Abstract
Churg–Strauss syndrome (CSS) is a rare, systemic, necrotizing, small- and middle-sized vessel vasculitis which is accompanied by blood eosinophilia, eosinophil infiltration of various tissues, and bronchial asthma. The lungs are the organs most often involved in CSS. The aim of this study was a retrospective evaluation of the pulmonary findings in chest X-rays and high resolution computed tomography (HRCT) in CSS patients at the time of initial diagnosis and to determine their frequency, character, and location. Seventeen CSS patients were studied (12 women; 5 men; aged 29–56 years). In all patients at the time of initial diagnosis, chest X-rays were performed, and in 15 patients, HRCT was performed additionally. The radiological images were evaluated independently by two radiologists who reached a decision by consensus. Out of 17 patients studied, chest X-rays revealed parenchymal abnormalities in 11, pleural effusion in three, and bronchial wall thickening in one. In five patients, no abnormalities in chest X-rays were found. In HRCT, abnormalities were found in all patients (15 patients, 100%). Predominant HRCT findings consisted of: ground-glass opacities and consolidations found in 13 patients (86.7%). Additionally, in four patients, pulmonary micronodules were described; in ten, interlobular septal thickening; in three, linear opacities; in ten, bronchial wall thickening and/or bronchial dilatation; and in three, pleural effusions. Ground-glass opacities and consolidation abnormalities distribution pattern were peripheral in seven and random in six patients. In patients with CSS, the most common pulmonary radiological findings are parenchymal opacities, which may be peripheral or random in distribution. Pathologic changes were found in 70.6% of patient in chest X-rays, and in 100%, when HRCT was performed. These changes are nonspecific; however, they should be not overlooked, as they may help in establishing the diagnosis and suggest the prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Churg–Strauss syndrome (CSS) is a rare disease (morbidity rate of 0.5–6.8/1 million persons), in the course of which a granulomatous inflammation of the respiratory tract with small- and medium-sized vessel vasculitis is accompanied by eosinophil infiltration of tissues and eosinophilia in peripheral blood [1–3]. In almost all cases, these changes are associated with asthma, and frequently, with other allergic diseases: sinusitis and allergic rhinitis [4]. Currently, the disease is diagnosed based on criteria introduced in the year 1990 by the American College of Rheumatology (ACR) [5], in which six key features were distinguished. The presence of four of the mentioned criteria makes the diagnosis highly probable: asthma, peripheral blood eosinophilia higher than 10%, mono- or polyneuropathy, paranasal sinus abnormality, eosinophilic accumulation in tissues revealed on biopsy, and migratory infiltrates in lungs. The latter were traditionally observed in chest X-rays as transient patchy infiltrates, often with non-necrotic nodules of noncharacteristic location [6]. Lately, in a few studies, chest CT scans were analyzed; however, due to the variability of the nonspecific lesions and small groups of patients, no characteristic findings in CSS were discovered. The most frequently described lesions were interstitial ground-glass opacities and bronchial wall thickening [7–11].
The aim of this study was a retrospective evaluation of radiologic findings based on chest X-rays and CT scans of 17 patients with CSS at the moment of diagnosis, as well as determining the frequency, character, and location of the lesions. We also compare the results obtained by us with previous reports on this subject.
Materials and methods
Subjects
In the Chest Diseases and Allergy and Immunology Departments of the Jagiellonian University in Cracow, medical databases were searched for patients diagnosed with CSS between years 1999–2009. All patients fulfilled at least four out of six classic ACR criteria of CSS. Seventeen patients have been found, and their medical documentation at the time of diagnosis was analyzed retrospectively. The group consisted of 12 female and five male patients aged 29 to 56 years (mean age 40.4 ± 8.6 years). The activity of the disease was assessed using the Birmingham vasculitis activity score (BVAS scale [12]; range: 0–63 pts.; above 4 assumed as active disease), and the severity of asthma using the Global Initiative for Asthma 2008 protocol [13].
Imaging examinations
In all patients posteroanterior and lateral chest X-rays, and in 15, additionally high resolution computed tomography scanning (HRCT) were performed. The scans covered the total lung area, with 1-mm thick sections at 10-mm intervals, using a maximum lamp voltage of 125–140 kVp (Elscint, CT-twin) or 1-mm sections at 1-mm intervals with a spiral technique and maximum lamp voltage of 110–120 kVp (Toshiba, Aquilion-64). High resolution algorithm was applied.
In the study, the radiographs were evaluated independently by two experienced radiologists. The final interpretation included the character of the alterations (parenchymal or bronchial lesions, pleural effusion, enlarged lymph nodes), as well as their location. The lesions were defined according to the established general guidelines [14]. Parenchymal abnormalities in HRCT included: ground-glass opacities, consolidation, pulmonary nodules, interlobular septal thickening, and linear opacities. Distribution of parenchymal abnormalities on CT scans was categorized as: unilateral or bilateral; peripheral (subpleural), central or random in the transverse plane; upper, middle, lower zones or all zones in the longitudinal plane. Bronchial lesions in HRCT included bronchial dilatation and/or bronchial wall thickening.
