Abstract
Intra-articular hyaluronic acid has been used in treatment of patients with knee osteoarthritis. Though its effect on pain has been well studied, it is not clear how it affects the articular cartilage. This is a preliminary study to evaluate the kinetics of urinary collagen type-II C-telopeptide (CTX-II) as a biomarker of collagen breakdown in response to intra-articular hyaluronic acid injection in patients with symptomatic knee osteoarthritis. Intra-articular injections of hyaluronan were administered to ten patients with symptomatic knee osteoarthritis. Urine collection for urinary CTX-II was obtained at baseline, before each injection and once every other week for a total of 6 months. Urine CTX-II was measured using a CartiLaps© ELISA kit. There was a statistically significant increase (p = 0.0136) in CTX-II a week after the third intra-articular injection of hyaluronic acid (6,216 ng/mmol ± 4,428) compared with baseline (2,233 ng/mmol ± 1,220). This increase in CTX-II was sustained throughout the entire 6 months follow-up period (repeated measures ANOVA, p < 0.015). This is the first study of changes in an osteoarthritis biomarker after intra-articular hyaluronic acid injections in patients with symptomatic knee osteoarthritis. Contrary to our initial hypothesis that CTX-II levels should decrease after intra-articular hyaluronic acid injections, we found a significant increase in urinary CTX-II levels that was sustained throughout the study. These observations suggest that intra-articular hyaluronic acid injections may accelerate cartilage breakdown in patients with symptomatic knee osteoarthritis. The responsible mechanisms are unknown and warrant further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis is a degenerative joint disease characterized by articular cartilage breakdown, joint space narrowing, osteophyte formation and subchondral sclerosis. It is more common among the elderly and is the leading cause for physical disability, low quality of life and health-care costs in industrialized societies [1]. Approximately 13.9% of adults more than 25 years old and 33.6% (12.4 million) of those >65 are affected with OA. The prevalence of symptomatic knee OA accounts for 6.3% of adults more than 30 years old and 9.5% in 63 to 93 years old. In 1997, the estimated cost of hip and knee replacement was 7.9 billion dollars and accounted for 7.1 million of all arthritis-related ambulatory care visits [2]. Osteoarthritis is a heterogenious disease with varied clinical presentations, clinical course and outcomes. Many efforts have been made to prevent or slow the progression of this condition without much long-term success.
Cartilage has been the target for most of the therapeutic approaches in osteoarthritis. It is composed of chondrocytes surrounded by an extra-cellular matrix containing collagen fibers and aggregates which include hyaluronic acid (hyaluronate) and aggrecans. The primary role of hyaluronate in the matrix is to maintain its viscoelasticity and provide loading protection to the cartilage. Hyaluronate was FDA approved in 1997 for intra-articular injection to provide viscosupplementation for the treatment of knee osteoarthritis. Intra-articular hyaluronic acid is indicated for patients who have failed conservative management, patients who cannot tolerate non-steroidal anti-inflammatory drugs because of renal and/or gastrointestinal intolerance, and patients who refuse or in whom surgery is contraindicated [3]. A recent review of randomized placebo-controlled trials suggests that intra-articular hyaluronic acid injections are safe, tolerable, and effective in reducing pain in patients with symptomatic osteoarthritis of the knee [4]. Other studies comparing intra-articular hyaluronic acid and intra-articular corticosteroids concluded that HA may provide superior treatment for chronic osteoarthritis [5]. In vitro studies have shown Fas-induced apoptosis of chondrocytes in patients with osteoarthritis is suppressed by intra-articular hyaluronic acid [6]. The anti-apoptotic effect of hyaluronic acid is mediated by its binding to specific receptors, CD44 and ICAM, suggesting that HA may decrease progression of chondrocyte apoptosis in OA.
Molecular markers have been identified that reflect joint damage and changes in cartilage metabolism. Christgau et al. first described an immunoassay for the measurement of urinary collagen type-II C-telopeptide (CTX-II) as an indicator of cartilage degradation; this study demonstrated a significant increase in urinary CTX-II in patients with RA and OA [7]. CTX-II values in patients with knee OA compared with healthy controls are significantly higher [8]. Hence, CTX-II has been considered to be a biomarker that monitors progression of OA [8–10]. A relationship between radiographic changes in OA and CTX-II levels has also been described [11, 12]. Furthermore, elevation of CTX-II has been associated with subchondral bone changes identified by MRI in patients with OA of the knee [13, 14].
