Abstract
This case describes a patient who developed diffuse fasciitis with eosinophilia (DFE) after an exaggerated local response to radiation following excision of a lymph node-negative breast cancer. Our patient’s fasciitis was diffuse, involving both upper and lower extremities and the trunk at sites distant from the irradiation and tumor site. The fasciitis progressed after curative excision of the breast cancer rather than concurrently with active breast cancer and persisted despite therapy; there was no tumor reoccurrence. With three published cases linking localized eosinophilic fasciitis with breast cancer, and with the literature suggesting that DFE commonly presents after a traumatic trigger, the possibility that radiation therapy for breast cancer could be one such trigger is an important insight for clinicians treating the many patients who undergo breast cancer treatment each year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
A 67-year-old Caucasian female with a history of breast cancer status post lumpectomy and local radiation was referred to Johns Hopkins Scleroderma Center in February 2002 for evaluation of pain, swelling, erythema, and sclerosis of her skin with concomitant eosinophilia.
The patient developed biopsy-proven giant cell arteritis and associated polymyalgia rheumatica in 1995 that responded without visual sequelae to a 1-year, tapering course of oral prednisone. The patient returned to her usual state of good health until June 2000, when a screening mammogram demonstrated a 0.5-cm mass in the upper outer quadrant of the left breast. A fine needle aspiration yielded malignant cells, and a sentinel node biopsy and wide local excision of the mass were performed in July 2000, exactly 1 month after the original mammogram. The sentinel node was negative, and histopathology of the 0.9-cm excised nodule revealed a 0.4-cm infiltrating ductal carcinoma, with an in situ component and clear margins. The tumor was estrogen receptor positive and progesterone receptor negative; Her-2-neu was 2-plus. From August to September 2000, the patient underwent a 6-week course of local radiation therapy. She received doses of radiation that totaled 4,500 cGy for the left breast and 1,440 cGy for the cone down component.
Following radiation treatment to the left breast and 3 days of tamoxifen treatment in February 2001, the patient’s irradiated left breast developed swelling and erythema. During the next month, the adjacent chest wall also became swollen and erythematous. In April 2001, she was given a 2-week course of cephalexin for presumed cellulitis, but the swelling and erythema persisted and were soon accompanied by sclerosis of the skin over the left breast and, to a lesser extent, the adjacent chest wall. Since the symptoms did not improve, cephalexin was switched to linezolid and furosemide. The patient then developed what was considered to be a diffuse hypersensitivity reaction with generalized erythema, and prednisone 80 mg PO daily was initiated on April 19, 2001.
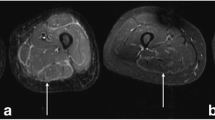
The patient continued on antibiotic therapy for several months, including courses of dicloxacillin and tetracycline. In May 2001, the patient developed painful bilateral distal upper extremities with swelling and erythema similar to that on the chest wall and breast areas, and was found to have 40% eosinophils in her peripheral blood count. Eventually, the affected skin thickened, and the inflammatory skin changes progressed to involve the proximal upper extremities, back, abdomen, and legs. The lower extremities were affected from the hip to the foot bilaterally, with the greatest degree of involvement distally, at the foot and ankle. During this time, prednisone was decreased to 15 mg PO daily, and topical muprocin ointment and betamethasone dipropionate 0.05% gel were prescribed for BID application to the left breast. Symptoms did not improve on this regimen. Magnetic resonance imaging (MRI) of the patient’s musculature at this time revealed an increased T2 signal typical of fluid or inflammation in the fascial layer of the tissues (Figs. 1 and 2) and was interpreted as consistent with fasciitis. Given the physical findings and striking MRI findings, a diagnosis of fasciitis was made, and the patient refused tissue biopsy. Treatment was initiated with a 250 mg pulse of methylprednisolone and methylprednisolone 8 mg PO BID on May 31, 2001. While on steroid therapy, the patient noted improvement in the skin over the breasts and distal upper extremities, but continued to have stiffness in the arms and progressing discomfort around the feet and ankles. Signs of inflammation persisted, and methotrexate 22.5 mg per week was added to a tapering regimen of methylprednisolone.