Results
Patients
The patients were in an active phase of the disease (BVAS 22.76 ± 7.2 points) during examination. All of them were diagnosed with severe bronchial asthma (average duration of 4.7 ± 7.5 years). Each of them complained of dyspnea on admission, 15 patients (88.2%) additionally had dry cough, and 16 (94.1%) reported symptoms of rhinitis or paranasal sinusitis. Lung biopsies were performed in three patients (17.6%); the findings were typical for CSS histopathology. The CT scans of paranasal sinuses of all the patients revealed thickening of the sinus mucosa, and two (11.8%) additionally had polyps. The skin prick tests for standard inhaled allergens were positive in 11 subjects (64.7%). Constitutional symptoms (fever, weakness, weight loss) were observed in 16 (94.1%) of the examined patients. Antineutrophil cytoplasmic antibodies with perinuclear pattern (p-ANCA) were detected in the serum of five (29.4%) of the patients. The mean absolute eosinophilia in peripheral blood at the moment of diagnosis of CSS was 6,124 cells/μl (normal, <440). None of the patients had clinical nor laboratory features of a respiratory tract infection; the bacteriology and mycology results of sputum and/or bronchial lavage were also negative. The clinical characteristics are presented in Table 1.
Radiologic findings
Chest X-rays
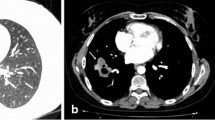
All patients had chest X-rays performed. In 11 patients (64.7%), bilateral patchy parenchymal opacities were observed, especially in the lower and middle lung areas (Fig. 1); one person had bronchial wall thickening in parahiliar areas, and in three patients, pleural effusions were detected. Among five patients (29.4%), no pathological lesions were found; in one of them, HRCT was not performed, and in the remaining four, the CT scans revealed changes which were not visible in chest X-rays (Table 2).
Chest HRCT
HRCT was performed in 15 patients, and in all of them, pathologic findings were discovered. The most frequently observed anomalies were parenchymal attenuations in the form of ground-glass opacities and consolidations (13 persons, 86.7%; Fig. 2). In seven patients (53.8%), the lesions were located mainly peripherally (subpleurally); in six patients (46.2%), the changes had a random distribution; nonetheless, they were always bilateral. The prevailing location of the lesions, depending on the lung zone was as follows: in five persons (38.4%) in the lower zones, in three persons (23%) in the lower and middle zones, in one person in the middle zone, in one person in the middle and upper zones, in two persons (15.4%) in the upper zones, and in one person in all of the lung zones. Furthermore, in four persons (26.6%), pulmonary non-necrotic nodular opacities of 3 mm to 1 cm in diameter were found (Fig. 3), located usually peripherally and most often in lower and middle lung zones. In two patients they consisted of isolated centrilobular micronodules; in others—multiple, diffuse, several-millimeter nodules overlapping the parenchymal lesions were predominant in the HRCT image. Moreover, in ten patients (66.6%), interlobular septal thickening (Fig. 4), and in three subjects (20%), linear opacities were observed. The bronchial wall thickening and/or bronchial dilatation (Fig. 4) concerned ten patients (66.6%), and the changes depended mostly on the duration of asthma. Bronchial lesions were present mainly in the lower and middle lung zones (Fig. 5). In one patient, the mediastinal lymph nodes were slightly enlarged with a diameter of about 10 mm. Moreover, in three patients (17.6%), the imaging examinations revealed pleural effusion. Comparison of our results with previous studies is presented in Table 3.
Discussion
The natural history of CSS usually consists of three stages. In the first, prodromal period, which lasts several years, allergic rhinitis appears, later joined by asthma; in the second stage, eosinophilia in peripheral blood prevails with eosinophil tissue infiltrations, progressing next into the third phase with the life-threatening vasculitis [3]. The disease is usually diagnosed at the point when significant eosinophilia is already present, and multiple organs are affected accompanied by asthma—by then, changes in the respiratory system are present in most persons, as it was in our patients.
Most of the radiological observations published were limited to routine X-rays, while HRCT imaging was performed only in a few recent reports [7–11]. These reports are restricted in number and scanty, because the disease is so rare; it affects one person in a million. Our study, therefore, adds to the existing knowledge, pointing to the value of HRCT in early detection of pulmonary changes in CSS syndrome.
Medical documentation of all 17 patients at the initial diagnosis, during active phase of the disease, was analyzed. All of the patients were in a severe exacerbation of the disease, and in most patients, the treatment was not yet administered due to the diagnostic process in progress (Table 2). It is important, while in other vasculitides, the pulmonary lesions tend to regress after introducing treatment with glucocorticosteroids [9, 15], hence, most certainly the multiple pathologic findings in the studied group.