Early disease detection in osteoarthritis remains a challenge. Symptoms of osteoarthritis correlate poorly with radiographic changes [15]. However, a recent study suggests that urinary CTX-II levels correlate with pain in patients with symptomatic knee osteoarthritis [16]. Though there are many studies evaluating pain after treatment with intra-articular hyaluronan, there are none that relate changes in biomarkers with this therapy. The objective of this pilot study was to evaluate the relationship between urinary CTX-II as a biomarker of collagen breakdown and intra-articular treatment with hyaluronic acid in patients with symptomatic knee OA. Given prior evidence of the relationship of CTX-II levels and disease progression, we postulated that the levels of urinary CTX-II would decrease after intra-articular HA injection.
Materials and methods
Patients
We recruited ten patients with symptomatic knee osteoarthritis who failed treatment with oral analgesics and prior steroid injections who were not considered sufficiently advance to warrant knee arthroplasty. Patients had a mean age of 59.4 and a mean BMI of 28.93 (Table 1). We also included one healthy control for internal validation purposes. Inclusion criteria included subjects more than 18 years old with radiographic evidence of osteoarthritis who were dissatisfied with prior attempts at non-surgical management, including non-steroidal anti-inflammatory drugs (NSAIDs), other oral analgesics, physical therapy, knee braces, and nutritional supplements. Patients who were taking NSAIDs were allowed to maintain their regular dose but not to change the dose for the duration of study, given that NSAIDs may affect OA biomarker measurements [17]. If during the study subjects experience knee pain, they were allowed to use Tramadol 50 to100 mg every 6–8 h as needed for pain control. Tramadol is not known to have anti-inflammatory effects that affect OA biomarkers.
Patients were excluded if radiographs showed bone-on-bone arthritis, chondrocalcinosis, and physical examination demonstrated any insufficiency of the anterior or posterior ligaments, collateral ligaments, and history of crystalline, inflammatory or, neuropathic arthropathy or infection in the affected extremity. Patients who had intra-articular knee injection with corticosteroids or hyaluronic acid within the previous three months, had allergies or hypersensitivity to eggs, feathers, avian proteins or chicken, pregnant or lactating women, and patients who carried a diagnosis of diabetes mellitus or severe obesity defined as a body mass index more than 40 were also excluded.
Study protocol
This 6-month prospective pilot study was conducted at the Veterans Affair Hospital in Houston Texas. The study was approved by the institutional review board, all participating patients provided informed written consent and the study was performed in accordance to the Declaration of Helsinki.
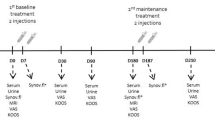
We obtained weight-bearing anteroposterior, lateral and patellar skyline knee radiographs from all patients. Patients then received an intra-articular hyaluronic acid injection on the affected knee or knees weekly for three consecutive weeks. A total of 16 knees were injected. Patients 1, 4, 6, 7, 8, and 10 received intra-articular injections in both knees, patients 3, 5, and 9 on the left knee and patient 2 on the right knee. Each injection was given with the use of the manufacturer's prefilled 2.5-ml syringe, containing 25 mg of sodium hyaluronate (molecular weight of 620,000–1,170,000 daltons) dissolved in a physiological saline, with a pH of 6.8–7.8 [3]. If the patient had a significant knee effusion, the synovial fluid was aspirated prior to intra-articular injection of hyaluronan. No additional intra-articular injections were allowed during the 6 months study. All injections were performed under sterile conditions with anatomical guidance of an orthopedic physician, rheumatology fellow or an orthopedic-trained physician assistant. After the procedure patients were instructed to refrain from any strenuous activity for 24 h.
Urine for osteoarthritis biomarker CTX-II was collected at baseline, then weekly before each intra-articular injection and then every 2 weeks until completion of 6 months of study. Given the evidence of strongest correlation between radiographic severity, knee osteoarthritis and biomarkers when samples were collected in the first 4 h after getting up from bed, subjects were instructed to collect the urine samples 4–6 h after getting up in the morning [18]. Reminders for urine collection the day prior each urine collection was given to each patient by phone. Patients were instructed to store urine samples at temperatures lower than −5°C at their respective homes [19]. Aliquots of urine were frozen at −80°C within 24 h of collection. At the end of the 6 months of study, all urine samples were thawed and assayed for CTX-II by a specific competitive ELISA using a mouse monoclonal antibody, MAbF46, directed against a linear six amino acid epitope (EKGPDP) of human type-II collagen C-telopeptide (CartiLabs ©ELISA kit). CTX-II levels were then corrected for patients' creatinine and analysed using Kruskall–Wallis statistics for one-way repeated ANOVA, with the aid of Statistica Release 6 from Statsoft, Tulsa, OK, US.