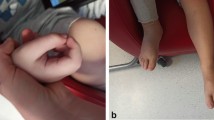
At the time of presentation to Johns Hopkins, approximately 12 months after the onset of skin disease, the patient’s main complaint was stiffness and pain in the involved areas. She denied any symptoms of systemic disease. She had a history of “cold handedness” without Raynaud’s phenomenon. There was significant thickening of the skin over the dorsum of the hands, distal and proximal upper extremities, chest, abdomen, proximal, and distal lower extremities, as well as dorsum of the feet. The most dramatic skin thickening was seen from the shins to the dorsum of the feet. Puckering of the tissues consistent with fibrosis in the fascial layer (“peau d’orange”) was evident between the shoulders and elbows (Fig. 3), and over the abdomen, particularly over the anterior and lateral trunk bilaterally. The left breast, which was the site of previous radiation therapy, had significant tissue atrophy with sclerosis and multiple telangectasias, sparing the nipple. The skin was normal over the fingers and palmar surface of the hands as well as on the face. There was mild erythema of the conjunctivae, but no arthropathy, lympadenopathy, or hepatosplenomegaly. The remainder of her examination was unremarkable, including normal nailfold capillaries. On presentation, the only abnormal laboratory finding was a 5% eosinophilia (total eosinophil number of 350/mm3) with a normal erythrocyte sedimentation rate of 15 mm/h while on daily alternating doses of 2 and 4 mg of methylprednisolone, totaling 20 mg of methylprednisolone per week. A diagnosis of diffuse fasciitis with eosinophilia was confirmed based on the clinical presentation, MRI findings, and laboratory data.
In the 2 years subsequent to presentation at Johns Hopkins, the patient has had no evidence of recurrent breast cancer. The signs of fasciitis were intense and involved areas of the trunk and limbs, becoming quite disabling. She was intolerant to continued corticosteroids due to side effects of weight gain and Cushingoid syndrome. Because of continued disease activity, steroid sparing anti-inflammatory treatments were used. Her treatment regimen in sequence included various doses of methylprednisolone, methotrexate, mycophenolate mofetil, intravenous immunoglobulin, and tumor necrosis factor inhibition. Each trial was shortened secondary to intolerance without serious toxicity. No other systemic disease is currently evident, and laboratory data is normal except for a persistently elevated eosinophil count at 18%. Gradually, the process has improved with softening of the tissues and improved normal activity, including a regular exercise program. Her main complaint is continued stiffness in the hands, wrists, feet, and ankles, with signs of intense sclerosis of the skin persisting in these areas. She has now been cancer free for 8 years.
Discussion
Our case demonstrates a striking temporal association of the onset of diffuse fasciitis with eosinophilia and very local radiation treatment following lumpectomy for adenocarcinoma of the breast. While local fibrosis of the skin and underlying tissues is known to occur following radiation therapy, diffuse fasciitis with eosinophilia has not previously been linked to antecedent local radiation. Likewise, no case of breast cancer preceding diffuse fasciitis with eosinophilia involving such large areas of the body is reported. The only published associations between the two are a case report of two sisters who developed focal areas of eosinophilic fasciitis and contemporaneous breast cancer [1] and a case of a patient with breast cancer and angioimmunoblastic lymphadenopthy who developed sleeve-like fasciitis of the calves [2]. There have been no published associations between DFE and tamoxifen use.
