptoms or if it was in response to a decline in annual screening PFTs. The primary outcomes were FVC and transfer factor (DLCO) at approximately 6, 12 months after starting i.v. cyclophosphamide.
Disease and demographic characteristics were summarized using mean (SD) for continuous and n (%) for categorical variables. Continuous and categorical variables were compared using t test and chi-square test, respectively. The PFTs were analyzed post hoc to establish if i.v. cyclophosphamide associates with better respiratory outcome in SSc-ILD diagnosed on screening PFTs, than in those diagnosed due to respiratory symptoms. Covariate analysis was used to compare FVC and DLCO between the two groups at baseline and at 6 and 12 months after adjusting for age and gender. Kaplan–Meier curves were plotted to estimate survival. If the patient was not dead or lost to follow-up, data were censored on 31/01/2010. Log rank test was used to ascertain differences in survival between the two groups (vide supra). Statistical analysis was carried out using SPSS (v14). Statistical significance was set at p ≤ 0.05 (two-tailed).
Results
We identified 36 patients (27 women) who received i.v. cyclophosphamide for SSc/MCTD-ILD. Of these, 12 had limited SSc, 19 had diffuse SSc and five had MCTD. Thirty-five were antinuclear antibody (ANA) positive. Ten had anti-Scl-70, eight anti-centromere, three anti-nucleolar pattern ANA and five had anti-RNP antibody. One person had asthma. None had COPD, congestive cardiac failure or pulmonary arterial hypertension. With the inclusion of a patient who received 2 cycles of i.v. cyclophosphamide >3 years apart, data for 37 i.v. cyclophosphamide treatment cycles were analysed.
Twenty-nine treatment cycles were completed (78.4%). Fourteen (37.8%) treatment cycles associated with side effects (Table 1). Nausea and vomiting despite regular ondansetron or granisetron was the commonest side effect. Side effects did not vary according to age (p = 0.94) or gender (p = 0.17). Eight i.v. cyclophosphamide treatment cycles were terminated prematurely (median dose and duration 1.75 g, 1 month). Six of these were subsequently commenced on an immunosuppressant (oral cyclophosphamide 2, azathioprine 2, tacrolimus 2 patients each). One patient withdrew consent for further treatment, and information regarding immunosuppressant use could not be ascertained for one. Patients who could not complete an i.v. cyclophosphamide treatment cycle were more likely to be women (p = 0.05), and young (mean (SD) 46.38(14.42), 56.43(13.36) year, p = 0.07). However, two patients with severe neutropenia requiring treatment discontinuation were older (65, 68 years) and had chronic kidney disease 3 (eGFR 30–60 ml/min).
The HRCT chest which showed changes of ILD was performed in response to respiratory symptoms in 13, and in response to decline in monitoring PFTs in 24 instances respectively. There was no difference between these two groups for demographic features, disease subtype and treatment (Table 2). SSc-ILD patients diagnosed with symptomatic ILD had lower FVC and DLCO at baseline (Table 3).
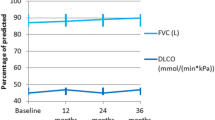
There was 0.8% increase in FVC and 2.4% decline in DLCO at 12 months compared to baseline (Table 3). However, SSc-ILD patients presenting with respiratory symptoms showed 7.0% decline in FVC over 12 months, while those diagnosed on screening had a minimal (0.4%) increase in FVC in the same time period. After adjusting for age and gender, the FVC at 6 and 12 months was significantly lower and decline in DLCO larger in SSc-ILD patients presenting with respiratory symptoms. After i.v. cyclophosphamide, immunosuppression was continued with oral cyclophosphamide (2), azathioprine (17), mycophenolate mofetil (11), tacrolimus (2) and ciclosporin (1).
Eight patients have died over 159.2 patient years. The mean (95% confidence interval (95% CI)), survival after treatment with i.v. cyclophosphamide was 7.7 (6.3–9.2) years overall, 4.7 (3.3–6.1) years for SSc-ILD presenting with respiratory symptoms and 8.9 (7.6–10.2) years for SSc-ILD diagnosed due to decline in screening PFTs. Overall survival was 76.1% at 5 years. The 5-year survival for SSc-ILD diagnosed due to symptoms and SSc-ILD diagnosed due to decline in screening PFTs was 62.9% and 91.5%, respectively (p = 0.05, log rank test; Fig. 1).