Results
All patients were able to collect their urine samples in the first 4 hours after getting up from bed and store them at a temperature −5°C. After 6 months of urine collection frequent measurements of urinary CTX-II were obtained (Table 2). One urine collection from patient #7 at week 12 was missing. The majority of the patients experienced pain relief after the 3rd intra-articular injection. Only one patient reported no relief from the treatment.
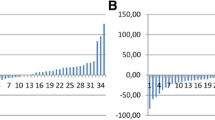
CTX-II values were significantly increased from the 3rd week after injections began. Baseline measurement showed 2,233 ng/mmol ± 1,221; at week three the average level was 6,216 ng/mmol ± 4,428 (p = 0.014). This increase in CTX-II was sustained throughout the entire 6-month follow-up period (Fig. 1). Values were corrected for differences in plasma creatinine. Both analyses showed a statistically significant increase in urinary CTX-II levels that persisted through the 6th month of the study (p = 0.015) (Fig. 1).
Discussion
There is a large amount of clinical evidence showing symptomatic improvement with viscosupplementation in patients with knee OA [2]. However, we do not know whether this treatment slows the progression of knee OA. Urinary CTX-II had been shown to be an excellent marker of cartilage breakdown; levels correlate with OA disease severity. Repeated courses of viscosupplementation had been given only when pain from knee OA recurs; the treatment is effective in relieving pain for four to six months. Though viscosupplementation is not considered to be disease modifying in OA, there are some animal studies showing that it may also be “chondroprotective”. Since pain in knee OA does not correlate well with radiographic changes and pain does not arise from the articular cartilage, we postulated that cartilage breakdown may precede pain. Hence, when patients have recurrence of pain, significant amount of cartilage breakdown could have already occurred. We reasoned that if intra-articular HA injections protect cartilage we would find that OA biomarkers decrease after treatment and then, as the product is metabolized, they would begin to increase once more. We postulated that if we knew the kinetics of urinary CTX-II after intra-articular HA injections we could time injections appropriately to forestall further cartilage injury and prevent recurrence of pain. However, contrary to what we had expected, we found a highly significant increase in urinary CTX-II levels 3 weeks after the injection in all ten patients that was sustained throughout the remainder of the study. These observations suggest that intra-articular HA injections accelerate cartilage breakdown in patients with symptomatic knee osteoarthritis.
The main symptom in patients with osteoarthritis that brings them to a physician is pain. It is the stimulation of nociceptor nerve endings located at the joint capsule, synovium, and subchondral bone, not the cartilage, which causes pain in OA. Multiple animal studies support the idea that high molecular weight HA intra-articular injections reduce pain. This result is attributed to the decrease in concentration of inflammatory substances and reduction of transmission of mechanical forces to nociceptor nerve endings by increasing synovial fluid viscosity [20–22]. Even though pain is the main symptom in OA, it is also important to reduce cartilage breakdown. Our study suggests that HA provides temporary relief of pain at the expense of hastening OA progression.
One possible explanation for our findings is that even though the substance injected mainly contains high molecular weight (HMW) HA, it may also contain short HA fragments which displace HMW HA from CD44, the major chondrocyte cell receptor for HA. This may make the extra-cellular matrix unstable and cause chondrolysis. Knudson et al. demonstrated that exposure to hyaluronan hexasaccharides will cause chondrolysis and loss of proteoglycan-rich matrix [23]. Termeer et al. suggested that low molecular weight and not high molecular weight HA fragments stimulate dendritic cell maturation, pro-inflammatory cytokine production and T cell activation [24]. These may increase matrix metalloproteinase production and cartilage breakdown [25].
Another possibility is that insertion of a needle in the joint triggered a response that resulted in sustained matrix degradation. While acute joint injury may cause inflammation sufficient to increase urinary CTX-II levels [26, 27], it is unlikely that this increase in urinary CTX-II would be delayed until the 3rd week after the start of the treatments or that shedding of CTX-II would persist for more than 5 months after the treatments were stopped. If needle injury was the cause of CTX-II release, we would expect levels to increase after the first week not 3 weeks or more after the first intra-articular injection. In addition, if needle injury was the cause of CTX-II increase, we would expect CTX-II to decline after a couple of weeks, but in all patients these measurements remained elevated for 6 months.