While the cause of diffuse fasciitis with eosinophilia (DFE) is unknown, it has been associated with vigorous exercise and tissue trauma. A temporal association has also been noted with various hematologic abnormalities including aplastic anemia, myelomonocytic and chronic lymphocytic leukemia, thrombocytopenia, monoclonal gammopathy, myeloproliferative syndrome, and lymphomas [3]. Naschitz et al. have suggested that eosinophilic fasciitis is an idiopathic form of a group of fasciitis–panniculitis syndromes [2]. The literature documents that a variety of insults including post-irradiation, late graft-versus-host disease [4], and cancer including solid tumors such as prostate cancer [5] are associated with fasciitis–panniculitis reactions. Our case is unique in that diffuse fasciitis with eosinophilia began soon after localized radiation, and at a time when there was no evidence of active breast cancer. In addition, it progressed despite the lack of any new radiation therapy or evidence of recurrent breast disease.
A review of the literature revealed several described cases as “localized scleroderma” of the breast following radiation treatment for breast cancer. In these cases, post-irradiation fibrosis was confined to the region that received direct radiation. One study cited the incidence of radiation-induced “localized scleroderma” as one in 500 patients who receive radiation for breast cancer [6]. In the reported cases of radiation-induced localized scleroderma, the breast that received radiation treatment becomes indurated, retracted, and erythematous to violaceous with progressive pigmentation within the treatment field [6]. Other authors use the term “postirradiation morphea” to describe “morphea” developing at the site of previous radiation, from several months to 32 years after radiation therapy for breast cancer [7]. The natural history of these lesions includes an inflammatory phase resolving over the course of approximately 1 year with residual fibrosis remaining [7]. Chronic progressive localized areas of radiation-induced fibrosis of tissues is also reported as a frequent complication following radiation therapy to the thorax and pelvis [8]. Treatment with both topical and intralesional steroids has been cited as effective in these patients [9].
Only two cases of fibrosing skin reactions at sites distant from the areas of radiation treatment for breast cancer have been reported. One case report describes a patient who, 13 years after receiving radiotherapy for bilateral breast cancer, developed “morphea” both over the previously irradiated regions of the chest wall as well as in a stocking distribution over both feet and ankles [10]. In these areas, the skin was markedly hyperpigmented, thickened, and tight. A similar case was reported in 2003 of a patient who received radiation therapy for endometrial cancer followed by radiation for breast cancer several years later who subsequently developed “localized scleroderma” over the previously irradiated breast and abdominal wall as well as on the lower extremities bilaterally [11]. In these two reported cases, the only distant sites of localized tissue sclerosis involved the lower extremities. The terms “morphea” and “localized scleroderma” in these cases likely represent local tissue sclerosis rather than true immune mediated localized scleroderma or morphea; no cases are reported of widespread radiation-induced tissue sclerosis following localized radiation in a previously normal individual.
Clinically, DFE can be distinguished from systemic sclerosis (scleroderma) in that DFE exhibits a characteristic “peau d’orange” puckering of the skin due to an inflammatory fibrotic reaction in the fascial layer of the tissues. Sclerosis then extends into the muscle and skin, mimicking the skin findings of scleroderma, and causing restricted motion of the involved tissues. In terms of distribution, DFE and scleroderma differ in that scleroderma typically is most intense on the distal limbs and involves the fingers and face, while in DFE these regions are spared. As seen in our case, patients with DFE are characteristically free from internal organ involvement.
The mechanism for inducing exaggerated fibrosis of tissue following radiation is unknown. It is interesting to speculate that non-specific tissue injury from radiation therapy could represent a trigger for diffuse fasciitis similar to other reported cases of tissue injury that may occur following exercise or trauma thought to precede the onset of DFE.
While our case does not have a tissue diagnosis through biopsy, the clinical presentation and imaging findings yield a convincing diagnosis of diffuse fasciitis with eosinophilia: “peau d’orange” cutaneous findings, lack of Raynaud’s phenomenon or internal organ involvement, hypereosinophilia, and classic MRI findings of fascial T2 uptake. There are published cases of DFE diagnosed based on peripheral eosinophilia with classic MRI findings of fascial T2 uptake [12, 13]. Baumann et al. reviewed six biopsy-proven DFE cases retrospectively and demonstrated that MRI demonstrating fascial inflammation correlated with the fascial biopsies that were obtained at baseline and with subsequent clinical remission [14].