Discussion
This audit is among the largest to report on outcome of SSc-ILD treated with i.v. cyclophosphamide [16, 21]. It enables evaluation of respiratory outcome of SSc-ILD using routine clinical data. Utilising the outcomes of routine clinical practice can lead to important treatment observations in rare disorders, and this approach has been adopted by European Scleroderma Observational Study, a study of outcomes in Scleroderma [24].
I.V. cyclophosphamide stabilized lung function overall. This is likely to be a treatment effect, and not due to regression to the mean (period of slow or no disease progression after a period of deterioration), as untreated SSc-ILD patients show a decline in PFTs [25]. As the initial doses of cyclophosphamide and subsequent oral immunosuppression may exert a treatment effect, the eight treatment cycles where cyclophosphamide was stopped prematurely were included. A similar approach was used in FAST trial [4].
SSc-ILD diagnosed due to decline in serial PFTs associated with a better respiratory outcome than that diagnosed due to respiratory symptoms. This is a novel observation, and there are several explanations for this—including lead time bias—early diagnosis resulting in prompt treatment. There may be differences in natural history, and in the extent of pulmonary involvement, which was not quantified. The lack of difference between disease and demographic features in the two groups in part argues against any substantial differences in their natural history. However, patients with comparable characteristics may still experience different pattern of end organ damage.
The improved respiratory outcome at 12 months in those with SSc-ILD diagnosed on screening PFTs may be confounded by a greater proportion continuing oral immunosuppression after i.v. cyclophosphamide in this group. SSc-ILD patients who improve continue on treatment, and patients who fail to improve, or deteriorate stop all treatment, and due to paucity of evidence for treatment of SSc-ILD refractory to cyclophosphamide, are difficult to persuade otherwise.
Neutrophilic alveolitis [8] and high-dose prednisolone [12] associate with better response to i.v. cyclophosphamide in SSc-ILD. However, the latter study was confounded by non-random allocation and severe lung fibrosis in low-dose prednisolone group [12].
Five-year survival of 76.1% highlights the poor prognosis associated with SSc-ILD. This is comparable with a previously reported 2-year survival of 88.8% [16]. Another study reported a 5-year survival of 48% in SSc-ILD treated with oral cyclophosphamide [26]. SSc-ILD diagnosed due to a decline in screening PFTs had a better 5-year survival than those presenting with respiratory symptoms. However, we do not have information about the cause of death and are unable to say if the survival benefit is due to improved respiratory outcome. None of the eight deaths reported here were directly related to i.v. cyclophosphamide treatment. Respiratory failure is the most frequent cause of death in other reports of SSc-ILD treated with i.v. cyclophosphamide [7, 12, 15–17, 23, 26]. Studies with <12-month follow-up do not report any deaths [8, 10, 11, 13, 18, 27].
The proportion of patients who could not complete treatment was comparable to that in the FAST trial [4]. However, side effects were more common than previous reports [4, 7–20, 22]; none of which reported severe neutropenia. Two patients with severe neutropenia had impaired renal function, and failure to reduce i.v. cyclophosphamide dose may have been a contributing factor. Lymphopenia responding to i.v. cyclophosphamide dose reduction has been reported [22].
This audit reflects clinical events and outcomes in the real world. The main strength is the inclusion of every patient with SSc-ILD treated with i.v. cyclophosphamide at three hospitals over 10 years, irrespective of disease severity, treatment outcome and their long-term follow-up.