In chest X-rays, abnormalities were present in 12 patients (70.6%), which corresponds with earlier publications, among them the study by Lehman, which was a systematic review of available case reports (collected from 138 patients with CSS), in which lesions in chest X-rays were present in 74% of subjects [16]. Even though in four cases the chest X-rays were normal, the HRCT revealed significant pathologic findings.
In HRCT images prevailed parenchymal alterations in the form of ground-glass opacities and consolidations, which were present among 86.7% of patients. They were situated peripherally or had random distribution, and were always present bilaterally. The character and location of the mentioned lesions is in accordance with previous studies [9, 10, 17]. Among four patients (26.7%), apart from the dominant findings, non-necrotic micronodular opacities were discovered. They were identified more frequently in earlier publications [9]. It might be caused by the fact that nodular lesions in pathological examination correlate with the presence of places of bleeding from small vessels and necrotic granulomas. They are more common among patients with positive p-ANCA antibodies [9, 17], whereas in our group, p-ANCA was detected only in five persons (all of these patients presented with myeloperoxidase p-ANCA type).
Bronchial wall thickening and/or bronchial dilatation in HRCT were observed among ten patients (66.6%). It was most likely due to the coexistence of severe asthma. Such changes are often described in severe forms of asthma [18]. In accordance with this, the findings were more intense among patients whose asthma preceded for a long time the diagnosis of the disease. The most advanced changes concerned a patient with a 30-year-long asthma history.
While describing the bronchial findings, it should be noted that they were not caused by infection. Heart insufficiency must also be taken into consideration, which also occurs in the course of CSS, and may be responsible for causing the mentioned changes and interlobular septal thickening or pleural effusion.
In differential diagnosis of the presented parenchymal changes, first of all other interstitial lung diseases, especially eosinophilic pneumonitis and other vasculitis syndromes, should be considered.
Resuming, in this study, chest X-rays and HRCT images of 17 patients at the moment of diagnosis of CSS were analyzed. The types of radiologic findings and their frequency remain in accordance with previous publications. The findings are not pathognomonic for CSS, but their frequency along with the coexistence of certain clinical features of the disease may facilitate the diagnosis.
References
Churg J, Strauss L (1951) Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 27(2):277–301
Pagnoux C, Guilpain P, Guillevin L (2007) Churg-Strauss syndrome. Curr Opin Rheumatol 19(1):25–32
Noth I, Strek ME, Leff AR (2003) Churg-Strauss syndrome. Lancet 361(9357):587–594
Abril A, Calamia KT, Cohen MD (2003) The Churg Strauss syndrome (allergic granulomatous angiitis): review and update. Semin Arthritis Rheum 33(2):106–114
Masi AT et al (1990) The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 33(8):1094–1100
Chumbley LC, Harrison EG Jr, DeRemee RA (1977) Allergic granulomatosis and angiitis (Churg-Strauss syndrome). Report and analysis of 30 cases. Mayo Clin Proc 52(8):477–484
Worthy SA et al (1998) Churg-Strauss syndrome: the spectrum of pulmonary CT findings in 17 patients. AJR Am J Roentgenol 170(2):297–300
Choi YH et al (2000) Thoracic manifestation of Churg-Strauss syndrome: radiologic and clinical findings. Chest 117(1):117–124
Kim YK et al (2007) Pulmonary involvement in Churg-Strauss syndrome: an analysis of CT, clinical, and pathologic findings. Eur Radiol 17(12):3157–3165
Silva CI et al (2005) Churg-Strauss syndrome: high resolution CT and pathologic findings. J Thorac Imaging 20(2):74–80
Furuiye M et al (2010) Churg-Strauss syndrome versus chronic eosinophilic pneumonia on high-resolution computed tomographic findings. J Comput Assist Tomogr 34(1):19–22
Luqmani RA et al (1994) Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 87(11):671–678
Bateman ED et al (2008) Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 31(1):143–178
Austin JH et al (1996) Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 200(2):327–331
Szczeklik W et al (2010) Lung involvement in Churg-Strauss syndrome as related to the activity of the disease. Allergy
Lanham JG et al (1984) Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore) 63(2):65–81
Ando Y et al (2004) Thoracic manifestation of myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA)-related disease. CT findings in 51 patients. J Comput Assist Tomogr 28(5):710–716
Silva CI, Colby TV, Muller NL (2004) Asthma and associated conditions: high-resolution CT and pathologic findings. AJR Am J Roentgenol 183(3):817–824
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szczeklik, W., Sokołowska, B., Mastalerz, L. et al. Pulmonary findings in Churg–Strauss syndrome in chest X-rays and high resolution computed tomography at the time of initial diagnosis. Clin Rheumatol 29, 1127–1134 (2010). https://doi.org/10.1007/s10067-010-1530-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-010-1530-3