While three cases have been published that suggest an association between breast cancer and DFE, our case differs from these in two significant ways. First, our patient’s fasciitis was diffuse rather than limited to focal areas of sclerosis as in the previously published cases in patients with breast cancer. Second, our patient’s fasciitis occurred after curative excision of the breast cancer rather than concurrently with active breast cancer—she has now been cancer free for 8 years. While DFE as a paraneoplastic process cannot be definitively excluded in this patient, the development of diffuse fasciitis occurring soon after an exaggerated response to local radiation—7 months after the breast cancer was removed—is striking. With three published cases linking localized eosinophilic fasciitis with breast cancer, and with the literature suggesting that DFE commonly presents after a traumatic trigger, the possibility that radiation therapy for breast cancer could be one such trigger is an important insight for clinicians treating the many patients who undergo breast cancer treatment each year.
References
Watts RA, Merry P (1994) Familial eosinophilic fasciitis and breast cancer. Br J Rheumatol 33:93–94
Naschitz JE, Boss JH, Misselevich I, Yeshurun D, Rosner I (1996) The fasciitis–panniculitis syndromes. Medicine 75:6–15
Doyle JA (1984) Eosinophilic fasciitis: extracutaneous manifestations and associations. Cutis 34:259–261
Janin A, Socie G, Devergie A, Aractingi S, Esperou H, Verola O, Gluckman E (1994) Fasciitis in chronic graft-versus-hosts disease. Ann Intern Med 120:993–998
Naschitz JE, Yeshrun D, Zuckerman E, Rosenbaum M, Misselevich I, Shajrawi I, Boss JH (1994) Cancer-associated fasciitis–panniculitis. Cancer 73:231–235
Bleasel NR, Stapleton KM, Commens C, Ahern VA (1999) Radiation-induced localized scleroderma in breast cancer patients. Aust J Dermatol 40:99–102
Schaffer JV, Carroll C, Dvoretsky I, Huether MJ, Girardi M (2000) Postirradiation morphea of the breast: presentation of two cases and review of the literature. Dermatology 200:67–71
Murray JC (1994) Radiation-induced fibrosis: the structure/function relationship. Scanning Microsc 8:79–87
Gollob MH, Dekhoven JG, Bell MJ, Assaad D, Rao J (1998) Postradiation morphea. J Rheumatol 25:2267–2269
Ardern-Jones MR, Black MM (2003) Widespread morphoea following radiotherapy for carcinoma of the breast. Clin Exp Dermatol 28:160–162
Trattner A, Figer A, David M, Lurie H, Sandbank M (1991) Circumscribed scleroderma induced by postlumpectomy radiation therapy. Cancer 68:2131–2133
Sugimoto T, Nitta R, Kashiwai A (2007) Usefulness of magnetic resonance imaging in eosinophilic fasciitis. Rheumatol Int 27:791–792
Agnew KL, Blunt D, Francis ND, Bunker CB (2005) Magnetic resonance imaging in eosinophilic fasciitis. Clin Exp Dermatol 30:435–436
Baumann F, Bruhlmann P, Andreisek G, Michel B, Marincek B, Weishaupt D (2005) MRI for diagnosis and monitoring of patients with eosinophilic fasciitis. Am J Roentgenol 184:169–174
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author participated in the preparation of the manuscript. No financial support was received by any of the above authors in the preparation of this manuscript. There are no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Sherber, N.S., Wigley, F.M. & Paget, S.A. Diffuse fasciitis with eosinophilia developing after local irradiation for breast cancer. Clin Rheumatol 28, 729–732 (2009). https://doi.org/10.1007/s10067-009-1122-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-009-1122-2