However, there are several caveats. Firstly, the findings are limited by retrospective nature of routine data. However, large prospective studies examining predictors of outcome in SSc-ILD are difficult due to its rarity. Alternative approaches, based on observational methods, are needed if people with rare diseases are to receive safe and effective treatments [28, 29]. Secondly, we do not have information about subtype of ILD because HRCT scans were not reported according to ILD subtypes at our hospitals in early 2000s. However, similar to this report, patients in FAST or SLS were not classified according to their ILD subtypes [3, 4]. We are unable to access HRCT films, as many scans predate electronic archiving and are difficult to locate. Thirdly, HRCT scans have not been scored for ground glass changes and fibrosis. Again, this reflects clinical practise in our hospitals. Although this is a significant limitation, we have no reason to believe that it undermines the clinical message conveyed in this paper, that treatment of SSc-ILD diagnosed on screening PFTs may associate with a better respiratory outcome. Indeed, one of the potential explanations for this observation is that patients with SSc-ILD diagnosed on screening PFTs have limited or mild ILD. Finally, we do not have information about the cause of death. This was not documented in the case notes, as patients may have died at care homes or at another hospital.
In conclusion, treatment of SSc-ILD with i.v. cyclophopshamide stabilised lung function. Regular PFTs and advising patients to seek medical opinion at an early stage for any new-onset cough or dyspnoea may facilitate early diagnosis, treatment and improve respiratory outcome in SSc-ILD. However, further prospective studies are required to firmly establish this approach.
References
Steen VD, Medsger TA (2007) Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 66:940–944. doi:10.1136/ard.2006.066068
Avouac J, Kowal-Bielecka O, Landewe R, Chwiesko S, Miniati I, Czirjak L et al (2009) European league against rheumatism (eular) scleroderma trial and research group (eustar) recommendations for the treatment of systemic sclerosis: methods of elaboration and results of systematic literature research. Ann Rheum Dis 68:629–634. doi:10.1136/ard.2008.095299
Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE et al (2006) Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 354:2655–2666. doi:10.1056/NEJMoa055120
Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS et al (2006) A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 54:3962–3970. doi:10.1002/art.22204
de Groot K, Adu D, Savage CO (2001) The value of pulse cyclophosphamide in anca-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant 16:2018–2027. doi:10.1093/ndt/16.10.2018
Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM et al (2008) Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 177:1248–1254. doi:10.1164/rccm.200706-877OC
Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P (2002) Systemic sclerosis and interstitial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol 29:2371–2378
Kowal-Bielecka O, Kowal K, Rojewska J, Bodzenta-Lukaszyk A, Siergiejko Z, Sierakowska M et al (2005) Cyclophosphamide reduces neutrophilic alveolitis in patients with scleroderma lung disease: a retrospective analysis of serial bronchoalveolar lavage investigations. Ann Rheum Dis 64:1343–1346. doi:10.1136/ard.2004.033076
Airo P, Danieli E, Rossi M, Frassi M, Cavazzana I, Scarsi M et al (2007) Intravenous cyclophosphamide for interstitial lung disease associated to systemic sclerosis: results with an 18-month long protocol including a maintenance phase. Clin Exp Rheumatol 25:293–296
Airo P, Danieli E, Parrinello G, Antonioli CM, Cavazzana I, Toniati P et al (2004) Intravenous cyclophosphamide therapy for systemic sclerosis. A single-center experience and review of the literature with pooled analysis of lung function test results. Clin Exp Rheumatol 22:573–578
Davas EM, Peppas C, Maragou M, Alvanou E, Hondros D, Dantis PC (1999) Intravenous cyclophosphamide pulse therapy for the treatment of lung disease associated with scleroderma. Clin Rheumatol 18:455–461
Pakas I, Ioannidis JP, Malagari K, Skopouli FN, Moutsopoulos HM, Vlachoyiannopoulos PG (2002) Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol 29:298–304