Throughout the study period, our subjects had reported minimal changes in their physical activities after having IA HA injections. Even if the subjects were more active after the injections, it would be highly improbable that the observed 2–3-folds rise in urinary CTX-II from weeks 3 to 24 has resulted from increased physical activities. Though serum cartilage oligomeric matrix protein (COMP) has been noted to increase in subjects undergoing strenuous exercises, the rise is lower (estimated to be 1.6 folds in subjects running marathons and ultra-marathons) and short-lived (lasting 2 days) [28]. Urinary CTX-II has not been studied in the same way but Ganero et al. has reported that urinary CTX-II is highly correlated to serum COMP as biomarkers of cartilage degradation [29].
Since majority of the HA preparations are derived from rooster combs, there is concern that avian protein impurities may contain chicken type-II collagen. From the manufacturers’ data, HA preparations like Orthovisc® and Supartz® contain minute amount of protein impurities (less than 2 ppm) [30]. Therefore, only 150 ng of avian protein would have been introduced in each set of three injections given to the patient. Taking an average urinary output of 2 l/day per subject, the excess urinary CTX-II peptide that each subject in our study would have excreted from weeks 3 to 24 compared with baseline amounted to approximately 1 mg. Thus it is impossible that the excess excreted CTX-II observed in the study could have originated from avian protein impurities from the HA preparations
This study had several limitations. Only ten patients were treated and followed. We used only one biomarker (CTX-II); nevertheless this was sufficient to show sustained evidence of matrix degradation.
Studies evaluating the impact of different therapeutic modalities like NSAID’s, calcitonin and bisphosphonates on joint matrix integrity have used CTX-II measurements to assess the ability of these agents to prevent progression of OA [17, 31–34]. However, this is the first study that looks at the impact of intra-articular HA injections on the release of joint matrix collagen peptides. While the mechanisms responsible for this loss of matrix remain obscure, these results suggest that investigations to identify the cause of this outcome are indicated to mitigate this surprising and unwarranted side effect of HA injections.
Abbreviations
- CTX-II:
-
Collagen type-II C-telopeptide
- OA:
-
Osteoarthritis
- HA:
-
Hyaluronic acid
- RA:
-
Rheumatoid arthritis
- HMW:
-
High molecular weight
References
Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF (1987) The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 30(8):914–918
National Center for Chronic Disease Prevention and Health Promotion (2008) http://www.cdc.gov/arthritis/arthritis/osteoarthritis.htm. Last revised: January 11
Supartz package insert. In: Smith and Nephew, Memphis; Revised: January 30 2007
Brzusek D, Petron D (2008) Treating knee osteoarthritis with intra-articular hyaluronans. Curr Med Res Opin 24(12):3307–3322
Caborn D, Rush J, Lanzer W, Parenti D, Murray C (2004) A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol 31(2):333–343
Lisignoli G, Grassi F, Zini N, Toneguzzi S, Piacentini A, Guidolin D et al (2001) Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) invovement. Arthritis Rheum 44(8):1800–1807
Christgau S, Garnero P, Fledelius C, Moniz C, Ensig M, Gineyts E et al (2001) Collagen type II C-telopeptide fragments as an index of cartilage degradation. Bone 29(3):209–215
Garnero P, Ayral X, Rousseau JC, Christgau S, Sandell LJ, Dougados M et al (2002) Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum 46(10):2613–2624
Garnero P, Conrozier T, Christgau S, Mathieu P, Delmas PD, Vignon E (2003) Urinary type II collagen C-telopeptide levels are increased in patients with rapidly destructive hip osteoarthritis. Ann Rheum Dis 62(10):939–943
Reijman M, Hazes JM, Bierma-Zeinstra SM, Koes BW, Christgau S, Christiansen C et al (2004) A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum 50(8):2471–2478
Jordan KM, Syddall HE, Garnero P, Gineyts E, Dennison EM, Sayer AA et al (2006) Urinary CTX-II and glucosyl-galactosyl-pyridinoline are associated with the presence and severity of radiographic knee osteoarthritis in men. Ann Rheum Dis 65(7):871–877
Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P (2006) Hellio Le Graverand MP, et al. Urinary CTX-II levels are associated with radiographic subtypes of osteoarthritis in hip, knee, hand, and facet joints in subject with familial osteoarthritis at multiple sites: the GARP study. Ann Rheum Dis 65(3):360–365
Bruyere O, Collette J, Kothari M, Zaim S, White D, Genant H et al (2006) Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis 65(8):1050–1054
Garnero P, Peterfy C, Zaim S, Schoenharting M (2005) Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: a three-month longitudinal study. Arthritis Rheum 52(9):2822–2829
Bedson J, Croft PR (2008) The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 9:116
Zhai G, Cicuttini F, Ding C, Scott F, Garnero P, Jones G (2007) Correlates of knee pain in younger subjects. Clin Rheumatol 26(1):75–80
Gineyts E, Mo JA, Ko A, Henriksen DB, Curtis SP, Gertz BJ et al (2004) Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis. Ann Rheum Dis 63(7):857–861
Kong SY, Stabler TV, Criscione LG, Elliott AL, Jordan JM, Kraus VB (2006) Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum 54(8):2496–2504
Qvist P, Munk M, Hoyle N, Christiansen C (2004) Serum and plasma fragments of C-telopeptides of type I collagen (CTX) are stable during storage at low temperatures for 3 years. Clin Chim Acta 350(1–2):167–173
Pozo MA, Balazs EA, Belmonte C (1997) Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res 116(1):3–9
Gomis A, Miralles A, Schmidt RF, Belmonte C (2007) Nociceptive nerve activity in an experimental model of knee joint osteoarthritis of the guinea pig: effect of intra-articular hyaluronan application. Pain 130(1–2):126–136
Gomis A, Miralles A, Schmidt RF, Belmonte C (2009) Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthritis Cartilage 17(6):798–804
Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB (2000) Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum 43(5):1165–1174
Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P et al (2000) Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol 165(4):1863–1870
Fernandes JC, Martel-Pelletier J, Pelletier JP (2002) The role of cytokines in osteoarthritis pathophysiology. Biorheology 39(1–2):237–246
Lohmander LS, Atley LM, Pietka TA, Eyre DR (2003) The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum 48(11):3130–3139
Nielsen RH, Stoop R, Leeming DJ, Stolina M, Qvist P, Christiansen C et al (2008) Evaluation of cartilage damage by measuring collagen degradation products in joint extracts in a traumatic model of osteoarthritis. Biomarkers 13(1):79–87
Kim HJ, Lee YH, Kim CK (2009) Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195 km) and an ultra-marathon (200 km) race. Eur J Appl Physiol 105(5):765–770
Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E (2001) Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis 60(6):619–626
Depuy Website: http://www.orthoviscline.com/replace-what-missing/safety
Bagger YZ, Tanko LB, Alexandersen P, Karsdal MA, Olson M, Mindeholm L et al (2005) Oral salmon calcitonin induced suppression of urinary collagen type II degradation in postmenopausal women: a new potential treatment of osteoarthritis. Bone 37(3):425–430
Manicourt DH, Azria M, Mindeholm L, Thonar EJ, Devogelaer JP (2006) Oral salmon calcitonin reduces Lequesne’s algofunctional index scores and decreases urinary and serum levels of biomarkers of joint metabolism in knee osteoarthritis. Arthritis Rheum 54(10):3205–3211
Manicourt DH, Bevilacqua M, Righini V, Famaey JP, Devogelaer JP (2005) Comparative effect of nimesulide and ibuprofen on the urinary levels of collagen type II C-telopeptide degradation products and on the serum levels of hyaluronan and matrix metalloproteinases-3 and -13 in patients with flare-up of osteoarthritis. Drugs R D 6(5):261–271
Lehmann HJ, Mouritzen U, Christgau S, Cloos PA, Christiansen C (2002) Effect of bisphosphonates on cartilage turnover assessed with a newly developed assay for collagen type II degradation products. Ann Rheum Dis 61(6):530–533
Acknowledgements
Reetakshi Arora, PhD
John I. Thornby, PhD
Conflicts of interest statement
None of the authors involve have any financial and personal relationships with other people or organizations that could inappropriately influence the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalez-Fuentes, A.M., Green, D.M., Rossen, R.D. et al. Intra-articular hyaluronic acid increases cartilage breakdown biomarker in patients with knee osteoarthritis. Clin Rheumatol 29, 619–624 (2010). https://doi.org/10.1007/s10067-010-1376-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-010-1376-8