Valentini G, Paone C, La Montagna G, Chiarolanza I, Menegozzo M, Colutta E et al (2006) Low-dose intravenous cyclophosphamide in systemic sclerosis: an open prospective efficacy study in patients with early diffuse disease. Scand J Rheumatol 35:35–38. doi:10.1080/03009740510026896
Giacomelli R, Valentini G, Salsano F, Cipriani P, Sambo P, Conforti ML et al (2002) Cyclophosphamide pulse regimen in the treatment of alveolitis in systemic sclerosis. J Rheumatol 29:731–736
Simeon-Aznar CP, Fonollosa-Pla V, Tolosa-Vilella C, Selva OCA, Solans-Laque R, Palliza E et al (2008) Intravenous cyclophosphamide pulse therapy in the treatment of systemic sclerosis-related interstitial lung disease: a long term study. Open Respir Med J 2:39–45. doi:10.2174/1874306400802010039
Berezne A, Ranque B, Valeyre D, Brauner M, Allanore Y, Launay D et al (2008) Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol 35:1064–1072
Yiannopoulos G, Pastromas V, Antonopoulos I, Katsiberis G, Kalliolias G, Liossis SN et al (2007) Combination of intravenous pulses of cyclophosphamide and methylprednizolone in patients with systemic sclerosis and interstitial lung disease. Rheumatol Int 27:357–361. doi:10.1007/s00296-006-0217-1
Ostojic P, Damjanov N (2006) Improvement of lung function in patients with systemic sclerosis after 6 months cyclophosphamide pulse therapy. Clin Rheumatol 25:819–821. doi:10.1007/s10067-005-0173-2
D’Angelo S, Cuomo G, Paone C, Colutta E, La Montagna G, Valentini G (2003) Low-dose intravenous cyclophosphamide in systemic sclerosis: a preliminary safety study. Clin Rheumatol 22:393–396. doi:10.1007/s10067-003-0756-8
Varai G, Earle L, Jimenez SA, Steiner RM, Varga J (1998) A pilot study of intermittent intravenous cyclophosphamide for the treatment of systemic sclerosis associated lung disease. J Rheumatol 25:1325–1329
Wanchu A, Suryanaryana BS, Sharma S, Sharma A, Bambery P (2009) High-dose prednisolone and bolus cyclophosphamide in interstitial lung disease associated with systemic sclerosis: a prospective open study. Int J Rheum Dis 12:239–242. doi:10.1111/j.1756-185X.2009.01417.x
Domiciano DS, Bonfa E, Borges CT, Kairalla RA, Capelozzi VL, Parra E et al (2010) A long-term prospective randomized controlled study of non-specific interstitial pneumonia (nsip) treatment in scleroderma. Clin Rheumatol. doi:10.1007/s10067-010-1493-4
Volpinari S, La Corte R, Bighi S, Ravenna F, Prandini N, Lo Monaco A et al (2010) Bronchoalveolar lavage in systemic sclerosis with lung involvement: role and correlations with functional, radiological and scintigraphic parameters. Rheumatol Int. doi:10.1007/s00296-010-1390-9
Herrick AL, Lunt M, Whidby N, Ennis H, Silman A, McHugh N et al (2010) Observational study of treatment outcome in early diffuse cutaneous systemic sclerosis. J Rheumatol 37:116–124. doi:10.3899/jrheum.090668
White B, Moore WC, Wigley FM, Xiao HQ, Wise RA (2000) Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and alveolitis. Ann Intern Med 132:947–954
Steen VD, Lanz JK Jr, Conte C, Owens GR, Medsger TA Jr (1994) Therapy for severe interstitial lung disease in systemic sclerosis. A retrospective study. Arthritis Rheum 37:1290–1296. doi:10.1002/art.1780370904
Schnabel A, Reuter M, Gross WL (1998) Intravenous pulse cyclophosphamide in the treatment of interstitial lung disease due to collagen vascular diseases. Arthritis Rheum 41:1215–1220. doi:10.1002/1529-0131(199807)41:7<1215::AID-ART11>3.0.CO;2-Y
Rawlins MD (2008) The harveian oration of 2008: on the evidence for decisions about the use of therapeutic interventions. Royal College of Physicians. London (UIK). ISBN: 9781860163470
Lagakos SW (2003) Clinical trials and rare diseases. N Engl J Med 348:2455–2456. doi:10.1056/NEJMe030024
Disclosures
None.
Details of funding
None of the authors have received any funding.
Details of the role of the study sponsors
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional information
Key message
• I.V. cyclophosphamide stabilises lung function in SSc-ILD.
• Treatment with i.v. cyclophosphamide may be more effective in less severe SSc-ILD.
Rights and permissions
About this article
Cite this article
Abhishek, A., Yazdani, R., Pearce, F. et al. Outcome of systemic sclerosis associated interstitial lung disease treated with intravenous cyclophosphamide. Clin Rheumatol 30, 1099–1104 (2011). https://doi.org/10.1007/s10067-011-1734-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1734-1